Abstract
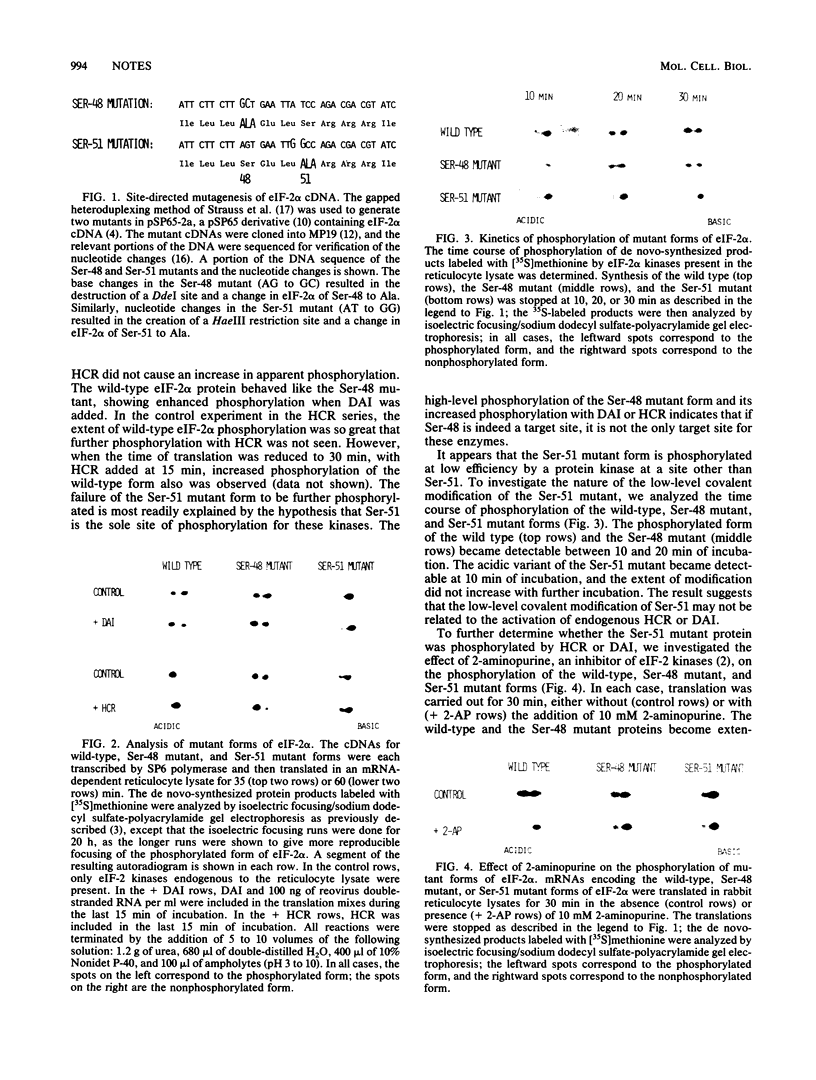
The phosphorylation of the alpha-subunit of initiation factor eIF-2 leads to an inhibition of protein synthesis in mammalian cells. We have performed site-directed mutagenesis on a cDNA encoding the alpha-subunit of human eIF-2 and have replaced the candidate sites of phosphorylation, Ser-48 and Ser-51, with alanines. The cDNAs were expressed in vitro by SP6 polymerase transcription and rabbit reticulocyte lysate translation, and the radiolabeled protein products were analyzed by high-resolution two-dimensional gel electrophoresis. The wild-type and Ser-48 mutant proteins became extensively phosphorylated by eIF-2 kinases present in the reticulocyte lysate, and when additional heme-controlled repressor or double-stranded RNA-activated kinase was present, phosphorylation of the proteins was enhanced. The Ser-51 mutant showed little covalent modification by the endogenous enzymes and showed no increase in the acidic variant with additional eIF-2 kinases, thereby suggesting that Ser-51 is the site of phosphorylation leading to repression of protein synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colthurst D. R., Campbell D. G., Proud C. G. Structure and regulation of eukaryotic initiation factor eIF-2. Sequence of the site in the alpha subunit phosphorylated by the haem-controlled repressor and by the double-stranded RNA-activated inhibitor. Eur J Biochem. 1987 Jul 15;166(2):357–363. doi: 10.1111/j.1432-1033.1987.tb13523.x. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Identification and quantitation of levels of protein synthesis initiation factors in crude HeLa cell lysates by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1983 Jun 10;258(11):7228–7235. [PubMed] [Google Scholar]
- Ernst H., Duncan R. F., Hershey J. W. Cloning and sequencing of complementary DNAs encoding the alpha-subunit of translational initiation factor eIF-2. Characterization of the protein and its messenger RNA. J Biol Chem. 1987 Jan 25;262(3):1206–1212. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Leroux A., London I. M. Site-specific phosphorylation of the alpha subunit of eukaryotic initiation factor eIF-2 by the heme-regulated and double-stranded RNA-activated eIF-2 alpha kinases from rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1286–1290. doi: 10.1073/pnas.77.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rynning J., Knish W. M. Evidence that the phosphorylation of eukaryotic initiation factor 2 alpha by the hemin-controlled translational repressor occurs at a single site. J Biol Chem. 1981 Jan 25;256(2):589–592. [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kudlicki W., Fullilove S., Read R., Kramer G., Hardesty B. Identification of spectrin-related peptides associated with the reticulocyte heme-controlled alpha subunit of eukaryotic translational initiation factor 2 kinase and of Mr 95,000 peptide that appears to be the catalytic subunit. J Biol Chem. 1987 Jul 15;262(20):9695–9701. [PubMed] [Google Scholar]
- Kudlicki W., Wettenhall R. E., Kemp B. E., Szyszka R., Kramer G., Hardesty B. Evidence for a second phosphorylation site on eIF-2 alpha from rabbit reticulocytes. FEBS Lett. 1987 May 4;215(1):16–20. doi: 10.1016/0014-5793(87)80105-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D., Raines R., Kawashima E., Knowles J. R., Gilbert W. Active site of triosephosphate isomerase: in vitro mutagenesis and characterization of an altered enzyme. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2272–2276. doi: 10.1073/pnas.82.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Site-specific phosphorylation if initiation factor 2 by three cyclic nucleotide-independent protein kinases. J Biol Chem. 1980 Nov 25;255(22):10954–10958. [PubMed] [Google Scholar]
- Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The NH2-terminal sequence of the alpha and gamma subunits of eukaryotic initiation factor 2 and the phosphorylation site for the heme-regulated eIF-2 alpha kinase. J Biol Chem. 1986 Sep 25;261(27):12444–12447. [PubMed] [Google Scholar]
- Zardeneta G., Kramer G., Hardesty B. Structure and function of peptide initiation factor 2: differential loss of activities during proteolysis and generation of a terminal fragment containing the phosphorylation sites of the alpha subunit. Proc Natl Acad Sci U S A. 1982 May;79(10):3158–3161. doi: 10.1073/pnas.79.10.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]





