Abstract
Purpose of review
This review summarizes the most recent evidence linking decreased sleep duration and poor sleep quality to obesity, focusing upon studies in adults.
Recent findings
Published and unpublished health examination surveys and epidemiological studies suggest that the worldwide prevalence of obesity has doubled since 1980. In 2008, 1 in 10 adults was obese, with women more likely to be obese than men. This obesity epidemic has been paralleled by a trend of reduced sleep duration. Poor sleep quality, which leads to overall sleep loss has also become a frequent complaint. Growing evidence from both laboratory and epidemiological studies points to short sleep duration and poor sleep quality as new risk factors for the development of obesity.
Summary
Sleep is an important modulator of neuroendocrine function and glucose metabolism and sleep loss has been shown to result in metabolic and endocrine alterations, including decreased glucose tolerance, decreased insulin sensitivity, increased evening concentrations of cortisol, increased levels of ghrelin, decreased levels of leptin, and increased hunger and appetite. Recent epidemiological and laboratory evidence confirm previous findings of an association between sleep loss and increased risk of obesity.
Keywords: ghrelin, leptin, obesity, sleep duration, sleep quality
Introduction
According to recent estimates, the worldwide prevalence of obesity has doubled since 1980 [1]. This obesity epidemic has been paralleled in modern society by a trend of reduced sleep duration [2]. Poor sleep quality, which is often associated with overall sleep loss, has also become a frequent complaint [2]. Growing evidence both from laboratory and epidemiological studies points to short sleep duration as a new risk factor for the development of obesity and its complications [3,4]. Sleep is an important modulator of neuroendocrine function and glucose metabolism and sleep loss has been shown to result in metabolic and endocrine alterations, including decreased glucose tolerance and alteration of appetite regulating hormone.
This brief review summarizes the most recent literature examining the link between sleep loss and obesity, focusing upon studies in the adults.
Sleep duration and obesity risk: epidemological evidence
Sleep ‘is a restorative process of the brain, by the brain, and for the brain’ [5], but it is now clear that sleep is important for health of the entire body. The decrease in sleep duration and increase in sleep complaints in modern society [2] raise concerns for a negative impact of chronic sleep disturbances on health in general, not only mental health.
Behavioral sleep curtailment is becoming endemic in modern times. Ours is a 24-h society with more evening and night-time work and leisure activities, which all lead to a sacrifice of hours available for sleep. This has had a major impact on sleep time, duration of dark exposure, and overall organization of circadian rhythms through the exposure to artificial light after sunset and often before sunrise, resulting in later bedtimes, reduced total sleep time, and the opportunity to be active and ingest food during the natural night.
Feeding represents a major synchronizer of peripheral circadian clocks, which have been found in virtually all tissues. Delayed feeding due to prolonged night-time wakefulness leads to desynchrony between central circadian and peripheral clocks [6].
Indeed circadian desynchrony as it occurs in shift workers is associated with cardiometabolic alterations [7] and increased risk of metabolic syndrome and cardiovascular disease [6,8–10]. On the basis of the link between circa-dian desynchrony and obesity and metabolic disorders, obesity could represent a ‘chronobiological disease’ [10].
To date, approximately 50 epidemiological studies done in different geographical regions have examined the association between sleep and obesity in adults and children. The majority found a significant association between short sleep (generally <6 h per night) and increased obesity risk [11–14]. A meta-analysis of 18 studies in 604 509 adults demonstrated a pooled obesity odds ratio (OR) of 1.55 (1.43–1.68; P < 0.0001) for less than 5 h of sleep and a dose effect of sleep duration such that for each additional hour of sleep BMI decreased by 0.35 kg/m2 [15].
Table 1 summarizes the most recent cross-sectional and prospective studies [16–19,20•,21•,22,23•–25•].
Table 1.
Summary of the recent epidemiological studies examining the association between sleep duration and obesity in adults
| Author | Description | Cohort | Sleep assessment | Results |
|---|---|---|---|---|
| Buxton and Marcelli [16] | Cross-sectional Data source: 2004–05 US National Health Interview Survey (US) |
56 507 M, F Age 18–85 years |
Self-reported sleep duration | <7 h 6% higher probability of obesity 7–8–h reference category >8 h 3% higher probability of obesity |
| Magee et al. [17] | Cross-sectional Data source: 45 and UP Study data (Australia) |
45 325 M, F Age 55–95 years |
Self-reported sleep duration | Age 55–64 years <6 h AOR obesity 1.52 (CI 1.21–1.89; P < 0.001) 6 h AOR obesity 1.42 (CI 1.26–1.61; P < 0.001) 7 h reference category ≥9 h AOR obesity 1.19 (CI 1.06–1.34; P < 0.001) Age >65: NS |
| Magee et al. [18] | Cross-sectional Data source: 45 and UP Study (Australia) |
16 951 M, F full-time workers Age 45–65 years |
Self-reported sleep duration | Inverse association between sleep duration and BMI (β = –0.615, P < 0.001) |
| Anic et al. [19] | Cross-sectional Data source: Collaborative Breast Cancer Study (US) |
5549 F Age 20–75 years |
Self-reported sleep duration | <6 h AOR of obesity 1.89 (CI 1.45–2.47; P < 0.0001); AOR extreme obesity 3.12 (CI 1.70–5.75; P = 0.0003) 6–6.9 h AOR obesity 1.52 (CI 1.23–1.89; P = 0.0003); AOR extreme obesity 2.22 (CI 1.27–3.87; P = 0.0003) 7–7.9 h reference category ≥9 h AOR obesity NS; AOR extreme obesity 2.53 (CI 1.10–5.78; P = 0.023) |
| Theorell-Haglow et al. [20•] | Cross-sectional Data source: Sleep and Health in Women Study (Sweden) |
400 F Age 29–70 years |
PSG recorded sleep duration | Inverse association between sleep duration and both waist circumference (Adj. β = –1.22 cm/h; P = 0.016) and sagittal abdominal diameter (Adj. β = –0.46cm/h; P = 0.001). |
| Watanabe et al. [21•] | Prospective 1 year Data source: workers of electric power company (Japan) |
31 477 M (mean age 40 ± 9 year old) and 3770 F (mean age 38 ± 9 years) | Self-reported sleep duration | M <5 h AOR of obesity 1.91 (CI 1.36–2.67; P < 0.001) 5–5.9 h AOR of obesity 1.5 (CI 1.25–1.8; P < 0.001) 7–8 h reference category F: NS |
| Bo et al. [22] | Prospective 6 years (Italy) | 1597 M, F Age 45–64 years |
Self-reported sleep duration | Each hour increase in total sleep time = 30% reduction in incident obesity (AOR 0.7/h; CI 0.57–0.86; P < 0.001) |
| Nishiura and Hashimoto [23•] | Prospective 4 years Annual health screen at a gas company (Japan) |
2362 M Age 40–59 years |
Self-reported sleep duration | <6 h AOR of obesity 2.46 (CI 1.41–4.31; P = 0.011) 7–7.9 h reference category ≥8 h NS |
| Hairston et al. [24•] | Prospective 5 years Three communities from the IRAS Family Study (USA) |
322 M, F African–Americans and 775 M, F Hispanic Americans Age 18–81 years |
Self-reported sleep duration | Age <40 years ≤5 h increase in BMI (+1.8 kg/m2, P < 0.001), SAT (+41 cm2, P < 0.0001), and VAT (+13cm2, P < 0.01) 6–7 h reference category ≥8 h increase in BMI (+0.8 kg/m2, P < 0.001), SAT (+20 cm2, P < 0.01), and VAT (+6 cm2, P < 0.05) Age >40: NS |
| Hayes et al. [25•] | Cross-sectional study Data source: Cleveland Family Study (USA) |
561 M, F Mean age 44.5 ± 16.1 years |
PSG recorded sleep duration | Each hour decrease in total sleep time: 6% increase in leptin levels (P = 0.01) 14% increase in visfatin levels (P = 0.02) Each hour decrease in REM sleep: 15% increase in leptin levels (P = 0.01) 31% increase in visfatin levels (P = 0.05) |
In each study, the association is expressed as higher probability or adjusted odds ratio (AOR) of obesity (BMI ≥30kg/m2) or increased waist circumference. Studies were conducted in different geographical regions. Sleep duration was self-reported or measured by overnight polysomnography (PSG). Adj. β, adjusted beta coefficient; CI, confidence interval; F, female; IRAS, Insulin Resistance Atherosclerosis Study; M, male; NS, not significant; REM, rapid eye movement; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Buxton and Marcelli [16] demonstrated a 6% increase in the probability of obesity in 56 507 US adults with a wide age range (18–85 years) for self-reported sleep duration of less than 7 h per night. An analysis of the data in 45 325 adults from the ‘45 and UP Australian Study’ confirmed an increased risk of obesity for short sleep duration in the 55–64 age group [adjusted OR (AOR) 1.52 for sleep <6 h, confidence interval (CI) 1.21–1.89, and 1.42 for sleep = 6 h, CI 1.26–1.61] but not in the elderly (>65 years) [17]. Additionally, in a smaller cohort from the same data set they demonstrated that in men, but not women, increased work hours were associated with higher BMI [18]. The association was partially mediated by sleep duration because men who worked longer hours slept less.
Anic et al. [19] in a cohort of 5549 US adult women demonstrated that the effect of short sleep duration was even stronger for extreme obesity (AOR of BMI ≥40 kg/m2 3.12 for <6 h of sleep, CI 1.70–5.75).
A recent single study objectively measured sleep duration by full-night polysomnography [20•] and demonstrated an inverse correlation between sleep time and both waist circumference [adjusted beta coefficient (Adj. β) –1.22 cm/h; P = 0.016] and sagittal abdominal diameter (Adj. β –0.46 cm/h; P = 0.001) [26]. These associations were stronger in women less than 50 years old.
The largest prospective study followed 35 247 Japanese workers (90% males) over 1 year [21•]. Short sleep duration (6 h or less) was associated with an increased risk of obesity in men only with an AOR 1.91 for the shortest sleep duration (<5 h; CI 1.36–2.67) and AOR 1.5 for 5–6 h of sleep (CI 1.24–1.8). The lack of a similar finding in women may be attributed to the small sample size. A 6-year Italian study demonstrated in 1597 male and female adults that every additional hour of sleep decreased the incidence of obesity by 30% [22]. In a prospective study, Nishiura and Hashimoto [23•] analyzed the dietary patterns of 2362 non-obese Japanese workers. The association between short sleep and obesity (AOR 2.46 for <6 h; CI 1.41–4.31) was only partially explained by dietary patterns (preference for fatty food, skipping breakfast, snacking, and eating out).
Hairston et al. [24•] performed abdominal computed tomography (CT) scans to evaluate visceral and subcutaneous adipose tissue (VAT and SAT, respectively) at baseline and 5 years in 322 African–Americans and 775 Hispanic Americans. A sleep duration of 5 h or less was associated with an increase in BMI (+1.8 kg/m2, P < 0.001), in VAT (+13 cm2, P > 0.01), and in SAT (+42 cm2, P < 0.0001), only in younger patients (≤40 years old).
The recent studies [17,20•,24•] confirm the previously published results of a stronger relationship between sleep duration and obesity risk in the younger cohorts, suggesting that age-related factors other than sleep disturbances may play a more important role in the development of obesity later in life.
Sleep duration and obesity risk: laboratories studies
The relationship between sleep and obesity is likely mediated by multiple pathways. An upregulation of the activity of orexin neurons and changes in appetite-regulating hormones may affect food intake. It has been previously shown that ghrelin, a hormone promoting hunger, increases with sleep restriction, whereas leptin, a hormone contributing to satiety perception, decreases [3,11,27–29]. More recently, Spiegel et al. [30] analyzed the 24 h ghrelin profile in relation to meal and sleep in 14 healthy young men and showed an inhibitory effect of sleep on ghrelin secretion.
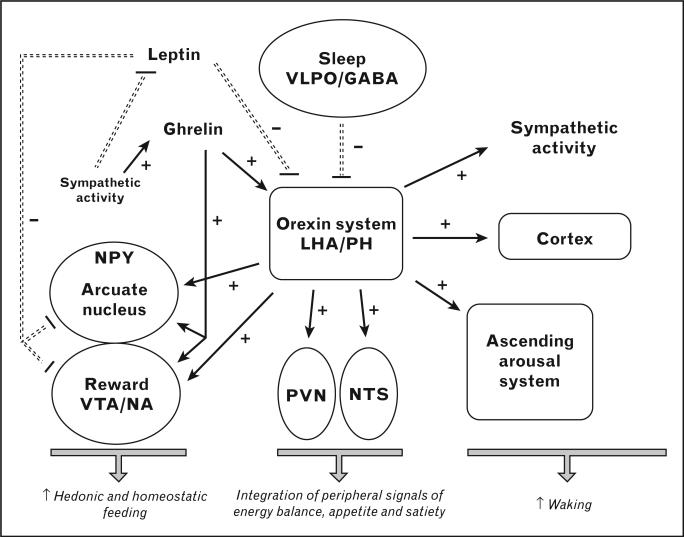
Figure 1 provides a schematic representation of the orexin system, which represents the link between sleep and feeding. Orexigenic neurons regulate the homeostatic feeding center in the hypothalamic arcuate nucleus, and concurrently affect hedonic feeding mediated by the ‘reward centers’ (ventro-tegmental area and nucleus accumbens) [11]. Leptin and ghrelin are peripheral signals directly interacting with the arcuate nucleus, and ultimately modulating the orexin system activity to decrease and increase food intake, respectively. Their secretion is also modulated by the autonomic nervous system activity. A shift of the sympathovagal balance to higher sympathetic activity has been observed in studies of sleep deprivation [27,31].
Figure 1. Main pathways connecting sleep–wake regulation and feeding and possible mechanisms for the adverse impact of sleep loss on energy homeostasis.
Central to this hypothesis is the role of the orexin system, which is inhibited by sleep-promoting neurons in the ventrolateral preoptic area (VLPO) containing gamma-aminobutyric acid (GABA). The orexigenic neurons, located in the lateral hypothalamic area (LHA) and posterior hypothalamus (PH), play a major role in the maintenance of arousal by activating the ascending arousal system and the entire cerebral cortex and modulating other central nervous system nuclei and structures involved in sleep–wake regulation. Orexin activity is also involved in the regulation of feeding by: (a) increasing the activity of the neuropeptide Y (NPY) neurons in the arcuate nucleus of the hypothalamus, thus, affecting homeostatic food intake; (b) stimulating the nucleus tractus solitarius (NTS) and the paraventricular nucleus (PVN), which integrate peripheral signals of energy balance, appetite, and satiety; (c) stimulating the dopaminergic ventrotegmental area (VTA) and nucleus accumbens (NA), the ‘reward centers’, which regulate nonhomeostatic food intake; (d) increasing sympathetic activity, which will in turn inhibit leptin release and stimulate ghrelin release. Lower leptin and higher ghrelin levels will act in concert to further activate orexin neurons resulting in an increased drive for both homeostatic and nonhomeostatic food intake. Adapted with permission from [11].
Additionally, sleep loss could affect energy balance by decreasing both exercise and nonexercise energy expenditure. Leptin by itself increases energy expenditure [32]; therefore, changes in leptin after sleep deprivation would affect both caloric intake and energy expenditure. Energy expenditure in sleep loss has been poorly studied, but it is conceivable that sleepiness and fatigue increase sedentary behavior and therefore decrease exercise-related energy expenditure under real life conditions [33]. Moreover, decreased body temperature has been shown in conditions of prolonged total sleep deprivation [34].
Jung et al. [35] recently demonstrated that after 40 h of sleep deprivation, participants showed an increase of 24-h energy expenditure, largely due to a 32% increase during the night. The decrease in leptin and increase in ghrelin observed in similar studies of total sleep deprivation [36,37] could be interpreted not only as a direct effect of sleep loss, but also as a short-term compensation to the increased energy expenditure rather than a direct effect of sleep loss.
Six recently published laboratory studies of sleep deprivation are summarized in Table 2 [38•,39–43]. These utilized various sleep interventions and caloric intake conditions but did not measure energy expenditure. The initial finding of a decrease in leptin levels after partial sleep deprivation was observed under conditions of strictly controlled caloric intake and BMI was unchanged [27,28]. When feeding is ad libitum an increase in weight generally occurs and has therefore the opposite effect on leptin levels, which may be more responsive to changes in adiposity than to changes in sleep duration. Ghrelin levels were measured in only one of the six studies.
Table 2.
Summary of the recent laboratory studies examining the effects of sleep deprivation on weight, hunger, food intake, and appetite regulating hormones
| Author | Intervention | Participants | Caloric intake | Changes with sleep deprivation |
|---|---|---|---|---|
| Nedeltcheva et al. [38•] | Partial sleep deprivation TIB 7 h × 2 days vs. 5.5 h × 14 days and 8.5 h × 14 days | 10 M, F; mean age 41 ± 5 years; mean BMI 27.4 ± 2 kg/m2 | Caloric content restricted to 90% of resting metabolic rate | 5.5 h (vs. 8.5) Same weight loss ↓ fat mass loss ↑ fat-free mass loss Leptin (24h profile): ←→ Acylated ghrelin (24 h profile): ↑ Hunger: ↑ |
| Omisade et al. [39] | Partial sleep deprivation TIB 10 h × 2 days vs. 3 h × 1 day | 15 F; age 18–25 years; BMI 18.3–51.9 kg/m2 | Matched meals | Weight: n/a Leptin (AM and PM assessment): ↑ Ghrelin: n/a Hunger: ←→ Caloric intake: n/a |
| Simpson et al. [40] | Partial sleep deprivation TIB 10 h × 2 days vs. 4 h × 5 days | 136 M, F; age 22–45 years; BMI 17.7–32.6 kg/m2 | Ad libitum food access | Weight: n/a Leptin (single AM assessment): ↑ Ghrelin: n/a Hunger: n/a Caloric intake: n/a |
| Pejovic et al. [41] | Total sleep deprivation TIB 8 h × 4 days 40 h sleep deprivation 8 h × 2 days (recovery) | 21 M, F; age 18–30 years; lean and overweight | Non-controlled food intake | TSD (vs. recovery): Weight: n/a Leptin (24h profile): ↑ Ghrelin: n/a Hunger: ←→ Food intake: ←→ |
| Brondel et al. [42] | Partial sleep deprivation TIB 8 h × 2 days vs. 4 h × 1 day | 12 M; age 18–29 years; BMI 19–24.6 kg/m2 | Ad libitum food intake after sleep restriction | Weight: n/a Leptin: n/a Ghrelin: n/a Hunger: ↑ before breakfast and dinner Caloric intake: ↑ |
| Tasali et al. [43] | Partial sleep deprivation TIB 8.5 h × 4 days vs. 4.5 h × 4 days | 10 M, F; age 18–28 years; BMI 20–25 kg/m2 | Matched meals/ad libitum buffet at the end | Weight: ←→ Leptin (single AM assessment): ↑ Ghrelin: n/a Hunger: n/a Caloric intake: n/a |
F, female; M, male; n/a, not available; TIB, time in bed; TSD, total sleep deprivation.
In 10 overweight middle-aged adults under condition of caloric restriction, Nedeltcheva et al. [38•] observed an increase in ghrelin levels and hunger, but not change in leptin levels, after 2 weeks of sleep restriction (–1.5 h per night) compared with 2 weeks of sleep extension (+1.5 h per night).
Two studies measured morning leptin after partial sleep deprivation [39,40] and demonstrated an increase, rather than a decrease. This finding could be explained by the delay of bedtime to the early morning with a shift forward of the nocturnal elevation of leptin. Simpson et al. [40] allowed ad libitum food intake throughout the study, but they did not evaluate any change in weight or total caloric intake.
Pejovic et al. [41] confirmed a 24-h circadian rhythm of leptin with a daytime suppression following nighttime sleep. After a night of total sleep deprivation, the leptin profile was flattened due to higher daytime levels. This phenomenon may be explained by the lack of suppression from the previous night-time sleep. Hunger, food intake, and preference were unchanged in condition of uncontrolled caloric intake. Ghrelin was not measured.
Higher leptin levels associated with decreased sleep time have also been reported in a recent cross-sectional study by Hayes et al. [25•]. Morning leptin levels were inversely associated with total sleep time; for each hour of decreased sleep there was a 6% increase in leptin levels after controlling for obesity and associated comorbidities (Table 1).
The Wisconsin Sleep Cohort study, consisting of 1024 volunteers, found that 5 h of habitual sleep time, as assessed by polysomnography, was associated with a 15% decrease in morning leptin levels and a similar increase in morning ghrelin levels [42].
The different results of the laboratory studies may be attributed to the difference in the study design such as the duration of the sleep restriction protocol and the caloric intake during the leptin and ghrelin sampling period (controlled [27,38•,39] vs. noncontrolled food intake [40,41]).
Eating behavior after sleep deprivation was also examined in two recent studies. Brondel et al. [42] described increased caloric intake and hunger after 4 h of night sleep in 12 normal weight young adults. Similarly, preliminary data in 10 healthy young adults by Tasali et al. [43] reported a 14% increase in caloric intake, particularly for carbohydrate-rich nutrients, during an ad libitum buffet, after four nights of 4.5 h in bed, compared with 8.5 h.
These findings suggest that excessive food intake associated with insufficient sleep may be a mechanism for increased obesity risk.
Sleep disturbance and obesity risk
Table 3 summarizes the most recent studies examining the relationship between sleep disturbance and obesity risk [44–46,47•,48].
Table 3.
Summary of the recent studies examining the relationship between poor sleep quality and measures of obesity and appetite regulation
| Author | Description | Cohort | Sleep assessment | Results |
|---|---|---|---|---|
| Eun et al. [44] | Interventional study (OSA surgery); patient from sleep center with obstructive sleep apnea (Korea) | 51 M; age 26–65 years; BMI 20.9–35.8 kg/m2 | PSG | Decreased leptin levels after surgery (before 8.1 ± 7.3 vs. after 6.1 ± 5.5, <0.001) without change in BMI |
| Ursavas et al. [45] | Cross-sectional Patient from Sleep Center (Turkey) |
55 apneic patients; mean age 51.1 ± 1.2 years; mean BMI 32.5 ± 0.9 kg/m2 15 nonapneic |
PSG | Ghrelin level significantly higher in OSA group (565 ± 44 pcm/ml vs. 403 ± 90 pcm/ml) Leptin: NS |
| Bidulescu et al. [46] | Cross-sectional Data source: Cardiovascular Health Epidemiology Study (USA) |
1515 M, F African–American Age 30–65 years |
Self-reported sleep quality (Pittsburgh Sleep Quality Index) | F Low sleep quality: AOR obesity 1.08 (CI 1.03–1.12) Score of Sleep disturbance: AOR obesity 1.48 (CI: 1.16–1.89) M: NS |
| Lyytikainen et al. [47•] | Prospective 5–7 years Data source: Helsinki Health Study (Finland) |
7332 M, F Age 40–60 years |
Self-reported sleep quality (Jenkins Sleep Questionnaire) | F Difficulty initiating sleep: AOR weight gain ≥5 kg; 1.65 (95% CI: 1.22–2.22) Wake up several time: AOR weight gain ≥5 kg; 1.48 (95% CI: 1.16–1.89) Trouble staying asleep AOR weight gain ≥5 kg; 1.41 (95% CI: 1.13–1.75) M: NS |
| Nordin and Kaplan [48] | Prospective 30 years Data source: Alameda County Study (USA) |
≈2700 M, F Age ≥17 years |
Self-reported sleep quality (continuity) | Consistent sleep discontinuity: 70% increase of obesity risk Both consistent sleep discontinuity and impaired sleep continuity: reduced chance for transitioning from obesity, increased risk of staying obese Improved sleep continuity: NS |
Sleep quality was self-reported or assessed by overnight polysomnography (PSG). AOR, adjusted odds ratio; CI, confidence interval; F, female; M, male; NS, not significant; OSA, obstructive sleep apnea.
Obstructive sleep apnea
Sleep loss occurs not only as a result of habitual behavior, but also in presence of pathological conditions associated with disturbed sleep, like obstructive sleep apnea (OSA). The increase in both the prevalence and the severity of obesity has translated into an increase in the prevalence of obesity-related comorbidities including OSA. The prevalence of OSA in the US adult population has been estimated to be 24% in men and 9% in women [49] but is increased in severe obesity by up to 93.6% among men and 73.5% among women [50].
OSA is characterized by recurrent episodes of complete or partial obstruction of the upper airway during sleep associated with progressive respiratory effort to overcome the obstruction. These events lead to cortical micro-arousals and oxygen desaturation and overall sleep fragmentation, chronic sleep loss, and increased sympathetic nervous activity [51]. Whether the sleep fragmentation secondary to OSA results in similar pathophysiological mechanisms like sleep deprivation [52] has not been well studied.
Although compelling evidence shows that obesity predisposes to OSA and that losing weight results in OSA improvement, recent studies suggest that OSA itself may cause weight gain. If sleep deprivation appears to be a risk factor for obesity, the sleep fragmentation, overall sleep loss, and daytime sleepiness associated with OSA could similarly favor weight gain, which then further worsens OSA. According to this new paradigm, OSA would cause a complex interaction of behavioral changes, leptin resistance, and increased ghrelin levels leading to decreased physical activity and/or an increase in unhealthier eating habits. A few studies have previously suggested that increased severity of OSA [53,54] and excessive daytime sleepiness [55] are associated with decreased physical activity by self-report. A recent cross-sectional study in a small sample of clinic patients [56] found that increased OSA severity was associated with objectively measured decreased physical activity, after controlling for age, sex, and daytime sleepiness. The small sample size and the study design are obvious limitations and, therefore, this study does not unequivocally prove that OSA leads to decreased physical activity but suggests a mechanism worth exploring.
Alternatively, OSA could directly affect appetite regulation and ultimately result in increased caloric intake. Studies in patients with OSA and similarly obese controls showed that those with OSA had gained weight in the year preceding the diagnosis [57] and had higher leptin levels than expected based on their percentage body fat [57–60] suggesting that OSA is associated with greater resistance to the weight-reducing effect of leptin than obesity alone. Multiple intervention studies in adults demonstrated a decrease in leptin levels in patients treated with continuous positive airway pressure (CPAP) [58,61–65]. Night-time levels of ghrelin are increased in obese patients [66]. One study showed that 2 days of CPAP treatment were sufficient to significantly decrease ghrelin levels in patients with OSA [65].
In addition to causing sleep fragmentation and overall sleep loss, OSA also involves respiratory distress, hypoxia, and hypercapnia with the result of an increase in sympathetic nervous activity. Leptin secretion in OSA could be triggered by hypoxia [67,68], whereas ghrelin release could be related to sympathetic activation [69].
Appetite regulation in OSA and the effect of OSA treatment are still the subject of a recent investigation. Eun et al. [44] showed that surgical treatment of OSA leads to a decrease in leptin levels in the absence of weight change, independently of the severity of OSA. Ursavas et al. [45] measured significantly higher serum ghrelin levels in 55 patients with newly diagnosed OSA compared with nonapneic age-matched and BMI-matched participants, despite no difference in total sleeping time and sleep efficiency. Ghrelin levels correlated with the apnea-hypoxia index (AHI) and with subjective sleepiness. In this study, adipokines such as leptin, adiponectin, and resistin did not differ between the groups.
The published studies have not measured subjective ratings of hunger, food preferences, or food intake. One of the abnormalities of sleep architecture seen in OSA is reduced slow wave sleep (SWS) or ‘deep’ sleep. Preliminary data showed that experimental suppression of SWS without affecting sleep duration in young healthy adults leads to increased hunger for calorie dense foods with high carbohydrate content particularly in the afternoon and evening hours [70]. In parallel with these early findings in the adults, a very recent study in 5–9-year-old obese children with and without OSA demonstrate that children with OSA ate 2.2 times more fast food and less healthy food such as fruits and vegetables, and they were 4.2 times less likely to be involved in organized sports [71]. Furthermore, OSA severity positively correlated with plasma ghrelin levels [71].
Poor sleep quality and excessive daytime sleepiness in the absence of obstructive sleep apnea
Severe obesity appears to be associated with marked sleep disturbances, even in individuals who do not have OSA [72–75]. Such sleep disturbances may equally predispose severely obese individuals to accumulate a sleep debt and may contribute to the dysregulation of appetite, limit the drive for physical activity, and further compromise weight maintenance.
Cross-sectional studies examining self-reported sleep quality have generally found that worse sleep quality is associated with higher BMI [76–78], but the longitudinal studies have been scarce and the results inconsistent [79,80]. A more recent cross-sectional analysis in 400 women participants in the Sleep and Health in Women Study showed that not only sleep duration but also sleep quality, as determined by sleep efficiency and sleep architecture (specifically minutes of SWS, the ‘deep restorative sleep’) were inversely related to waist circumference, after adjusting for age, level of physical activity, smoking status, alcohol consumption and AHI [20•]. Such associations were stronger in young women (age <50 years), suggesting that in older age the relationship between sleep quality and obesity may be less robust. The relationship between less time spent in SWS and central obesity could be mediated by a decrease in growth hormone level, which is secreted during SWS. Growth hormone deficiency is associated with visceral obesity, which can be reversed by growth hormone replacement [81,82]. Furthermore, a reduced amount of SWS leads to elevated cortisol levels, which favor central obesity [83].
In the Cardiovascular Health Epidemiology Study, which focused exclusively on African–Americans, an impressive 50% or more of the participants surveyed reported suboptimal sleep duration and low-sleep quality as assessed by the Pittsburgh Sleep Quality scale [46]. In a multivariate analysis model, the effect of sleep duration on obesity risk was rather modest, with a significant association between lower sleep quality and increased BMI in women only, and this association was modulated by perceived stress level as measured by the Cohen scale.
A sex difference in the association between poor-sleep quality and obesity risk has been confirmed in a longitudinal study of 7000 Finnish adults, aged 40–60 years. In women, sleep problems (difficulty initiating and maintaining sleep) predicted weight gain after 5–7 years. Moreover, there was a graded effect depending on the frequency of the sleep disturbances [47•].
Nordin and Kaplan [48] examined the effect of sleep discontinuity on the development of obesity over a 30-year period in approximately 7000 middle-age adults. Sleep quality was self-reported and assessed by the question ‘how often do you have any trouble getting to sleep or staying asleep’? On the basis of the answers compared from the first and last observation, the participants were divided into four categories of sleep continuity, consistently good continuity, consistent discontinuity, impaired continuity (worsened over time), and improved continuity. The main outcome was the risk of transition to and from obesity. Consistent sleep discontinuity was associated with 70% risk of conversion to obesity after adjusting for confounding variables related to demographics, pain, lifestyle, and health including sleep duration. Both consistent sleep discontinuity and impaired sleep continuity reduced the chance for transitioning from obesity, thus increasing the risk of staying obese. The major limitation of the study was that sleep quality and anthropometrics were self-reported and the analysis could not control the presence of a sleep disorder like OSA, which could in part account for the risk of weight gain.
Conclusion
Sleep curtailment has become a common behavior and an increasing number of adults report sleep complaints in modern society. Laboratory studies and multiple epidemiological studies have linked short-sleep duration and poor-sleep quality to obesity risk. With the growing prevalence of chronic sleep loss, any causal association between sleep alterations and obesity would have important public health implications. Currently, there is a lack of interventional studies in real life conditions aimed at increasing sleep duration and improving sleep quality in order to prevent weight gain or facilitate weight loss. A National Institute of Health (NIH)-funded randomized control trial [84] will enroll 150 US short sleeper adults (<6.5 h per night) and restore 7.5 h of sleep per night for 3 years to examine the effect of sleep extension on energy homeostasis and body weight.
Until results from such studies are available, the current evidence supports recommending sufficient amounts of habitual sleep and good sleep hygiene in subjects at risk of obesity.
Sleep is the ‘most sedentary activity’, yet may be the only one that protects from weight gain [85].
Key points.
The worldwide increase in the prevalence of obesity in the last several decades has been paralleled by a trend of reduced sleep duration in adults, as well as in children.
Evidence from both longitudinal and prospective epidemiological studies suggests that chronic partial sleep loss is associated with an increase in the risk of obesity.
Laboratory studies show that sleep restriction leads to hormonal alterations, which may favor an increase in calories intake and a decreased energy expenditure and ultimately lead to weight gain.
In addition to short sleep duration, evidence suggests that also sleep disturbance, such as obstructive sleep apnea and poor sleep quality, may increase obesity risk.
Prospective interventional studies are needed to clarify whether increasing sleep duration or improving sleep quality protects from weight gain or even favors weight loss.
Until results from such studies are available, the current evidence supports recommending sufficient amounts of habitual sleep and good sleep hygiene in patients at risk of obesity.
Acknowledgements
The preparation of this review was partly supported by NIH grants PO1 AG-11412, RR-024999, RO1-HL-075079, P50-HD-057796, P60-DK-20595, and by Department of Defense PR064727 grant.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 417–418).
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Unhealthy sleep-related behaviors – 12 States, 2009. MMWR Morb Mortal Wkly Rep. 2011;60:233–238. [PubMed] [Google Scholar]
- 3.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson JA. Sleep. Scientific American Library; New York: 1995. [Google Scholar]
- 6.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esquirol Y, Bongard V, Mabile L, et al. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559. doi: 10.1080/07420520902821176. [DOI] [PubMed] [Google Scholar]
- 9.De Bacquer D, Van Risseghem M, Clays E, et al. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 10.Garaulet M, Ordovas JM, Madrid JA. The chronobiology, etiology and patho-physiology of obesity. Int J Obes (Lond) 2010;34:1667–1683. doi: 10.1038/ijo.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannain S, Miller A, Van Cauter E. Sleep loss, obesity and diabetes: prevalence, association and emerging evidence for causation. Obes Metab-Milan. 2008;4:28–41. [Google Scholar]
- 12.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappuccio F, Miller M. The epidemiology of sleep and cardiovascular risk and disease. In: Cappuccio F, Miller M, Lockley S, editors. Sleep, health and society: from aetiology to public health. Oxford University Press; Oxford: 2010. pp. 83–110. [Google Scholar]
- 16.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Magee CA, Caputi P, Iverson DC. Is sleep duration associated with obesity in older Australian adults? J Aging Health. 2010;22:1235–1255. doi: 10.1177/0898264310372780. [DOI] [PubMed] [Google Scholar]
- 18.Magee CA, Caputi P, Iverson DC. Short sleep mediates the association between long work hours and increased body mass index. J Behav Med. 2011;34:83–91. doi: 10.1007/s10865-010-9287-3. [DOI] [PubMed] [Google Scholar]
- 19.Anic GM, Titus-Ernstoff L, Newcomb PA, et al. Sleep duration and obesity in a population-based study. Sleep Med. 2010;11:447–451. doi: 10.1016/j.sleep.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Theorell-Haglow J, Berne C, Janson C, et al. Associations between short sleep duration and central obesity in women. Sleep. 2010;33:593–598. [This cross-sectional study assessed sleep duration and architecture by polysomnography in a large cohort of 400 women. Decrease in either SWS or rapid eye movement (REM) sleep was independently associated with central obesity, and the associations remained significant after adjusting for BMI.] [PMC free article] [PubMed] [Google Scholar]
- 21•.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–167. doi: 10.1093/sleep/33.2.161. [This is the largest longitudinal study published in 2010, followed more than 35 000 workers (>90% men) over 1 year. Strengths of the study are the prospective design, the sample size, and the direct measurement of weight.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bo S, Ciccone G, Durazzo M, et al. Contributors to the obesity and hyperglycemia epidemics. A prospective study in a population-based cohort. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.5. [Epub ahead of print]. Doi:10.1038/ijo.2011.5. [DOI] [PubMed] [Google Scholar]
- 23•.Nishiura C, Hashimoto H. A 4-year study of the association between short sleep duration and change in body mass index in Japanese male workers. J Epidemiol. 2010;20:385–390. doi: 10.2188/jea.JE20100019. [This prospective study followed more then 2000 Japanese male workers over 4 years. The results are strongly significant, but women are not included.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Hairston KG, Bryer-Ash M, Norris JM, et al. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33:289–295. doi: 10.1093/sleep/33.3.289. [This prospective study is the only recent study that measured not only changes in BMI but also in subcutaneous and visceral fat accumulation, as determined by dual-energy x-ray absorptiometry, in relationship with sleep duration. The study cohort did not include all ethnic groups.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–152. doi: 10.1093/sleep/34.2.147. [This cross-sectional study used polysomnography to assess sleep duration and showed an association between total sleep time and leptin and visfatin levels. Notably, even REM sleep duration correlated with both leptin and visfatin levels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riserus U, Arnlov J, Brismar K, et al. Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care. 2004;27:2041–2046. doi: 10.2337/diacare.27.8.2041. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel K, Leproult R, L'Hermite-Baleriaux M, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 29.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegel K, Tasali E, Leproult R, et al. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96:486–493. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 32.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273(1 Pt 1):E226–E230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 33.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 34.Vaara J, Kyrolainen H, Koivu M, et al. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol. 2009;105:439–444. doi: 10.1007/s00421-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 35.Jung CM, Melanson EL, Frydendall EJ, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmid SM, Hallschmid M, Jauch-Chara K, et al. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 38•.Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [This interesting experimental study had a cross-over design comparing 5.5 and 8.5 h sleep duration, under caloric restriction for 14 days. The novelty of the study is to measure body composition by dual-energy x-ray absorptiometry scan. With adequate sleep time, more fat was lost from fat mass rather then fat-free mass.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–558. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brondel L, Romer MA, Nougues PM, et al. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 43.Tasali E, Broussard J, Day A, et al. Sleep curtailment in healthy young adults is associated with increased ad lib food intake [meeting abstract]. Sleep. 2009;32(Suppl):A163. [Google Scholar]
- 44.Eun YG, Kim MG, Kwon KH, et al. Short-term effect of multilevel surgery on adipokines and pro-inflammatory cytokines in patients with obstructive sleep apnea. Acta Otolaryngol. 2010;130:1394–1398. doi: 10.3109/00016489.2010.495134. [DOI] [PubMed] [Google Scholar]
- 45.Ursavas A, Ilcol YO, Nalci N, et al. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: role of obesity. Ann Thorac Med. 2010;5:161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES). BMC Public Health. 2010;10:581. doi: 10.1186/1471-2458-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Lyytikainen P, Lallukka T, Lahelma E, Rahkonen O. Sleep problems and major weight gain: a follow-up study. Int J Obes (Lond) 2011;35:109–114. doi: 10.1038/ijo.2010.113. [This prospective study had a long follow-up (5–7 years) and demonstrated that subjective sleep quality, particularly difficulty initiating and maintaining sleep, is associated with risk of weight gain.] [DOI] [PubMed] [Google Scholar]
- 48.Nordin M, Kaplan RM. Sleep discontinuity and impaired sleep continuity affect transition to and from obesity over time: results from the Alameda county study. Scand J Public Health. 2010;38:200–207. doi: 10.1177/1403494809357105. [DOI] [PubMed] [Google Scholar]
- 49.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2:349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sareli AE, Cantor CR, Williams NN, et al. Obstructive sleep apnea in patients undergoing bariatric surgery: a tertiary center experience. Obes Surg. 2011;21:316–327. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 51.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 53.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 54.Quan SF, O'Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 55.Chasens ER, Sereika SM, Weaver TE, Umlauf MG. Daytime sleepiness, exercise, and physical function in older adults. J Sleep Res. 2007;16:60–65. doi: 10.1111/j.1365-2869.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 56.Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res. 2011;2011:751819. doi: 10.4061/2011/751819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 58.Ip MS, Lam KS, Ho C, et al. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 59.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129:538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 60.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 61.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 62.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu K, Chin K, Nakamura T, et al. Plasma leptin levels and cardiac sympathetic function in patients with obstructive sleep apnoea-hypopnoea syndrome. Thorax. 2002;57:429–434. doi: 10.1136/thorax.57.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanner BM, Kollhosser P, Buechner N, et al. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23:601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 65.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 66.Yildiz BO, Suchard MA, Wong ML, et al. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tschop M, Strasburger CJ, Topfer M, et al. Influence of hypobaric hypoxia on leptin levels in men. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S151. doi: 10.1038/sj.ijo.0801309. [DOI] [PubMed] [Google Scholar]
- 68.Guerre-Millo M, Grosfeld A, Issad T. Leptin is a hypoxia-inducible gene. Obes Res. 2002;10:856. doi: 10.1038/oby.2002.116. author reply 857–858. [DOI] [PubMed] [Google Scholar]
- 69.Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- 70.Broussard J, Tasali E, Van Cauter E. Experimental suppression of slow wave sleep in healthy young men is associated with increased hunger and decreased vigor and mood. Sleep. 2008;31:A110. [Google Scholar]
- 71.Spruyt K, Serpero L, Sans Capdevila O, et al. Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr. 2010;156:724–730. 730.e1–730.e3. doi: 10.1016/j.jpeds.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 73.Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med. 2003;253:536–543. doi: 10.1046/j.1365-2796.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 74.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–675. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Bixler EO, Tan TL, et al. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–1337. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 76.Lauderdale DS, Knutson KL, Rathouz PJ, et al. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fogelholm M, Kronholm E, Kukkonen-Harjula K, et al. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond) 2007;31:1713–1721. doi: 10.1038/sj.ijo.0803663. [DOI] [PubMed] [Google Scholar]
- 78.van den Berg JF, Knvistingh Neven A, Tulen JH, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32:1083–1090. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 79.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 80.Janson C, Lindberg E, Gislason T, et al. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24:425–430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 81.Van Cauter E, Plat L. Physiology of growth hormone during sleep. J Pediatr. 1996;128:S32–S37. doi: 10.1016/s0022-3476(96)70008-2. [DOI] [PubMed] [Google Scholar]
- 82.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10–S17. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 84.Cizza G, Marincola P, Mattingly M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010;7:274–285. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaput JP, Klingenberg L, Sjodin A. Do all sedentary activities lead to weight gain: sleep does not. Curr Opin Clin Nutr Metab Care. 2010;13:601–607. doi: 10.1097/MCO.0b013e32833ef30e. [DOI] [PubMed] [Google Scholar]