Abstract
Background
Several studies have reported negative associations of polychlorinated biphenyls (PCBs), hexachlorobenzene (HCB) and mercury (Hg) with duration of gestation and foetal growth in fish eating populations. Docosahexaenoic acid (DHA) from fish, seafood and marine mammal intake has been reported to be positively related with pregnancy duration and foetal growth. So far, it remains unclear, however, if the associations of environmental contaminants (ECs) with growth are direct or mediated through their relation with the duration of gestation and the degree to which DHA intake during pregnancy attenuates the negative association of ECs with fetal growth.
Objectives
To investigate direct and indirect associations of in utero exposure to ECs with foetal growth and pregnancy duration while taking into account the possible positive effects of DHA.
Methods
Pregnant Inuit women (N = 248) from Arctic Quebec were recruited and cord blood samples were analyzed for PCBs, HCB, Hg and DHA. Anthropometric measurements were assessed at birth. Path models were used to evaluate direct and indirect associations.
Results
Cord concentrations of PCB 153, HCB and Hg were significantly associated with shorter duration of pregnancy (β varying from −0.17 to −0.20, p < 0.05). Path models indicated that the associations of PCBs, HCB and Hg with reduced foetal growth (β varying from −0.09 to −0.13, p < 0.05) were mediated through their relations with shorter gestation duration. Cord DHA was indirectly related to greater growth parameters (β varying from 0.17 to 0.20, p < 0.05) through its positive association with gestation duration.
Conclusion
Prenatal exposure to ECs was associated with reduced gestation duration, which is a recognized determinant of foetal growth. DHA intake during pregnancy appeared to have independent positive association with foetal growth by prolonging gestation. Whether these associations are causal remains to be elucidated.
Keywords: Gestation, growth, mercury, organochlorines, polyunsaturated fatty acids, prenatal exposure
1. Introduction
Benefits and risks associated with consumption of fish, seafood and marine mammals is a public health issue of great relevance for many populations around the world. These food sources often contain environmental contaminants (ECs) that cross the placenta, thereby exposing the embryo and the foetus during conception and prenatal development, periods of well-documented vulnerability to exogenous chemicals. On the other hand, fish, seafood and marine mammals are good sources of nutrients, such as n-3 polyunsaturated fatty acids (n-3 PUFAs), for which there is increasing evidence of positive effects on foetal growth and length of gestation in term infants (Makrides et al., 2006; Olsen et al., 1986; Szajewska et al., 2006).
ECs of concern for pregnant women and women of childbearing age that are consuming fish, seafood and marine mammals include methylmercury (MeHg) and organochlorine chemicals (OCs), such as polychlorinated biphenyls (PCBs) and hexachlorobenzene (HCB). OCs are a family of persistent hydrocarbon compounds used extensively in North American and European industry and agriculture from 1930 through the mid-1980’s. Due to their high lipophilicity and resistance to biodegradation, OCs bioaccumulate in fatty tissues of organisms and are biomagnified through the food chain (Dewailly et al., 1993a)) In the aquatic environment, mercury (Hg), which comes from both natural and anthropogenic sources, is transformed by bacteria into methylmercury (MeHg) and accumulates in seafood.
Relationships between prenatal exposure to background levels of OCs and MeHg with foetal growth and duration of gestation have been studied in several prospective and retrospective cohort studies of fish eating populations. To date, ten PCB studies have been conducted with frequent consumers of fish and/or marine mammals (Bjerregaard and Hansen, 2000; Dewailly et al., 1993b; Fein et al., 1984; Grandjean et al., 2001; Karmaus and Zhu, 2004; Murphy et al., 2010; Rylander et al., 1998; Vartiainen et al., 1998; Weisskopf et al., 2005; Wojtyniak et al., 2010). PCB exposure was associated with smaller birth weight in five of those studies (Fein et al., 1984; Karmaus and Zhu, 2004; Murphy et al., 2010; Rylander et al., 1998; Wojtyniak et al., 2010).Dewailly et al. (1993b) have found shorter body length among boys, without association with head circumference. However, smaller head circumference was observed inFein et al. (1984) study. Shorter gestation was found to be related to cord PCB concentrations in one study (Fein et al., 1984), but not with maternal preconceptional PCBs concentrations in the New York anglers study (Murphy et al., 2010). The association of in utero exposure to HCB with foetal growth was reported only in one fish eating cohort (Dewailly et al., 1993b), where this OC was related to shorter body length.
Six studies in fish-eating populations have focused on in utero Hg exposure relationships with birth weight (Bjerregaard and Hansen, 2000; Drouillet-Pinard et al., 2010; Foldspang and Hansen, 1990; Lee et al., 2010; Mendez et al., 2010; Ramon et al., 2009), two on body length (Drouillet-Pinard et al., 2010; Ramon et al., 2009), one on head circumference (Drouillet-Pinard et al., 2010) and four on gestation duration (Bjerregaard and Hansen, 2000; Drouillet-Pinard et al., 2010; Foldspang and Hansen, 1990; Xue et al., 2007). Significant associations were reported with smaller birth weight in four studies, and with reduced birth length in one of the Spanish cohorts (Ramon et al., 2009) Newborn head circumference was not related to maternal Hg levels in hair among French pregnant women (Drouillet-Pinard et al., 2010). In the Pregnancy Outcome and Community Health (POUCH) study, increasing risk of preterm delivery was reported among women with hair Hg concentrations above the 90th percentile (Xue et al., 2007), while the other studies did not evaluate or find a relation between Hg exposure and pregnancy duration.
Several randomized controlled trials have documented benefits of prenatal n-3 PUFAs, particularly docosahexaenoic acid (DHA), on gestation duration, reduced risk of preterm deliveries (Olsen et al., 2000) and in some instances foetal growth (Campoy et al., 2012; Helland et al., 2001; Olsen et al., 1992). Although the hypothesis that the adverse reproductive effects of OCs and MeHg might be attenuated by a high maternal intake of n-3 PUFAs was formulated two decades ago, it has received little direct scientific scrutiny. The vast majority of cohort studies conducted to date have focused either on ECs or n-3 PUFAs and did not obtain biomarkers of both. Moreover, in observational studies, a failure to control for prenatal n-3 PUFAs could lead to an underestimation of the toxicity of ECs on the outcomes of interest (Choi et al., 2008; Davidson et al., 2008).
We conducted a prospective longitudinal study to examine the potential associations of pre- and postnatal exposure to moderately high levels of OCs and Hg on duration of gestation, physical growth as well as cognitive and behavioral development in a sample of Inuit infants in Northern Quebec, Canada (Jacobson et al., 2008; Muckle et al., 2001). This paper focuses specifically on the associations of prenatal exposure to OCs and Hg with foetal growth and duration of pregnancy and addresses two research questions: If EC concentrations are negatively associated with growth parameters, are the associations direct or mediated through shortened duration of gestation? Are n-3 PUFA levels positively related to gestation duration and foetal growth, and if so, do they mitigate the potential negative effects of ECs on gestation duration and growth?
2. Materials and Methods
2.1 Population
Pregnant Inuit women from the Arctic Quebec region known as Nunavik were invited to participate in a study focusing on infant health and development. Nunavik is a region located north of the 55th parallel, in which 10 000 Inuit live in 14 villages scattered along a 2000-km coastline on the Hudson Bay, Hudson Strait, and Ungava Bay. Participants were recruited from the three largest communities on the Hudson Bay coast.
2.2 Procedures and Variables
From November 1995 to November 2001 pregnant women in these communities were invited to participate in the study at their first prenatal visit to the village nursing station. The recruitment procedure has previously been described (Muckle et al., 2001). A detailed informed consent was obtained from each participating mother. The research procedures were approved by the human subjects committees of Laval University and Wayne State University. Maternal interviews were conducted in the community’s nursing station at mid-pregnancy and at 1-month postpartum by trained research assistants to assess the mothers’ socioeconomic and personal characteristics. Among the 417 Nunavik women invited to participate in this study, 47 were excluded because a newborn from the same mother had been previously recruited, 9 could not be contacted by our research assistants to schedule the prenatal interview, and 110 refused to participate. Among the 251 women interviewed prenatally, 3 were subsequently excluded due to miscarriage or perinatal or postnatal mortality. Overall, the participation rate for the study was 69%, for a total of 248 pregnant women and their newborns for whom we have information regarding environmental exposure and/or growth parameters.
2.3 Biomarkers and laboratory procedures
A 30-ml blood sample was drawn from the umbilical cord after it was severed. Concentration of the 14 most prevalent PCB congeners (IUPAC nos. 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187) and HCB, were measured in cord plasma samples. The detection limit was 0.02 μg/L for all compounds. Hg concentrations were determined in cord whole blood, as well as in the maternal hair sample collected postnatally, which was cut into three segments of 3 cm, each corresponding to a trimester of pregnancy. The detection limits were 1.0 nmol/L and 1.0 nmol/g for blood and hair Hg analysis, respectively. Fatty acids including n-3 PUFAs and DHA were determined in cord plasma phospholipids and expressed as percentages of all fatty acids from C14:0 to C24:1 (% weight). Total cholesterol, free cholesterol, triglycerides, and phospholipids in plasma were also determined. Concentrations of total plasma lipids were estimated according to the formula developed byAkins et al. (1989). Detailed laboratory procedures for ECs, n-3 PUFAs and lipids quantification and quality control data have been described previously (Jacobson et al., 2008; Muckle et al., 2001).
2.4 Outcomes
Weight, length, and head circumference at birth were measured by a midwife or nurse, who had been trained by the investigators to follow a standard protocol. Two measurements were performed for each growth parameter, and a third one was performed when a discrepancy of more than 5 g for birth weight and 0.3 cm for length and head circumference was noted. Final growth parameters were based on the average of the two closest measurements. Because determination of gestational age by ultrasound was not available for the majority of participants, duration of pregnancy was based primarily on the Ballard Examination for Foetal Maturity (N = 192) (Ballard et al., 1991). This instrument estimates gestation duration by assessing physical and neuromuscular maturity. When the Ballard examination data were not available, duration of gestation was based on results of the ultrasound performed before 15 weeks of pregnancy (N = 10) or if missing, on the date of the last menstrual period and a medical exam performed by a midwife at the first prenatal visit (N = 28).
2.5 Statistical analyses
2.5.1 Control variables
Pre- and postnatal maternal interviews were conducted to assess potential confounding variables pertaining to demographic background, pregnancy history, and foetal exposure to other toxicants, such as nicotine and alcohol. Maternal height was measured by our research assistants; maternal pre-pregnancy weight was obtained from medical charts or, when missing, from maternal recall at prenatal interview. An initial screening of potential confounding variables was performed by assessing the bivariate correlation of each control variable with each outcome measure. Control variables tested were maternal age, weight, parity, maternal tobacco (number of cigarettes/day) and alcohol consumption (average absolute alcohol per day during pregnancy), socioeconomic status, sex of the baby, and cord blood lead concentrations. Covariates were initially retained as potential confounders if they were correlated with outcomes at p ≤ 0.20.
2.5.2 Model testing
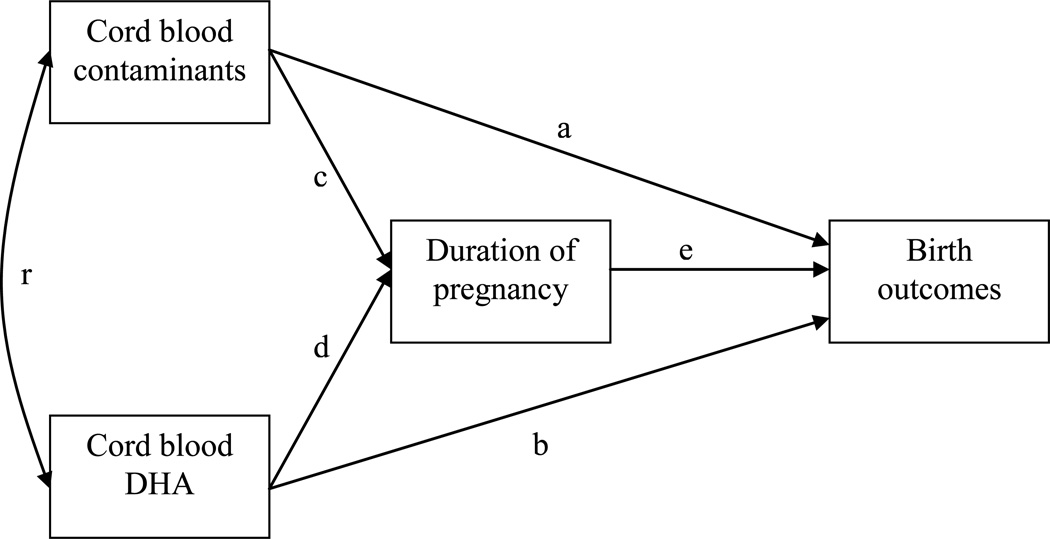
Cord blood concentrations of ECs were log-transformed to reduce skewness. Relationships between growth measurements and PCB 153, Hg, and HCB were examined. Multiple regression models were used to test direct associations of PCB 153, HCB, and Hg on weight, length, head circumference and duration of pregnancy. Because duration of pregnancy may be in the causal pathway between contaminant levels and growth outcomes, this variable was considered as an outcome and was not adjusted for in the multiple linear models of growth parameters. Each potential confounder was entered individually in the linear model for each outcome to which it was related, starting with the potential confounder with the strongest association, and retained if its inclusion modified the β associated with the contaminant by at least 10%. Sex of the baby was included as an obligatory confounder in all predictive models of birth outcomes. Control variables retained in each final model are presented in footnotes of Table 3. Path models were used to test indirect associations of ECs and DHA on growth parameters through their relationship with pregnancy duration. They estimate simultaneously a series of regressions with multiple dependant variables (Kline, 2010). The path model tested in this study is shown in Figure 1. Confounding variables included in path models were the same as those retained in multiple regression models. A bilateral p-value < 0.05 was considered statistically significant. Models were tested with the statistical package Mplus 5.21.
Table 3.
Association between cord concentrations of OCs and mercury and birth outcomes
| Contaminant | N | Standardized regression coefficient |
|
|---|---|---|---|
| Birth weight | PCB 153a | 248 | −0.13 |
| Hexachlorobenzeneb | 248 | −0.15 | |
| Mercuryc | 248 | −0.06 | |
| Length | PCB 153d | 245 | −0.16* |
| Hexachlorobenzened | 245 | −0.18* | |
| Mercuryd | 245 | −0.09 | |
| Head circumference | PCB 153e | 248 | −0.01 |
| Hexachlorobenzenee | 248 | −0.05 | |
| Mercurye | 248 | −0.08 | |
| Duration of pregnancy | PCB 153f | 230 | −0.17* |
| Hexachlorobenzenef | 230 | −0.20* | |
| Mercuryf | 232 | −0.18* |
p ≤0.05
Abbreviations: PCB 153, polychlorinated biphenyl congener 153.
Confounders included in final models are:
maternal weight, cord DHA, absolute alcohol per day in late pregnancy, parity, socioeconomic status, sex of baby;
maternal pre-pregnancy weight, cord DHA, absolute alcohol per day in late pregnancy, socioeconomic status, sex;
cord DHA, absolute alcohol per day in late pregnancy, parity, sex;
maternal weight, cord DHA, sex;
maternal weight, parity, absolute alcohol per day in late pregnancy, cord DHA, sex;
cord DHA.
Figure 1. Representation of the indirect effects of cord blood contaminant measures and DHA.
Indirect effect of contaminant on outcome = c*e
Indirect effect of DHA on outcome = d*e
Birth outcome tested are birth weight, length and head circumference; cord blood measures of contaminants tested are PCB 153, HCB and Hg
2.5.3 Missing Data
The proportion of missing data for the predictive and outcome variables ranged from 0 – 56.5%. The highest number of missing data was observed for the biomarkers of ECs and DHA because cord blood samples were not available from 138 newborns. Although the proportion of missing cord blood data was high, there was no reason to think that the cause of missingness was related to the concentrations of ECs and DHA. Thus, the assumption that the data were missing at random (MAR) was considered reasonable even though this assumption can never be formally tested (Schafer and Graham, 2002). Assuming MAR, all models were tested with the full information maximum likelihood procedure, a state-of-the-art procedure for estimating models with missing data (Graham, 2009). Auxiliary variables can be included in these models. These variables are not predictive variables but are entered in the model because they are highly correlated with the test variables with missing data. Their role is to reduce bias in parameter estimates, including departures from the MAR assumption, and restore some of the power lost because of missing data. In the present study, the measures of contaminants and DHA obtained from maternal blood and breast milk sample analyses were used as auxiliary variables in models with missing cord values (see intercorrelations in Table 1, Supplemental Material). A series of t-tests revealed that there was no significant difference in concentrations of ECs and DHA in blood of mothers of newborns with or without available cord blood samples, except that the HCB blood level was lower for mothers of newborns for whom cord blood samples were not taken (p = 0.02).
Table 1.
Characteristics of participants
| Characteristics | N | % | Mean | SD | Range |
|---|---|---|---|---|---|
| Maternal | |||||
| Age | 248 | 24.9 | 5.8 | 14.1–40.7 | |
| Education (years) | 248 | 9.0 | 1.7 | 5.5–14.3 | |
| Marital status | 248 | ||||
| Single | 29.0 | ||||
| Married | 18.2 | ||||
| Cohabiting not married | 50.0 | ||||
| Divorced, separated, widowed | 2.8 | ||||
| Socioeconomic status (12 months)a | 176 | ||||
| No occupation | 60.8 | ||||
| Unskilled labourers | 6.8 | ||||
| Semiskilled labourers | 8.5 | ||||
| Skilled craftmen, clerical, sales | 12.5 | ||||
| Technical, small business | 10.3 | ||||
| Professionals | 1.1 | ||||
| Lives with parents | 248 | 39.5 | |||
| Language of interview | 248 | ||||
| English | 70.9 | ||||
| French | 16.2 | ||||
| Inuktituk | 12.9 | ||||
| Parity | 248 | 2.1 | 1.9 | 0–9.0 | |
| Pre-pregnancy weight (kg) | 243 | 61.5 | 11.9 | 53.8–66.8 | |
| Pregnancy smoking (% smokers) | 248 | 90.3 | |||
| Pregnancy smoking | 248 | 9.9 | 6.9 | 0–27.5 | |
| (# cigarettes/day) | |||||
| Average absolute alcohol per dayb | 216 | 0.03 | 0.15 | 0–2.02 | |
| Number of fish meals/week | 215 | 3.4 | 4.0 | 0–28.6 | |
| Number of marine mammal meals/week | 215 | 1.5 | 2.4 | 0–23.0 | |
| Neonates | |||||
| Sex of baby (% male) | 230 | 57.0 | |||
| Cord blood lead (μmol/L) | 110 | 0.22 | 0.17 | 0.03–0.86 | |
| Cord blood selenium (μmol/L) | 107 | 3.7 | 1.5 | 1.9–11.6 | |
| Cord DHA (% total phospholipids) | 108 | 3.6 | 1.2 | 1.1–7.5 | |
| Outcomes | |||||
| Birth weight (g) | 238 | 3427 | 581 | 1620–4800 | |
| Lenght (cm) | 210 | 50.2 | 5.0 | 41.0–56.0 | |
| Head circumference (cm) | 211 | 34.4 | 1.6 | 29.0–40.0 | |
| Duration of pregnancy (# weeks) | 230 | 38.7 | 1.8 | 32.0–42.0 |
The data come from the prenatal interview unless specified otherwise. The N represents the total number of participants for whom data were available.
Hollingshead Index (1975) for the mother and her partner or, if she was not selfsupporting, for her primary source of support (usually her parents);
During the 2nd and 3th trimesters of pregnancy.
3. Results
3.1 Descriptive statistics
Sociodemographic characteristics are summarized in Table 1. About 11% of women were younger than 18 years and 6% were older than 35. Only 20% of participants had completed high-school. Twenty-three percent of the participating women were primiparous, and 34% had already delivered three or more children. Most women smoked during pregnancy. Ninety-one percent of women had eaten at least one fish meal/month and 74% one marine mammal meal/month. About 15% of neonates were born before 37 weeks of gestation, and 7% weighted less than 2500 grams.
Concentrations of the 14 PCB congeners, HCB, and Hg measured in cord plasma are presented in Table 2. PCB 153 was the most prevalent congener and was highly correlated with each of the other congeners detected in at least 70% of the samples (r = 0.87 – 0.98). This finding supports the use of PCB 153, the most prevalent congener, as a marker of exposure to the PCB mixture in the Arctic (Ayotte et al., 2003). HCB was detected in more than 70% of sample. Furthermore, it was less correlated with PCB 153 (r = 0.72) and was the only one with previously reported evidence of association with foetal growth. Cord blood Hg concentrations were moderately correlated with PCB 153 (r = 0.28, p ≤ .001).
Table 2.
Descriptive statistics for cord plasma OC (µg/kg) and Hg (µg/L) concentrations detected in at least 70% of samples (N = 110)
| Percent detected |
Arithmetic mean |
Geometric mean |
SD | Range | |
|---|---|---|---|---|---|
| PCB 128 | 5.5 | ||||
| PCB 52 | 22.7 | ||||
| PCB 99 | 92.7 | 21.7 | 16.8 | 18.3 | 2.8–116.8 |
| PCB 101 | 17.3 | ||||
| PCB 105 | 12.7 | ||||
| PCB 118 | 84.5 | 16.7 | 12.8 | 14.5 | 2.8–97.4 |
| PCB 128 | 0 | ||||
| PCB 138 | 100 | 70.0 | 53.7 | 56.1 | 9.8–313.1 |
| PCB 153 | 100 | 113.7 | 84.0 | 95.2 | 12.0–550.9 |
| PCB 156 | 41.8 | ||||
| PCB 170 | 75.5 | 17.5 | 12.2 | 15.8 | 2.8–73.0 |
| PCB 180 | 99.1 | 44.2 | 32.36 | 37.4 | 4.3–164.2 |
| PCB 183 | 35.5 | ||||
| PCB 187 | 92.7 | 20.7 | 16.5 | 14.6 | 3.7–84.8 |
| Σ 14 PCB congenersa | 343.8 | 273.8 | 258.3 | 70.8–1420.1 | |
| Hexachlorobenzene | 100 | 53.3 | 43.9 | 37.9 | 10.6–233.6 |
| Mercuryb | 100 | 21.3 | 16.7 | 15.6 | 2.4–97.3 |
p ≤0.01;
p ≤0.001.
Abbreviations: PCBs, polychlorinated biphenyls.
Sum of all 14 congeners including congeners not detected in at least 70% of samples for which a score corresponding to half the detection limit was given;
Hg arithmetic mean = 106.1 µmol/L.
3.2 Direct predictive models
Because of the high intercorrelations among the ECs, their associations with each outcome were tested in separate multiple regression models. DHA was associated with ECs (β = 0.29 – 0.32, p ≤ 0.01) as well as with duration of pregnancy and included in all models. Cord blood concentrations of PCB 153 and HCB were significantly associated with shorter height but not with weight and head circumference (Table 3). Cord Hg concentrations were not associated with the growth parameters. Cord concentrations of PCB 153, HCB and Hg were significantly associated with shorter duration of pregnancy.
3.3 Indirect predictive models
The degree to which the associations of the ECs and DHA on growth were mediated by their associations on pregnancy duration was tested using the path model illustrated in Figure 1 and reported in Table 4. An indirect association mediated by pregnancy duration was calculated by the product of the coefficients of the path from the EC or DHA to duration of pregnancy and the path from duration of pregnancy to the outcome, adjusted for the other variables in the model. Models were tested separately for each EC and for each outcome with cord DHA concentrations included in all models. We found significant negative indirect associations of cord concentrations of PCB 153, HCB and Hg mediated by pregnancy duration on the three growth outcomes. Removing two newborns with birth weight under 2000 grams from the models of birth weight did not change the results. Additionally, significant positive indirect associations of cord blood DHA on fetal weight, height, and head circumference mediated by pregnancy duration were observed. Specific estimates of the associations of ECs and DHA on length of gestation and the anthropometric parameters are presented in Table 2 (Supplemental Material). The predicted duration of gestation was increased by 3 days for each increase of 1% change of DHA/total fatty acids; gestation was shortened by 3 days for the difference between the first and third quartile of exposure to prenatal PCB 153 and HCB and by 8 days for that difference in exposure to prenatal Hg. Depending on the ECs, decrease of birth weight ranged from 102 to 198 g, whereas length and head circumference reduction ranged from 0.45 to 0.98 cm and 0.22 to 0.55 cm, respectively.
Table 4.
Indirect associations of OCs, mercury and DHA on foetal growth mediated by duration of pregnancya
| N | Models | Standardized regression coefficients for effects of contaminants on outcome |
Standardized regression coefficients for effects of DHA on outcome |
|||
|---|---|---|---|---|---|---|
| Direct | Indirect | Direct | Indirect | |||
| Birth weight | 248 | PCB 153 / DHA | 0.00 | −0.13** | −0.01 | 0.18** |
| HCB / DHA | −0.01 | −0.13* | 0.04 | 0.20** | ||
| Mercury / DHA | 0.07 | −0.12* | −0.04 | 0.20** | ||
| Length | 245 | PCB 153 / DHA | −0.05 | −0.10* | 0.04 | 0.15** |
| HCB / DHA | −0.06 | −0.10* | 0.06 | 0.17** | ||
| Mercury /DHA | −0.01 | −0.10* | 0.05 | 0.17* | ||
| Head circumference | 248 | PCB 153 / DHA | 0.09 | −0.09* | −0.08 | 0.15** |
| HCB / DHA | 0.06 | −0.09* | −0.06 | 0.14** | ||
| Mercury / DHA | 0.02 | −0.10* | −0.09 | 0.16** | ||
p ≤0.05,
p ≤0.01.
Abbreviations: DHA, docosahexaenoic acids; HCB, hexachlorobenzene; PCB 153, polychlorinated biphenyl congener 153.
Confounders are the same as those included in the models reported in Table 4.
4. Discussion
Findings from this cohort study of Inuit newborns exposed to PCBs, organochlorine pesticides, and MeHg from environmental sources revealed that in utero exposure to moderately high levels of these contaminants was associated with a shortening of the period of gestation which, in turn, was associated with reduced birth weight, length and head circumference. By contrast, prenatal DHA was associated with an increase in the duration of gestation, which contributed to increased foetal growth.
In our study, cord DHA, which was positively correlated with both EC exposure and pregnancy duration, acted as a suppressor variable in the direct models. When DHA was not entered in a model, none of the contaminants were significantly associated with duration of pregnancy. In the Faroe Islands study (Grandjean et al., 2001), adjustment for DHA made the weak positive association between cord PCB concentrations and gestational length disappear, whereas in our study, inclusion of DHA in direct models revealed significant negative associations of ECs with pregnancy duration, meaning that DHA obscured the relation between ECs and growth outcomes in observational studies. It is not clear why the inclusion of DHA in the statistical models of the two studies had not the same effects on associations. Potential explanations for this discrepancy between studies are differences in plasma concentrations of both PCBs and DHA, and in intercorrelations between those variables since fish and marine mammal species consumed in these populations are not the same. To our knowledge, no other studies have considered the potential suppressor effects of essential fatty acids from fish on the effects of ECs on foetal growth. These findings are consistent with other studies that have demonstrated negative bias from inclusion of n-3 PUFAs in analyses of effects of toxicants on neurodevelopment and cardiovascular diseases (Choi et al., 2008).
Positive association of DHA with pregnancy duration and foetal growth were also found in the indirect models. Indeed, gestational length was prolonged with increasing cord DHA concentrations, which indirectly had a significant positive effect on birth weight, length, and head circumference. Thus, increased foetal growth was apparently related to prolongation of gestation and not directly by DHA action on growth parameters. In this study, gestation duration was estimated to be 3 days longer for newborns found in the third quartile of exposure to ECs compare to the first quartile (see Supplemental Material). This fatty acid was also found to prolong the gestational period in the Faroe Islands study but did not necessarily increase foetal growth (Grandjean et al., 2001). In that study, very high levels of n-3 PUFAs actually appeared to be detrimental to birth weight. However, in our study, mean DHA concentration was 3.7%, half the level found in the Faroe Islands. Consistent with our results, meta-analyses of randomised controlled trials assessing effects of fish oil supplementation during pregnancy on obstetrical and neonatal outcomes suggest that the modest increase in birth weight and length associated with increased fish oil in the diet is mediated by the prolongation of gestation (Makrides et al., 2011). It has been suggested that reduction of prostaglandin E2 and F2α by fatty acids could be the mechanism leading to delay in cervical ripening and initiation of labor (McGregor et al., 2001).
Prenatal exposures to PCBs and HCB in this cohort of newborns were associated with reduced birth length in the direct models. However, the indirect models revealed that these associations were actually attributable to shorter gestation duration. Two other studies, including one among Inuit participants, have also found negative associations between PCBs and pregnancy duration (Fein et al., 1984; Wojtyniak et al., 2010). Another study reported reduced birth weight among neonates born to mothers consuming contaminated fish from Lake Michigan (Karmaus and Zhu, 2004), Lake Ontario (Murphy et al., 2010) and the Baltic Sea (Rylander et al., 1998), without specifically affecting length of gestation. In fact, duration of gestation was considered as a confounder variable in most of those studies and adjusted for in multiple regression models focusing on birth weight as the main outcome. Because length of gestation could be on the causal pathway between ECs exposure and anthropometric birth outcomes, controlling for this variable can cause overadjustment bias (Schisterman et al., 2005). Absence of associations with growth parameters was also reported from the consortium study of Great Lakes sport-caught fish (Weisskopf et al., 2005), Finland (Vartiainen et al., 1998), Faroe Islands (Grandjean et al., 2001) and Greenland (Bjerregaard and Hansen, 2000).
Possible reduction of gestational length by PCBs was explored only in the New York Angler study (Murphy et al., 2010) and the first Michigan study (Fein et al., 1984). Maternal preconception PCB levels were not related to pregnancy duration in the New York Angler study, even though significant associations were observed with birth weight. On the other hand, newborns from the Michigan study with cord serum concentration above PCB detection limits were born about 9 days earlier than infants with PCB levels below the detection limit. This association is much stronger than the 3-day discrepancy found here between the first and third quartiles of PCB exposure, but median concentration of PCB 153 in the Michigan study was 1/3 higher (120 ng/g lipids) than in our study (80 ng/g) (Longnecker et al., 2003). One possible mechanism by which PCBs can affect gestation duration is by activating the release of arachidonic acid from myometrial cells (Brant and Caruso, 2006). This fatty acid is known to induce prostaglandin secretion that contributes to cervical softening and effacement, as well as uterine contraction (Gibb, 1998).
Cord blood HCB and PCB 153 had similar associations with pregnancy duration. Although HCB was less strongly correlated with PCB congener than the other OCs, the correlation was still quite high. Consequently the HCB pregnancy duration association might be entirely or partly attributable to PCB congeners or other OCs. A negative association between maternal blood HCB concentration and gestation length was also reported among Mexican immigrants from the CHAMACOS study in California, who were exposed at levels similar to those in our sample (Fenster et al., 2006). Associations of cord HCB concentrations with birth outcomes could be explained by its capacity to disrupt thyroid hormone homeostasis (van Raaij et al., 1993), a well known risk factor for preterm birth (Casey et al., 2005).
In this study, we also found a significant negative association of cord Hg levels with pregnancy duration, which is in agreement with results from the POUCH study (Xue et al., 2007), the largest birth cohort study to investigate the association of Hg with pregnancy outcomes. In that low exposure cohort, maternal hair mercury levels above the 90th percentile (≥ 0.55 µg/g) at mid-pregnancy were associated with an increase risk to deliver before 35 weeks of gestation. Although we did not investigate the association with prematurity, Hg levels were associated with a reduction of almost 8 days in gestational length on average, an association that might have resulted in an increased prematurity rate in a larger sample. A similar relationship of Hg on duration of pregnancy was also reported among Greenlanders born to mothers with a high intake of fish and marine mammals (Foldspang and Hansen, 1990). However, these results were not replicated in another larger Greenlandic study (Bjerregaard and Hansen, 1996), in a previous cohort of Nunavik Inuit newborns (Lucas et al., 2004), or in the Faroe Islands study (Grandjean et al., 2001), which had levels of exposure to Hg that were similar to our study population. Other studies among fish-eaters have observed negative associations of prenatal Hg exposure to birth weight and/or length when adjusted on gestational age (Lee et al., 2010; Ramon et al., 2009) but did not report specific associations with gestational length. Effects of Hg on pregnancy duration remain unclear, but animal studies have revealed that this metal can promote platelet aggregation and thromboxane production (Caprino et al., 1983) and cause oxidative stress and endothelial dysfunction that may increase vascular resistance and hypertension (Wiggers et al., 2008). Whether this mechanism can account for the reduced gestation length observed in this study remains to be determined, but hypertensive disorders are well known factors of prematurity (Ferrazzani et al., 2011).
Our study has a number of strengths. First, in cord blood, we quantified both contaminants (Hg and several OCs) and essential nutrient levels derived from fish, seafood and sea mammal consumption and considered them simultaneously in the statistical analysis. Also of interest is our statistical analysis using path models which enabled us to identify direct and indirect effects of ECs on pregnancy duration and anthropometric measurements at birth. The percentage of missing data on ECs and DHA concentrations is a limitation of the study, but the use of the full information maximum likelihood procedure for missing data allowed us to reduce biased estimates, compared to the “complete data” approach (Graham, 2009). Although the Ballard examination is less precise than ultrasound for determination of gestational age, it was the best alternative due to the unavailability of ultrasound evaluation for most participating women. Also, data on maternal pre-pregnancy weight were partially based on maternal recall which could have led to random measurement errors, since weight in Inuit women is not an issue of social desirability. These measurement errors probably underestimate the associations between ECs and foetal growth. Other weaknesses of this study are the small sample size, the exclusion of women with at-risk pregnancies who do not deliver in Nunavik, and the use of a single time-point for exposure assessment, preventing us from identifying specific windows of critical vulnerability for foetal growth.
Altogether, these results indicate that n-3 PUFA content in fish, seafood and marine mammals may mask the negative association of prenatal Hg and OCs with foetal growth in observational studies and that it is crucial to consider this beneficial nutrient in statistical analyses of risk assessment of maternal fish and seafood consumption during pregnancy. Our data suggest that moderately high levels of ECs derived from fish, seafood and marine mammals can affect foetal growth by reducing pregnancy duration. On the other hand, n-3 PUFAs may mitigate these adverse effects by prolonging pregnancy duration. These findings underscore the importance of promoting consumption of fish species with low EC content and high n-3 PUFA concentrations in order to optimize foetal growth and development.
Supplementary Material
Highlights.
-
➢
Associations of contaminants on duration of gestation and foetal growth are equivocal.
-
➢
Fatty acids from fish appear to prolong gestation and promote foetal growth.
-
➢
We evaluated their associations on gestation duration and foetal growth in Inuit newborns.
-
➢
Contaminants negatively associated with pregnancy duration, reducing foetal growth.
-
➢
Positive association of fatty acids with gestation duration, improving foetal growth.
Acknowledgments
We are grateful to the Nunavik population and their organisations for their participation in this research. We also thank Germain Lebel, Edna Lachance, Christine Bouffard, Karine Poitras, Carole Vézina, Jocelyne Gagnon, Renee Sun, Neil Dodge, and Brenda Tuttle for their committed involvement in this study.
Funding sources:
This study was supported by grants from the National Institute of Environmental Health Sciences/NIH (R01-ES07902); Northern Contaminants Program, Indian and Northern Affairs Canada (Health Canada); Environmental Child Health Initiative, Hydro-Québec. RD was supported through a postdoctoral fellowship from Canadian Institutes of Health Research.
Abbreviations
- DHA
Docosahexaenoic acids
- ECs
Environmental contaminants
- HCB
Hexachlorobenzene
- Hg
Mercury
- MAR
Missing at random
- MeHg
Methylmercury
- n-3 PUFAs
n-3 polyunsatured fatty acids
- OCs
Organochlorine compounds
- Pb
Lead
- PCBs
Polychlorinated biphenyls
- Se
Selenium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics:
The participation of human subjects did occur after informed consent was obtained. The research procedures were approved by the human subjects committees of Laval University and Wayne State University.
References
- Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic 'summation' method. Clin Chim Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit Cohort Study. Environ Health Perspect. 2003;111:1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- Bjerregaard P, Hansen JC. Effects of smoking and marine diet on birthweight in Greenland. Arctic Med Res. 1996;55:156–164. [PubMed] [Google Scholar]
- Bjerregaard P, Hansen JC. Organochlorines and heavy metals in pregnant women from the Disko Bay area in Greenland. Sci Total Environ. 2000;245:195–202. doi: 10.1016/s0048-9697(99)00444-1. [DOI] [PubMed] [Google Scholar]
- Brant KA, Caruso RL. PCB 50 stimulates release of arachidonic acid and prostaglandins from late gestation rat amnion fibroblast cells. Reprod Toxicol. 2006;22:591–598. doi: 10.1016/j.reprotox.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Campoy C, Escolano-Margarit MV, Anjos T, Szajewska H, Uauy R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr. 2012;107(Suppl 2):S85–S106. doi: 10.1017/S0007114512001493. [DOI] [PubMed] [Google Scholar]
- Caprino L, Togna AR, Cebo B, Dolci N, Togna G. In vitro effects of mercury on platelet aggregation, thromboxane and vascular prostacyclin production. Arch Toxicol Suppl. 1983;6:48–51. doi: 10.1007/978-3-642-69083-9_8. [DOI] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- Choi AL, Cordier S, Weihe P, Grandjean P. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38:877–893. doi: 10.1080/10408440802273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JM, Robson PJ, Duffy EM, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Clarkson TW. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Ayotte P, Bruneau S, Laliberte C, Muir DC, Norstrom RJ. Inuit exposure to organochlorines through the aquatic food chain in arctic Quebec. Environ Health Perspect. 1993a;101:618–620. doi: 10.1289/ehp.93101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Bruneau S, Ayotte P, Laliberte C, Gingras S, Bélanger D, Ferron L. Health status at birth of Inuit newborn prenatally exposed to organochlorines. Chemosphere. 1993b;27:359–366. [Google Scholar]
- Drouillet-Pinard P, Huel G, Slama R, Forhan A, Sahuquillo J, Goua V, Thiebaugeorges O, Foliguet B, Magnin G, Kaminski M, Cordier S, Charles MA. Prenatal mercury contamination: relationship with maternal seafood consumption during pregnancy and fetal growth in the 'EDEN mother-child' cohort. Br J Nutr. 2010;104:1096–1100. doi: 10.1017/S0007114510001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. J Pediatr. 1984;105:315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- Fenster L, Eskenazi B, Anderson M, Bradman A, Harley K, Hernandez H, Hubbard A, Barr DB. Association of in utero organochlorine pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2006;114:597–602. doi: 10.1289/ehp.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazzani S, Luciano R, Garofalo S, D'Andrea V, De Carolis S, De Carolis MP, Paolucci V, Romagnoli C, Caruso A. Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev. 2011;87:445–449. doi: 10.1016/j.earlhumdev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Foldspang A, Hansen JC. Dietary intake of methylmercury as a correlate of gestational length and birth weight among newborns in Greenland. Am J Epidemiol. 1990;132:310–317. doi: 10.1093/oxfordjournals.aje.a115660. [DOI] [PubMed] [Google Scholar]
- Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30:235–241. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol. 2001;30:1272–1278. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108:E82. doi: 10.1542/peds.108.5.e82. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatr. 2008;152:356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Zhu X. Maternal concentration of polychlorinated biphenyls and dichlorodiphenyl dichlorethylene and birth weight in Michigan fish eaters: a cohort study. Environ Health. 2004;3:1. doi: 10.1186/1476-069X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Third Edition. New York: The Guilford Press; 2010. [Google Scholar]
- Lee BE, Hong YC, Park H, Ha M, Koo BS, Chang N, Roh YM, Kim BN, Kim YJ, Kim BM, Jo SJ, Ha EH. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ Health Perspect. 2010;118:437–443. doi: 10.1289/ehp.0900731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, Korrick SA, Rogan WJ, Weisglas-Kuperus N, Hertz-Picciotto I, Ayotte P, Stewart P, Winneke G, Charles MJ, Jacobson SW, Dewailly E, Boersma ER, Altshul LM, Heinzow B, Pagano JJ, Jensen AA. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Dewailly E, Muckle G, Ayotte P, Bruneau S, Gingras S, Rhainds M, Holub BJ. Gestational age and birth weight in relation to n-3 fatty acids among Inuit (Canada) Lipids. 2004;39:617–626. doi: 10.1007/s11745-004-1274-7. [DOI] [PubMed] [Google Scholar]
- Makrides M, Collins CT, Gibson RA. Impact of fatty acid status on growth and neurobehavioural development in humans. Matern Child Nutr. 2011;7(Suppl 2):80–88. doi: 10.1111/j.1740-8709.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;3:CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- McGregor JA, Allen KG, Harris MA, Reece M, Wheeler M, French JI, Morrison J. The omega-3 story: nutritional prevention of preterm birth and other adverse pregnancy outcomes. Obstet Gynecol Surv. 2001;56:S1–S13. doi: 10.1097/00006254-200105001-00001. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Plana E, Guxens M, Foradada Morillo CM, Albareda RM, Garcia-Esteban R, Goni F, Kogevinas M, Sunyer J. Seafood consumption in pregnancy and infant size at birth: results from a prospective Spanish cohort. J Epidemiol Community Health. 2010;64:216–222. doi: 10.1136/jech.2008.081893. [DOI] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LE, Gollenberg AL, Buck Louis GM, Kostyniak PJ, Sundaram R. Maternal serum preconception polychlorinated biphenyl concentrations and infant birth weight. Environ Health Perspect. 2010;118:297–302. doi: 10.1289/ehp.0901150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, Knudsen LB. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet. 1986;2:367–369. doi: 10.1016/s0140-6736(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team. BJOG. 2000;107:382–395. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, Grant A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- Ramon R, Ballester F, Aguinagalde X, Amurrio A, Vioque J, Lacasana M, Rebagliato M, Murcia M, Iniguez C. Fish consumption during pregnancy, prenatal mercury exposure, and anthropometric measures at birth in a prospective mother-infant cohort study in Spain. Am J Clin Nutr. 2009;90:1047–1055. doi: 10.3945/ajcn.2009.27944. [DOI] [PubMed] [Google Scholar]
- Rylander L, Stromberg U, Dyremark E, Ostman C, Nilsson-Ehle P, Hagmar L. Polychlorinated biphenyls in blood plasma among Swedish female fish consumers in relation to low birth weight. Am J Epidemiol. 1998;147:493–502. doi: 10.1093/oxfordjournals.aje.a009476. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- van Raaij JA, Frijters CM, van den Berg KJ. Hexachlorobenzene-induced hypothyroidism. Involvement of different mechanisms by parent compound and metabolite. Biochem Pharmacol. 1993;46:1385–1391. doi: 10.1016/0006-2952(93)90103-4. [DOI] [PubMed] [Google Scholar]
- Vartiainen T, Jaakkola JJ, Saarikoski S, Tuomisto J. Birth weight and sex of children and the correlation to the body burden of PCDDs/PCDFs and PCBs of the mother. Environ Health Perspect. 1998;106:61–66. doi: 10.1289/ehp.9810661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Anderson HA, Hanrahan LP, Kanarek MS, Falk CM, Steenport DM, Draheim LA. Maternal exposure to Great Lakes sport-caught fish and dichlorodiphenyl dichloroethylene, but not polychlorinated biphenyls, is associated with reduced birth weight. Environ Res. 2005;97:149–162. doi: 10.1016/j.envres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Wiggers GA, Pecanha FM, Briones AM, Perez-Giron JV, Miguel M, Vassallo DV, Cachofeiro V, Alonso MJ, Salaices M. Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am J Physiol Heart Circ Physiol. 2008;295:H1033–H1043. doi: 10.1152/ajpheart.00430.2008. [DOI] [PubMed] [Google Scholar]
- Wojtyniak BJ, Rabczenko D, Jonsson BA, Zvezday V, Pedersen HS, Rylander L, Toft G, Ludwicki JK, Goralczyk K, Lesovaya A, Hagmar L, Bonde JP. Association of maternal serum concentrations of 2,2′, 4,4′5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p′-DDE) levels with birth weight, gestational age and preterm births in Inuit and European populations. Environ Health. 2010;9:56. doi: 10.1186/1476-069X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect. 2007;115:42–47. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.