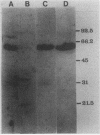
Abstract
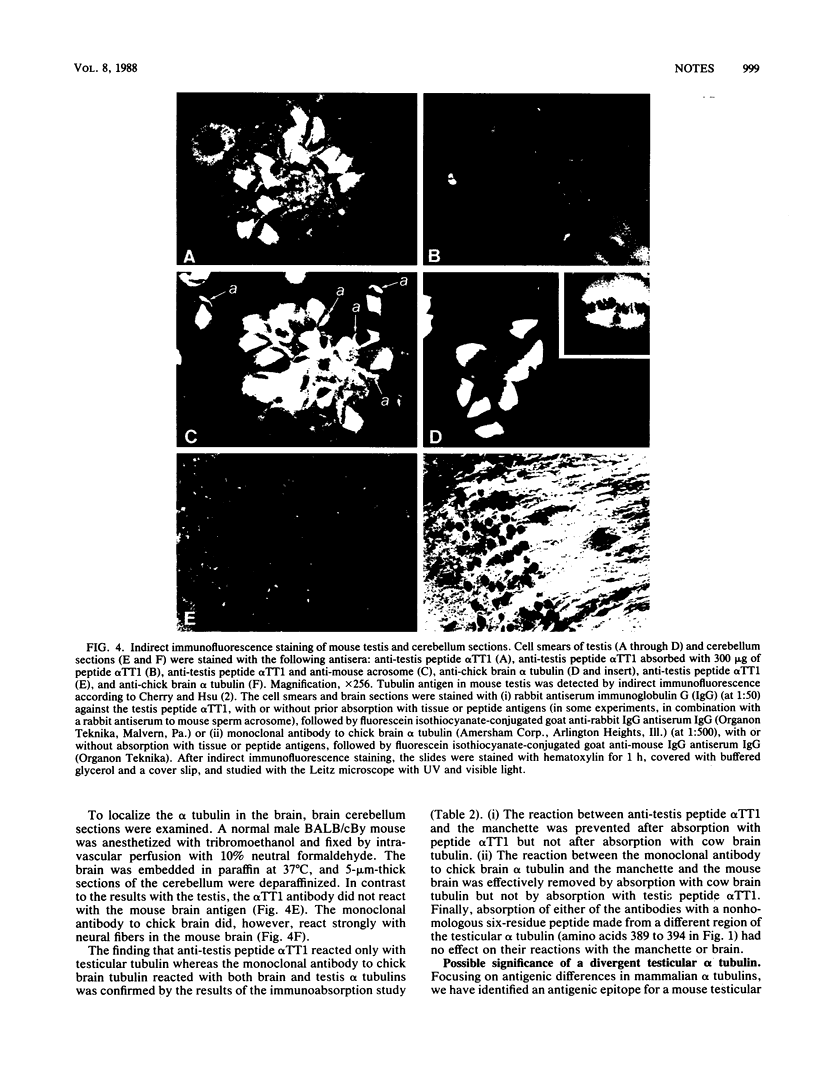
Sequence analysis of a mouse testicular alpha-tubulin partial cDNA, pRD alpha TT1, reveals an isotype that differs from both the somatic and the predominant testicular alpha tubulins at approximately 30% of the 212 amino acid residues determined. Although this mouse testicular cDNA retains the highly conserved sequence, Glu-Gly-Glu-Glu, found in the carboxyl termini of many alpha tubulins, the protein extends substantially beyond this sequence and does not terminate with a C-terminal tyrosine. Using rabbit antiserum prepared to a novel synthetic peptide predicted from this mouse testis alpha-tubulin cDNA, we have have detected by immunoblot and indirect immunofluorescence an antigenic epitope present in testicular alpha tubulin that is not detectable in brain alpha tubulins. We find that the antiserum specifically binds to the manchettes and meiotic spindles of the mouse testis but not with neural fibers or tubulin extracts of the adult mouse brain. These results demonstrate that at least one of the multiple alpha-tubulin isotypes of the mammalian testis is expressed and used in male germ cells but not in the brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cherry L. M., Hsu T. C. Antitubulin immunofluorescence studies of spermatogenesis in the mouse. Chromosoma. 1984;90(4):265–274. doi: 10.1007/BF00287034. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Kleene K. C., Hecht N. B. Haploid expression of a mouse testis alpha-tubulin gene. Science. 1984 Apr 6;224(4644):68–70. doi: 10.1126/science.6701535. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Nucleotide sequence of a cDNA clone encoding mouse protamine 1. Biochemistry. 1985 Jan 29;24(3):719–722. doi: 10.1021/bi00324a027. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee A. C., Powell J. E., Tregear G. W., Niall H. D., Stevens V. C. A method for preparing beta-hCG COOH peptide-carrier conjugates of predictable composition. Mol Immunol. 1980 Jun;17(6):749–756. doi: 10.1016/0161-5890(80)90145-5. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gu W., Cowan N. J. Free intermingling of mammalian beta-tubulin isotypes among functionally distinct microtubules. Cell. 1987 May 22;49(4):539–548. doi: 10.1016/0092-8674(87)90456-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pratt L. F., Okamura S., Cleveland D. W. A divergent testis-specific alpha-tubulin isotype that does not contain a coded C-terminal tyrosine. Mol Cell Biol. 1987 Jan;7(1):552–555. doi: 10.1128/mcb.7.1.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Baum H., Bo J., Wensink P. C. Tissue-specific and constitutive alpha-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8477–8481. doi: 10.1073/pnas.83.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat T. E., Shelton J. A., Gonzales-Prevatt V., Goldberg E. The antigenicity of synthetic peptide fragments of lactate dehydrogenase C4. Mol Immunol. 1985 Oct;22(10):1195–1199. doi: 10.1016/0161-5890(85)90008-2. [DOI] [PubMed] [Google Scholar]