Abstract
Epigenetic medicine is still in its infancy. To date, only a handful of diseases have documented epigenetic correlates upstream of gene regulation including cancer, developmental syndromes and late-onset diseases. The finding that epigenetic markers are dynamic and heterogeneous at tissue and cellular levels, combined with recent identification of a new form of functionally distinct DNA methylation has opened a wider window for investigators to pry into the epigenetic world. It is anticipated that many diseases will be elucidated through this epigenetic inquiry. In this review, we discuss the normal course of DNA methylation during development, taking alcohol as a demonstrator of the epigenetic impact of environmental factors in disease etiology, particularly the growth retardation and neurodevelopmental deficits of fetal alcohol spectrum disorders.
Keywords: 5-hydroxymethylcytosine, developmental syndromes, DNA methylation, fetal alcohol syndrome, late-onset disease, transgenerational epigenetics
Epigenetics is characterized by its ability to modify genetic outputs through the alteration of 3D chromatin structure and subsequent accessibility of transcriptional machinery. This capacity endows the important biological function of intrinsically steering the progression of cell and tissue development or steadily maintaining a cell-specific program. The backbone of epigenetics is cytosine methylation (methylcytosine hereafter referred to as 5mC) and the chemical coding of the histone protein. Recent discovery of 5-hydroxymethylcytosine (5hmC), a 5mC derivative, and high-throughput DNA methylation analyses have provided insight into cellular and organismal development, maintenance and plasticity. Contrary to pre-existing beliefs, DNA methylation is earning a new reputation as a dynamic and programmable epigenetic marker, consequently allowing for a greater role in development and plasticity. The first part of this review examines these new features more closely, particularly in the context of nervous system development, which is spatiotemporally organized, protracted and greatly influenced by environmental factors.
Perhaps the most intriguing facet of epigenetics is its potential to internalize external impacts to potentially redirect an established genetic course. In other words, epigenetics can selectively record and accumulate environmental information to alter default transcriptional schemes. An increasing number of environmental factors, such as nutritional disparity, stress, pollutants, substances of abuse and maternal care [1-5] are evidently capable of altering epigenetics in this way and laying groundwork for future studies of environmentally linked diseases. How these external inputs are mechanistically translated remains poorly understood. The second part of the review will tackle how alcohol abuse as an environmental teratogen can affect epigenetics at the cellular and genomic level to alter gene transcription and elicit alcohol-associated disease phenotypes. We specifically emphasize the role of altered epigenetics in fetal alcohol syndrome (FAS; occurs in 1–2 of 1000 newborns and is a leading cause of nongenetic mental retardation in the western world) in which children born to women who drink during pregnancy suffer from severe dysmorphism, and brain and body retardation with psychosocial disability. Approximately ten-times more children escape diagnosis owing to a lack of obvious dysmorphism. These patients suffer from a slew of neurodevelopmental deficits and are now categorized into a broader class termed fetal alcohol spectrum disorders (FASD). Aside from primary abnormalities, some victims of fetal alcohol exposure do not present distinct phenotypes until later life when, typically, impaired cognitive plasticity and/or maladaptive behaviors emerge. On the other hand, FASD and related abnormalities have been identified in subjects without intrauterine exposure [6,7]. Along these lines, we explore evidence for the novel case of ‘epigenetic memory’, which may shed light on environmentally linked late-onset and transgenerational diseases. Elucidating the epigenetic mediation of FASD (including FAS) will pave the way for the understanding of the epigenetic mechanisms of environmental exposures that underlie other developmental deficits. To date, there is no treatment for the disease. This is largely attributed to the lack of understanding of the mechanisms underlying complex FASD phenotypes. Epigenetic investigation will expand the pathogenic scope and shed new light on the underpinnings of this and other developmental syndromes. It will also broaden the pharmacotherapeutic horizon for intervention and treatment in future medicine.
DNA methylation during development
DNA methylation dynamics
Genomic methylation influences the 3D conformation of chromatin and consequent DNA packing. Such changes further affect the accessibility of bioactive proteins that are essential for transcription. Methylation predominantly occurs at CpG sites by DNA methyl transferases (DNMTs) in the somatic cells of vertebrates while non-CpG methylation prevails in embryonic stem (ES) cells [8,9]. CpG methylation in promoter regions has been canonically associated with condensed DNA packing and inhibited transcription of genes, ncRNA and transposable elements [10,11]. Dynamic transcriptional regulation has been believed by some to arise from the differential distribution patterns of methyl cytosines within a locus [12]. For example, a distinct gene–body (intragenic) hypermethylation was shown in highly expressed genes in human cells [13]. Other works have described hypermethylation at exonic regions, which regulate alternative splicing [14]. The recent discovery of 5hmC, the hydroxylated form of 5mC, provides a vital alternative that challenges this view of DNA methylation in transcriptional regulation. In a process of active demethylation, 5mC is oxidized by Tet 1/2/3 enzymes into 5hmC, which can be further demethylated into 5-formyl cytosine and 5-carboxylcytosine or deaminated by cytidine deaminase into 5-methyluracil [15]. This is significant because developmental demethylation has been previously thought to occur only through replication-dependent or passive pathways [16]. This developmental, active demethylation pathway significantly alters the functional equation of DNA methylation in two major ways. First, because of 5hmC abundance during neural development [17,18], a major shift in the initial 5mC profile (demethylation) is expected, which could alter gene expression. Second, beyond a nonfunctional intermediate, 5hmC is garnering evidence as a standalone epigenetic player that might serve to prepare or mediate gene transcription [19].
5hmC is found as an enduring form of DNA methylation in ES and zygotic cells [20,21]. Global ana lysis has indicated that while 5mC is associated with suppression of transcription, 5hmC is associated with genes that are transcriptionally active or transitioning towards activation in ES cells [22] and in the developing brain [17]. In other reports, 5hmC in genes transitioning from silenced to expressed were found to be bivalently affiliated with activating and suppressive histone 3 modifications in ES cells [23,24]. The functional implications of 5mC versus 5hmC are further supported by their genomic distribution, in which 5mC is preferentially distributed in promoter regions while 5hmC is associated with gene bodies (as indicated above) and promoter regions of developmental regulatory genes (Figure 1) [22] and enriched in regions associated with an activating histone code [19,25]. Finally, 5hmC and 5mC are independently tied to unique methyl binding proteins (transcriptional regulators) [26] and differentially colocalized to eurochromatin and heterochromain, respectively [19]. Collectively, this evidence advocates that the conversion of 5mC to 5hmC offers a critical means for transcriptional transition. Thus, the dynamic turnover of 5mC and 5hmC shed new light on gene regulation, which is essential in the context of the ever-changing developmental landscape. In the next section, we review DNA methylation dynamics in the differentiation of neural stem cells during neural tube and brain development.
Figure 1. Two distinct forms of DNA methylation.
DNMT transfers a methyl group to cytosine bases to form 5-methylcytosine (5mC). 5mC is found at CpG rich islands in the promoter regions of the gene. Canonically, CpG methylation is associated with silenced gene activation (represented by the ‘X’) [10,11], often through methyl CpG-binding proteins (i.e., MBD1), which recognize 5mC sites and recruit negative transcriptional regulatory proteins. The Tet family enzymes can further bind 5mC and hydroxylate the methyl group into 5-hydroxylmethylcytosine (5hmC). A shift of 5hmC from promoter to the gene-body regions is genomically observed. 5hmC have shown preference for differential methyl CpG-binding proteins (e.g., MBD3) [26] and increasingly found to link to transcriptionally activating complexes [17,19,22]. The complimentary distribution and differential role of 5mC and 5hmC provide a new dynamic for epigenetic regulation of the complex development.
C: Cytosine; DNMT: DNA methyl transferase; Me: Methylation; TSS: Transcription start site.
DNA methylation program
Despite many studies describing the timely expression of cohorts of genes in spatially precise manners, there is still no satisfactory explanation for how tens of thousands of genes are orchestrated in their exclusive expression during differentiation. Although the answer to the question remains wide open, upstream epigenetic regulation is an inviting step forward in that direction. It is now clear that DNA methylation is neither random nor fixed throughout the epigenome. Instead, DNA methylation throughout development is dynamic, yet patterned like a program as the stem cell undergoes differentiation.
In the zygote, both 5mC and 5hmC are parentally inherited through the gametes but are lost as cells undergo rapid divisions in the absence of a DNMT. Thereafter, a less understood, widespread, de novo remethylation, mediated by DNMT, occurs to ensure the epigenetic regulation of genomic function [27]. Totipotent ES cells (capable of producing any cell type) also go through methylation reprogramming, in which a distribution ratio of 75% CpG methylation to 25% non-CpG methylation is shifted to 99% CpG methylation [9]. These events may be connected to 5hmC’s involvement in programming and maintenance of the pluri potency of ES cells and the totipotency of zygotes [20,21,28].
The doctrine of epigenetics suggests that DNA methylation is modifiable and this modification can be inherited through cell divisions. While this view is indisputable, new findings also tell us that many newborn progenitor cells are not obligated to the parental epigenome. In other words, a methylation pattern can either be inherited completely (e.g., reinstated in symmetrical daughter cells) or partially modified (e.g., heterogeneous in asymmetrical daughter cells). The heterogeneity of DNA methylation may thus account for the diversification of cell fates. In addition to 5mC, recent findings indicate that 5hmC content in the methylome accounts for the major diversity of tissue specification [29]. Thus, the distribution of the 5mC and 5hmC are potential major upstream regulators of early cell fate determination.
Remarkably, during neural tube development, DNA methylation progresses in a precise spatiotemporal manner in neuroepithelial cells. This program coincides with neural differentiation, as recently demonstrated in our laboratory [30]. Undifferentiated neuroprogenitor cells are devoid of 5mC as indicated by immunocytochemistry, but they acquire DNA methylation at the beginning of differentiation and migration approximately at embryonic day 7-8. The DNA MBD1 and DNMT1 appear approximately 1 day behind 5mC in the mouse, following similar spatiotemporal patterns. In the anterior-posterior axis, a clear gradient of DNA methylation appears first in the hindbrain and progresses rostrally to the forebrain and caudally to the caudal neural tube and spinal cord. It is the same pattern that has long been known for the progression of differentiation in the neural axis. In the dorso–ventral division, DNA methylation first occurs ventrally and progresses toward the dorsal division. This is also matched by the differentiation gradation in the dorso–ventral aspect of the neural tube.
Since the finding of 5hmC, a much greater resolution of DNA methylation-associated neuroepithelial differentiation has been achieved. Although an increase of 5mC is a prerequisite for neural cell differentiation, the initiation of differentiation is not readily apparent at the presence of 5mC. By contrast, hours to a day after the arrival of 5mC, it is the appearance of (or transformation to) 5hmC that closely aligns with the beginning of differentiation in mouse embryos. The transition of 5mC to 5hmC may trigger this differentiation. This is in agreement with the associative transition of gene activation by 5hmC, which was recently reported in pluripotent stem cells [19] and in mouse neural tube [31]. It is also in agreement with the decrease of 5mC at CpG islands in promoter regions and the reported increase of 5hmC at the gene bodies of transcriptionally activated genes [17].
The DNA methylation program is also evident in the developing brain. Cellular 5mC and 5hmC are highly correlated with neural progenitor cells and their progression towards differentiation (Figure 2) in several developing regions (e.g., cortices, hippocampus and cerebellum) [32,33]. Epigenomic ana lysis has also demonstrated the association of 5hmC with transcription in the postnatal hippocampus and cerebellum [17]. It is worth mentioning that the DNA methylation program involves a continuous cycle of methylation and demethylation along the pathway of cellular differentiation until the neurons or glia reach full maturation. That is, the differentiating neurons containing high levels of DNA methylation may lose their 5mC and 5hmC after arriving at target regions of the brain or when transitioning into a stage of active wiring and synaptogenesis. This methylation and demethylation cycle can be repeated over the course of different stages of differentiation. These temporal changes indicate that DNA methylation and demethylation prevail throughout neural development as a program orchestrating the phenotype of specific neurons.
Figure 2. DNA methylation dynamics during neuronal differentiation.
A general view shows basic DNA methylation dynamics on the path to neuronal maturation from undifferentiated neuroepithelial cells (A-C). (A) While proliferating prior to differentiation, the neuroepithelial cell nucleus is spindle shaped and contains no DNA methylation marks (DMMs). However, during early differentiation, they increase in size and acquire cytosine methylation (5mC) followed by 5hmC. (B) Migration towards the transitional zone begins after the appearance of DMMs. By then, the nucleus has increased in size and become ellipsoid shaped and they acquire MBD1. Meanwhile, 5mC and 5hmC are complimentarily repackaged into heterochromatic and euchromatic regions, respectively. (C) As they approach a target region, the maturing neuron is characterized by both MBD1 and 5mC in the nuclear heterochromatic distribution and 5hmC in euchromatin. Upon reaching the target region, the cells appear almost fully rounded and establish more 5hmC while some mature neurons exhibit decreased 5mC. Left column: immunohistochemical representation of DMM distribution and nuclear morphology in each differentiation state; middle column: illustration of cell stage and DMMs; right column: DMM and nuclear morphology legend. Arrows indicate the direction of the differentiation path. Please see color figure at www.futuremedicine.com/doi/pdf/10.2217/epi.12.80. 5hmC: 5-hydroxymethylcytosine; 5mC: Methylcytosine; MBD: Methyl binding domain.
The DNA methylation progression of neural progenitor cells has been validated by genome-wide ana lysis of DNA methylation in embryos [34] and in neural differentiating stem cells (to control for cellular heterogeneity) [35]. Hyper- and hypo-methylation were identified in the embryonic genome during neurulation and in neural stem cell differentiation [35]. This is consistent with the transcriptional needs of a maturing cell in which many pluripotent genes are turned off (e.g., Oct 4), while neuron-specific genes are activated (e.g., Map2) [35]. The DNA methylation dynamic of 5mC and 5hmC occurs during normal development in a predictable program where 5mC and 5hmC appear and transition, in a spatiotemporal manner, to coincide with the phenotypic progression of neuroprogenitor cells towards differentiation of specific neurons and glia. This epigenetic program mostly likely underlies the transcriptional requirement for neural fate determination and specification [36]. DNA methylation dynamics walk a tightrope, navigating through the protracted and ever-changing developmental course to maintain normalcy – a feat that is challenged upon constant environmental impacts.
Alcohol as a modifier of epigenetics
Many environmental factors are known to significantly alter the intrauterine environment and contribute to the dysregulation of the fetal-growth processes. How these environmental challenges are recorded in active dividing cells and are translated to affect cellular and molecular processes during fetal development and in later life is not fully understood. DNA methylation offers an environmentally responsive plasticity in line with the fluctuant nature of transcriptional programs during cellular differentiation. Alcohol exposure during pregnancy, which can lead to FASD, has been used as a model to resolve the epigenetic pathway between environment and phenotype. Several laboratories have reported altered epigenetics, including DNA methylation, in multiple models of FASD. These studies have begun to shed light on the mechanisms by which environmental factors reshape epigenetic scripts to alter developmental phenotypes.
Alcohol and methyl metabolism
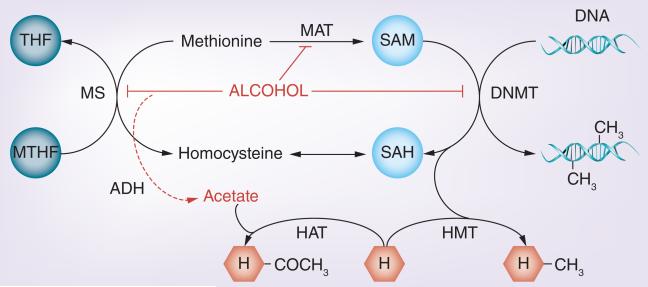
Alcohol can directly influence epigenetic marks through the alteration of the methionine pathway (Figure 3). The synthesis of methionine allows the production of an active methyl donor, S-adenosylmethionine (SAM), which is recruited by DNMTs for the transfer of a methyl group to the nucleotide cytosine. Dietary nutrients, methionine, folate and choline, all play substantial roles in the production of SAM and alcohol has been shown to affect each in an interdependent manner [37], thus allowing a biochemical pathway for alcohol to regulate DNA methylation. In turn, DNA methylation can influence histone modifications and ncRNA, all of which are capable of independently altering genomic expression. Next, we review alcohol-related methionine deficiencies and the associated epigenetic alterations in the context of developmental disease.
Figure 3. Alcohol and methyl metabolism.
DNA and histone methylation occurs mainly through the transfer of a methyl group to the substrate. SAM actively carries the methyl donor group. SAM is synthesized from methionine, which itself can be produced from the dietary intake of folate, choline and other methyl donors (only folate is shown here for simplicity). Alcohol can affect the methionine synthesis process by inhibiting metabolic enzymes [122], MAT [43] and DNMTs [123], both through direct and indirect processes [62,63]. This consequently induces a decrease in SAM production and hyperhomocysteinemia [124]. In addition, acetate, a by-product of alcohol metabolism (indicated by a dashed arrow), is involved in the acetylation of Hs.
DNMT: DNA methyl transferase; H: Histone; HAT: Histone acetyltransferase;
HMT: Histone methyltransferase; MTHF: Methyltetrahydrofolate;
SAH: S-adenosylhomocysteine; SAM: S-adenosylmethionine; THF: Tetrahydrofolate.
Dietary folate and vitamin B12 are cofactors of the enzyme methionine synthase, which is responsible for the conversion of homocysteine to methionine, a precursor of SAM. Betaine from dietary choline can also be utilized in the conversion of homocysteine to methionine by betaine homocysteine methyltransferase. Both micronutrients exhibit sensitivity to alcohol. Chronic alcohol consumption has been linked to decreases in folate absorption [38], uptake [39] and serum levels [40]. In fetal alcohol models, impaired folate transport to the fetus has also been reported [41]. Furthermore, while short-term ethanol exposure seems to increase betaine homocysteine methyltransferase levels (likely to compensate for folate metabolism deficiency [42]), they eventually fall, along with SAM, to coincide with hyperhomocysteinemia [43], a condition describing abnormally large levels of homocysteine in the blood. Alcohol-induced hyperhomocysteinemia itself has been linked to a host of hepatic [44], renal [45], cardiac [46] and retinal [47] disease phenotypes.
The role of methionine precursors in mediating the teratogenic effects of alcohol is further supported by fetal supplementation studies, which have demonstrated the rescuing capacity of folate and choline from alcohol-induced oxidative stress [48], hyperhomocysteinemia [49,50] and other developmental abnormalities [51-54] (including some that may not be evident until later life [55-57]). Gestational choline is particularly relevant since it has been shown to regulate methyl group availability and influence global and gene-specific DNA methylation in the fetal brain [58,59]. Interestingly, choline deficiency appears to increase the methylation of genes responsible for histone methylation, subsequently decreasing histone methylation marks, which are generally associated with gene inactivation (an effect reversible by choline supplementation)[60]. Finally, acetaldehyde and acetate, products of alcohol metabolism, have been independently shown to contribute to histone acetylation [61] and the inhibition of DNA methylation [62,63].
Epigenetic correlates of alcohol exposure
DNA methylation
Evidence of alcohol-induced epigenetic alteration has substantiated dysregulation of SAM as a mechanism by which environmental teratogens can achieve a range of disease phenotypes. Alcohol’s alteration of DNA methylation was exemplified by studies employing the agouti viable yellow (Avy) mouse mutant allele, which contains a methylation-sensitive element within the Avy locus, dictating coat color dynamics. In this way, coat color, ranging from yellow (unmethylated) to pseudoagouti (highly methylated) [64,65], could be used as an indicator of methylation status. Prenatal exposure to ethanol increased the incidence of pseudoagouti animals, indicating that the biosensor was highly methylated and ectopic expression was silenced [66]. It is now known that alcohol’s effects on DNA methylation can be locus specific and bidirectional. For example, alcohol has also demonstrated the ability to decrease DNA methylation at imprinting control sites of the H19 locus in sperm [67] and placenta [68], as well as at the glutamate receptor gene Nr2b [69] – areas that are appropriately tied to growth and neural function. These studies tell that fetal alcohol exposure has the capacity to alter developmentally significant regions of the genome, although it has also exhibited influence over other regions of biological relevance (Table 1) [70].
Table 1.
Epigenetic gene targets of alcohol.
| Biological function | Gene target |
|---|---|
| Epigenetic regulation | Mbd3, Dnmtl, Dnmt3b and Smarca2 |
| Transcriptional regulation | SIN3A, ZNF562, Cutl2 (Cux2), Pou4f3 and Sox7 |
| Metabolic regulation | Pcft, Rfc, Fpgs, Adh, Cytp4502c11, Lasdh and Hal |
| Cell cycle regulation | Bub1, E2f7, Ccnbl, Plk1, CDC23 and LIN37 |
| Cellular differentiation | Pard6a, Dgcr2, ERMAP, ACTR3C, Neurod6, Snail1 and Srf |
| Cell survival | Gst-yc2, Crh and Tnf |
| Neural communication | MAOA, GABRB3, HTR3A, HTR2B, DRD4, GRIN1, Nr2b, OPRM1, POMC, PDYN and Pnoc |
| Synaptic plasticity | NCAM1 |
| Embryonic development | Jag1, Elavl2, Igf1, Igf2, ABR, Aldh1a1, Enah, Ets2, Vax1, Lim2, Kif1a, Ncstn, Smyd1 and Efemp2 |
| Imprinted genes | H19/IGF2 locus, DLK1/GLK2 locus, H19, Cdkn1c,Ube3a, Grb10,Igf2r, Slc22a18, Gatm, PEG10 and Nnat |
| Developmental disease related | Wbscr22† Nipbl† Bsnd, Ptpn11† Pthlh, Pafah1b1, Vsx1, Vax2, Pdgfra, Ube3a, Dmpk, Idua, Kif21a and Foxl2 |
| Late-onset disease related | SNCA, App, Hmox and Trpm7 |
| Cancer | BLCAP and ACTR3C |
Demonstrates high-throughput and single-gene analysis of epigenetic alteration by alcohol exposure, including DNA methylation, histone methylation and acetylation, and miRNA targeting. Alcohol models and stringency of methylation/histone modification comparison vary by study. The genes (primarily listed by biological function) revealed by Ingenuity pathway analysis are shown. Supplementary validation methods were used in some studies. Additional details and references are detailed in Supplementary Table 1.
Genes with DNA methylation changes involved in developmental syndromes have considerable overlap with fetal alcohol syndrome, a severe form of fetal alcohol spectrum disorders based on CDC reports. These genes are WBSCR22 (William’s syndrome), NIPBL (Brachmann De Lange syndrome) and PTPN11 (Noonan’s syndrome).
The likelihood of epigenetic dysregulation in FASD etiology is furthered by evidence of alcohol-induced epigenetic alteration in neural systems. Both fetal alcohol exposure and choline supplementation in the third trimester alter total DNA methylation in the murine hippocampus and prefrontal cortex [71], while high-throughput embryo ana lysis has shown that ‘binge-like’ alcohol exposure alters the methylation profile of over 1000 genes at early neural development [34,35]. These alterations were further associated with changes in the expression of several genes, including some required for neural specification and neural growth. Genomic ana lysis of neural stem cells has revealed that alcohol retards moderately methylated genes from going toward the hypermethylation and hypomethylation necessary for differentiation, indicating alcohol’s disruption of the neuroprogenitor DNA methylation program [72]. The alteration of DNA methylation is consequential. The phenotypes of the embryos or neural stem cells treated with the methylation inhibitor 5-azacytidine are similar to those treated with alcohol [35]. These mirrored phenotypic aberrations implicate epigenetic mechanisms in alcohol-related developmental disease.
Histone code
Evidently, the epigenetic reach of alcohol is not limited to DNA methylation. Alcohol increases histone 3 acetylation [73], which is tied to gene upregulation [74]. It has also been shown to increase methylation at H3K4, while decreasing methylation at H3K9, transcriptionally activating and repressing modifications, respectively [75]. This bidirectional action speaks to the epigenetic heterogeneity of neural tissues [72]. For example, in an amygdala-specific study, alcohol increased H3K9 acetylation but decreased H3K27 trimethylation [76]. In a rat FASD model, histone 3 and 4 acetylation is decreased in the cerebellum as a consequence of a downregulated histone acetyl-transferase [77]. Alcohol and its metabolites have also been shown to impact non-neural systems via histone modification [50,78,79]. Furthermore, alcohol-independent choline studies have demonstrated the heritability of histone modifications [60,80], presenting yet another epigenetic pathway for FASD etiology.
miRNA & transposable elements
miRNAs are an epigenetic mechanism by which small ncRNA bind complimentary sequences of DNA and hinder access or stability of transcriptional machinery. Alcohol has been found to alter the expression of several miRNAs in cultured neural stem cells [81]. Evidence suggests that their sensitivity to alcohol is dependent on cell type and differentiation state [82]. Many of the affected miRNA families target cellular proliferation [83] and have developmental implications [84]. In cancer models, some miRNA families have been shown to act on genes regulating epigenetic elements [85], while themselves exhibiting sensitivity to epigenetic alterations, such as DNA methylation [86], although it is unclear if the same bidirectional relationship exists in models of alcohol-related disease.
Transposable elements (TEs) are repetitive, noncoding regions that move around the genome and may cause insertions, deletions and translocations to affect genome integrity or gene transcription. TEs are normally suppressed by DNA methylation. Differential methylation of the TEs LINE-1 and the Alu element, AluYb8, were recently found in human placental tissues of alcohol- and nicotine-exposed infants [87]. Identifying and understanding how TEs escape silencing will open yet another potential epigenetic pathway for FASD etiology.
Epigenetic phenotypes
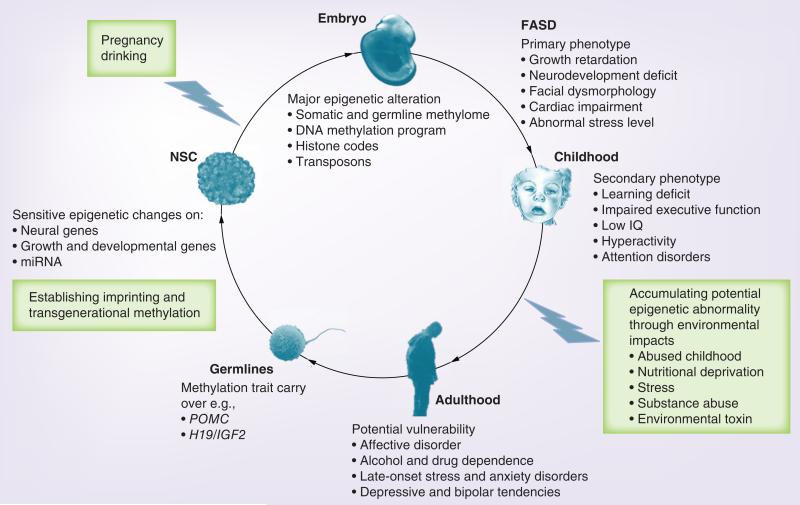
Although alcohol-induced epigenetic dysregulation has been reported for decades, we are only now uncovering the vast and complex epigenetic derivatives and their ties to human disease. Fetal alcohol exposure has been linked to a plethora of early- and late-onset FASD phenotypes (Figure 4). Cross-generational phenotypes have also been speculated in families without an immediate drinking history. A number of alcohol-altered epigenetic gene targets are correlated with these phenotypes (Table 1 & Supplementary Table 1; see www.futuremedicine.com/doi/suppl/10.2217/epi.12.80). A challenge remains the intractability of some phenotypes (particularly behavioral) to a defined and organized genetic scheme.
Figure 4. Epigenetic mechanisms and potential manifestations of fetal alcohol spectrum disorders.
Maternal alcohol exposure leads to expansive phenotypes of FASD but the mechanisms remain elusive. Besides immediate cellular effects, it is now understood that alcohol extensively alters epigenetics during fetal and NSC development through genomic DNA methylation, cellular DNA methylation programming (see text), histone modification, transposons and miRNA. These epigenetic changes are likely a major upstream disrupter of gene transcription leading to primary phenotypes of FASD (e.g., growth retardation and neurodevelopmental deficit) and collectively compromise brain function and mental faculty as secondary phenotypes in early life. It is not expected that all epigenetic changes lead to transcriptional and phenotypic changes but increasing evidence suggests that continuous environmental insults may lead to increased epigenetic abnormality [104]. The primary seeding of epigenetic errors and secondary, cumulative epigenetic abnormality via abrasive environment (e.g., childhood abuse and stress) may result in a potential manifestation of FASD beyond the classical diagnosis in adulthood. Moreover, epigenetic errors (e.g., POMC and H19 intergenic regulator) carried in the germlines that pass the checkpoint of global demethylation, may influence the next generations. Epigenetic medicine challenges clinicians to take a deeper as well as a broader view on FASD, using both the magnifying and telescopic lens of epigenetics.
FASD: Fetal alcohol spectrum disorders; NSC: Neural stem cell.
Child’s face ©The Board of Trustees of the University of Illinois, all rights reserved.
Early-onset phenotypes
A hallmark of FASD is growth retardation. Recall that the developmentally relevant imprinting control region H19 has exhibited sensitivity to prenatal alcohol. In one study, hypomethylation, although restricted to paternal alleles on exposed placentae, was accompanied by substantial growth restriction of both the placenta and embryo [88]. Another study found that on a particular region of the H19 locus, fetal alcohol evoked subtle hypomethylation in embryos as well [89]. The decreased methylation at this site corresponded with decreased Igf2 transcription – both of which were ameliorated with a methyl-supplemented diet.
Some of the earliest and most consequential fetal alcohol insults to the CNS occur at the level of progenitor cells, which impacts brain growth greatly. These primary phenotypes include compromised proliferation [90], migration [91], differentiation [92] and survival [93]. Several groups have demonstrated ethanol-induced epigenetic and transcriptional alterations of genes that are relevant to each of these biological processes (Table 1 & Supplementary Table 1). The effects of alcohol on basic, cellular processes can be further attested by the abnormal representation of neuronal populations and specific developmental deficits including cardiac [94], ocular [95], renal and hepatic [96] abnormalities that have been reported with fetal alcohol exposure. These can ultimately account for many of the secondary phenotypes of FASD, including attention deficits [97], motor impairments [98], impaired learning [99] and impaired cognitive ability [100].
Late-onset phenotypes
Many alcohol-related phenotypes do not manifest or are not recognized until later life (e.g., personality and mood disorders to schizoaffective disorders [97,101,102] and cancer [103]). These phenotypes may be the result of accumulated epigenetic alterations beset by the initial environmental insult, in this case fetal alcohol exposure, sustained in the lifespan by ‘epigenetic memory’ [104] and expanded by secondary environmental insults until a threshold for phenotypic manifestation is reached (Figure 4). A major challenge in disseminating the epigenetic background of these (a feat that must be accomplished in order to more clearly define their ties to fetal alcohol exposure) is that they are not easily defined in a genetic context. Nonetheless, preliminary advances have been made in the epigenetic examination of stress-regulating genes and their ties to anxiety disorders in adulthood [105]. Similarly, although not yet validated in fetal alcohol models, promising epigenetic gene targets have emerged for schizophrenia [106], depression [107] and various cancers [108].
It has been widely reported that mothers who consume alcohol exhibit altered behavior toward their offspring. Epidemiological studies have found a substantial correlation between neglectful and abusive parental behavior and substance abuse [109]. This type of early-life stress has been emulated in animal models and has revealed altered epigenetic outcomes in offspring. A parallel human and rat study found that early-life abuse altered DNA methylation in a locus-specific manner [2]. Conversely, in an animal model of early-life environmental enrichment (communal nest paradigm marked by high maternal care-giving behavior), BDNF expression is increased in the hippocampus and this may be attributable to increased histone 3 acetylation at the promoter [110]. Early maternal care (pup licking and grooming) has been linked to decreased Gad1 promoter methylation of male offspring and increased hippocampal expression of Gad1, a gene implicated in psychiatric diseases, such as schizophrenia and depression [5]. This is especially interesting given that FAS children have above average rates of psychiatric disease in adulthood [97,101,102]. It is an interesting hypothesis that neglectful parental behavior (as a consequence of alcoholism) may present an alternative epigenetic mechanism as a cause for FASD. A gap of knowledge in this area is yet to be filled and warrants further study.
Transgenerational phenotypes
The transgenerational heritability of epigenetic traits is circumstantial. A well-documented case reported that the disease states caused by the insecticide vinclozolin were transmitted across generations through sperm epigenetic mechanisms [111]. Recently, a cancer-associated germline epimutation was found to be transgenerationally inherited [112]. Can alcohol-induced epigenomic alterations also be passed to the offspring? This question was beckoned by early epidemiological studies revealing infants and adolescents bearing FASD-like features in the absence of maternal alcohol consumption where the fathers were alcoholics [6,7]. These studies suggest the existence of long-range and paternal epigenetic influences on FASD etiology. Work in the field has focused on the molecular mechansims behind these earlier findings and while the transmissibility of alcohol-induced epigenetic changes across generations has not been concretely demonstrated, ongoing studies have made strides toward this goal.
An early epigenetic study hypothesized that alcohol can change DNA methylation in sperm, and a chronic drinking model in rats undergoing spermatogenesis found that DNMT mRNA levels in sperm were lower than controls [113]. These findings suggest a hypomethylation of sperm DNA and support the hypothesis that the effects of paternal alcohol may be mediated by altered DNA methylation of sperm during spermatogenesis. Subsequent investigation has found hypomethylation at paternally imprinted loci in the DNA of isolated sperm from moderate to heavy drinkers [67]. Another reported similar findings at the H19 locus, although this time in the offspring DNA and not the paternal sperm [114]. Yet another ana lysis of this imprinting control region revealed hypomethylation at the paternal alleles in placental but not embryonic DNA [88]. Finally, Stouder and colleagues reported hypomethylation specifically at H19 CpGs in F1-generation sperm DNA and F2 offspring brain in a model of low-dose fetal ethanol exposure. This was accompanied by a decrease in the mean sperm concentration of male offspring [115], an effect which bears striking resemblance to intrauterine exposure to the potent demethylating agent 5-aza-2′-deoxycytidine [116].
Prenatal alcohol has also been linked to inherited epigenetic aberrations on nonimprinted genes. Neurons containing POMC-derived peptides have been functionally compromised in patients with a family history of alcoholism, raising the possibility that the alcohol effects on the POMC system may be transmissible across generations [117]. Functionally, alcohol increased the basal and lipopolysaccharide-induced stress response for both males and females of the F1 generation, although this only persisted in the F2 and F3 progeny of the established male germline [70]. These studies point to the potential heritability of environmentally induced epigenetic abnormalities and associated gene functionality across generations. More studies would be needed to confirm the site, nature, gene and stability of reprogrammed epigenetics in distant offspring. In addition, it will be important to consider early-life stressors (e.g., parental behavior) in the investigation of transgenerational epigenetics. More regions of transgenerational epigenomic inheritance are expected to be identified in the future. The outcomes could prove useful in furthering our understanding of many late-onset diseases of unknown etiology and their treatments.
Epigenetic therapies for FASD
There is no current disease-modifying therapy for FASD and clinicians agree that early intervention is the key to a positive therapeutic outcome. However, current diagnostic criteria are not entirely sensitive to the more subtle phenotypes of the disease spectrum. As such, there is a pressing need for a better understanding of the molecular underpinnings of the disease. Epigenetic research in FASD can further both the development of a more sensitive molecular diagnostic tool and disease-modifying therapies. The exploration of epigenetic regulators (e.g., methylcytidine analogs and inhibitors of histone-modifying enzymes) in the treatment of alcohol-related disease is progressing. Preliminary evidence, however, has presented cytotoxicity and nonspecificity as major roadblocks for their utility in developmental disease treatment [118]. As previously described, nutritional supplements, such as methionine, folate and choline, have demonstrated some capability to alleviate the teratogenic and hepatotoxic effects of ethanol exposure [34,48,49,53-57,119]. Although affecting much more than epigenetic mechanisms, carefully regulated nutritional supplements are well-tolerated in humans [120]. Many potential therapeutic agents and supplements can be developed in light of a better understanding of the epigenetic mechanisms of FASD. The safety and long-term potential of these should be explored.
Future perspective
Epigenetics is rapidly becoming the epicenter of future medicine [121]. The involvement of epigenetics in medicine expands beyond the conventional genetic focus of diseases and treatment. Cancer research has begun its movement toward epigenetic medicine. The next epigenetic wave is likely to occur in developmental medicine. These range from developmental syndromes without clear genetic errors (e.g., autistic spectrum disorders) to idiosyncratic developmental syndromes, late-onset, transgenerational diseases and aging. Developmental epigenetics may also contribute a better understanding of the diversity within the normal, ‘healthy’ population. There is no better time to emphasize environmental inputs and their long-term effects in developmental medicine. For example, early child care, stress, substances of abuse, environmental toxins can, through epigenetics, influence gene transcription and phenotype expression during development or continue throughout life. Many late-onset diseases not attributable to genetic error or polymorphisms may have been seeded during development. Their occurrence may be concurrent with or triggered upon further environmental insults over a life span. A greater comprehension of epigenetic medicine is still something to be attained but the pace of progress is improving and the elucidation of a host of human diseases through an epigenetic lens is imminent.
Supplementary Material
Executive summary.
Background
-
■
Epigenetics can be inherited, intrinsically evolved and externally modified. What and how an environmental factor impacts epigenetic processes is demonstrated by fetal alcohol-induced fetal alcohol spectrum disorders (FASD).
DNA methylation during development
-
■
DNA methylation during development is neither fixed nor random, but dynamically and specifically evolved to alter developmental processes.
-
■
5-hydroxymethylcytosine is increasingly believed to be functionally distinct from 5-methylcytosine and the dynamics of 5-methylcytosine and 5-hydroxymethylcytosine enrich the regulation of transcription.
Alcohol as a modifier of epigenetics
-
■
Alcohol can metabolically alter the availability of methyl donors to impact DNA and histone methylation, acetylation and/or ncRNAs.
-
■
Drinking during pregnancy alters DNA methylation and histone codification of many biologically relevant genes at the fetal stages, such changes may persist into later life and influence gene transcription.
Epigenetic therapies for FASD
-
■
Therapeutic advances in FASD should continue to focus on regulating the accessibility of alcohol to epigenetic precursors, such as methyl donors.
Future perspective
-
■
Since DNA methylation and histone modification appear to be cell and/or locus specific, future ana lysis of the epigenetics should include cellular and genomic approaches.
-
■
Underlying epigenetic causes of FASD serve as an excellent example of the environmental impact on epigenetics.
Acknowledgments
Studies on the epigenetic neural program in FC Zhou’s laboratory are supported by AA016698 and P50 AA07611.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suderman M, Mcgowan PO, Sasaki A, et al. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl Acad. Sci. USA. 2012;109(Suppl. 2):17266–17272. doi: 10.1073/pnas.1121260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madrigano J, Baccarelli A, Mittleman MA, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ. Health Perspect. 2011;119(7):977–982. doi: 10.1289/ehp.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J. Neurosci. 2010;30(39):13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little RE, Sing CF. Father’s drinking and infant birth weight: report of an association. Teratology. 1987;36(1):59–65. doi: 10.1002/tera.1420360109. [DOI] [PubMed] [Google Scholar]
- 7.Hegedus AM, Alterman AI, Tarter RE. Learning achievement in sons of alcoholics. Alcohol Clin. Exp. Res. 1984;8(3):330–333. doi: 10.1111/j.1530-0277.1984.tb05522.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramsahoye BH, Biniszkiewicz D, Lyko F, Clark V, Bird AP, Jaenisch R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl Acad. Sci. USA. 2000;97(10):5237–5242. doi: 10.1073/pnas.97.10.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 11.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 2007;213(2):384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 2008;9(6):465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 13.Ball MP, Li JB, Gao Y, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat. Biotech. 2009;27(4):361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastasiadou C, Malousi A, Maglaveras N, Kouidou S. Human epigenome data reveal increased CpG methylation in alternatively spliced sites and putative exonic splicing enhancers. DNA Cell Biol. 2011;30(5):267–275. doi: 10.1089/dna.2010.1094. [DOI] [PubMed] [Google Scholar]
- 15.Bhutani N, David DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146(6):866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 2010;11(9):607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14(12):1607–1616. doi: 10.1038/nn.2959. ■ Characterized the tissue-specific, transient presence of 5-hydroxymethylcytosine (5hmC) in the developing brain.
- 18.Song CX, Szulwach KE, Fu Y, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotech. 2011;29(1):68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubiura M, Okano M, Kimura H, Kawamura F, Tada M. Chromosome-wide regulation of euchromatin-specific 5mC to 5hmC conversion in mouse ES cells and female human somatic cells. Chromosome Res. 2012;20(7):837–848. doi: 10.1007/s10577-012-9317-9. ■■ Demonstrated mutually exclusive nuclear localization of methylcytosine (5mC) and 5hmC as well as propensity for 5hmC at transcriptionally active loci.
- 20.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science. 2011;334(6053):194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl Acad. Sci. USA. 2011;108(9):3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, D’alessio AC, Ito S, et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25(7):679–684. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastor WA, Pape UJ, Huang Y, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473(7347):394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams K, Christensen J, Pedersen MT, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473(7347):343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroud H, Feng S, Morey Kinney S, Pradhan S, Jacobsen SE. 5-hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011;12(6):R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildirim O, Li R, Hung JH, et al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147(7):1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005;14:R47–R58. doi: 10.1093/hmg/ddi114. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 28.Salvaing J, Aguirre-Lavin T, Boulesteix C, Lehmann G, Debey P, Beaujean N. 5-Methylcytosine and 5-hydroxymethylcytosine spatiotemporal profiles in the mouse zygote. PLoS ONE. 2012;7(5):e38156. doi: 10.1371/journal.pone.0038156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestor CE, Ottaviano R, Reddington J, et al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. 2012;22(3):467–477. doi: 10.1101/gr.126417.111. ■ A recent report on the variability of 5hmC in different tissues and the tendency of 5hmC to accumulate at intragenic regions, particularly regions of transcriptional activation.
- 30.Zhou FC, Chen Y, Love A. Cellular DNA methylation program during neurulation and its alteration by alcohol exposure. Birth Defects Res. A Clin. Mol. Teratol. 2011;91(8):W703–W715. doi: 10.1002/bdra.20820. ■ Described the spatiotemporal progression of 5mC during neural tube development and the delay of this program by ethanol exposure.
- 31.Zhou F. DNA methylation program during development. Front. Biol. 2012;7(6):485–494. doi: 10.1007/s11515-012-9246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Zhou FC. Alcohol alters cellular DNA methylation program in growth retarded cortex and hippocampus. Alcohol Clin. Exp. Res. 2012;36(Suppl. 1) Abstract 0789. [Google Scholar]
- 33.Ozturk N, Zhou FC. Alcohol markedly alters methyl binding proteins during neural development. Alcohol Clin. Exp. Res. 2012;36(Suppl. 1) Abstract 0459. [Google Scholar]
- 34.Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4(7):500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, Nephew KP. Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcohol Clin. Exp. Res. 2011;35(4):735–746. doi: 10.1111/j.1530-0277.2010.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khare T, Pai S, Koncevicius K, et al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat. Struct. Mol. Biol. 2012;19(10):1037–1043. doi: 10.1038/nsmb.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 2002;132(8):S2333–S2335. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 38.Wani NA, Nada R, Kaur J. Biochemical and molecular mechanisms of folate transport in rat pancreas; interference with ethanol ingestion. PLoS ONE. 2011;6(12):e28599. doi: 10.1371/journal.pone.0028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas A, Senthilkumar SR, Said HM. Effect of chronic alcohol exposure on folate uptake by liver mitochondria. Am. J. Physiol. Cell Physiol. 2012;302(1):C203–C209. doi: 10.1152/ajpcell.00283.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mcguffin R, Goff P, Hillman RS. The effect of diet and alcohol on the development of folate deficiency in the rat. Br. J. Haematol. 1975;31(2):185–192. doi: 10.1111/j.1365-2141.1975.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 41.Hutson JR, Stade B, Lehotay DC, Collier CP, Kapur BM. Folic acid transport to the human fetus is decreased in pregnancies with chronic alcohol exposure. PLoS ONE. 2012;7(5):e38057. doi: 10.1371/journal.pone.0038057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kharbanda KK. Alcoholic liver disease and methionine metabolism. Semin. Liver Dis. 2009;29(02):155–165. doi: 10.1055/s-0029-1214371. [DOI] [PubMed] [Google Scholar]
- 43.Lu SC, Huang Z-Z, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279(1):G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Kang MH, Min H. Folic acid supplementation reduces oxidative stress and hepatic toxicity in rats treated chronically with ethanol. Nutr. Res. Pract. 2011;5(6):520–526. doi: 10.4162/nrp.2011.5.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojeda ML, Barrero MJ, Nogales F, Murillo ML, Carreras O. Oxidative effects of chronic ethanol consumption on the functions of heart and kidney: folic acid supplementation. Alcohol Alcohol. 2012;47(4):404–412. doi: 10.1093/alcalc/ags056. [DOI] [PubMed] [Google Scholar]
- 46.Barrero MJ, Ojeda ML, Díaz Castro J, Nogales F, Murillo ML, Carreras O. The effects of ethanol upon hydric balance and arterial pressure in rats: folic acid as a possible hypotensor. Life Sci. 2012;90(9-10):337–342. doi: 10.1016/j.lfs.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Chirapapaisan N, Uiprasertkul M, Chuncharunee A. The effect of coenzyme Q10 and curcumin on chronic methanol intoxication induced retinopathy in rats. J. Med. Assoc. Thai. 2012;95(Suppl. 4):S76–S81. [PubMed] [Google Scholar]
- 48.Gundogan F, Elwood G, Mark P, et al. Ethanol-induced oxidative stress and mitochondrial dysfunction in rat placenta. Relevance to pregnancy loss. Alcohol Clin. Exp. Res. 2010;34(3):415–423. doi: 10.1111/j.1530-0277.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu YQ, Liu Y, Morita T, Sugiyama K. Methionine and serine synergistically suppress hyperhomocysteinemia induced by choline deficiency, but not by guanidinoacetic acid, in rats fed a low casein diet. Biosci. Biotechnol. Biochem. 2011;75(12):2333–2339. doi: 10.1271/bbb.110507. [DOI] [PubMed] [Google Scholar]
- 50.Chen YL, Yang SS, Peng HC, Hsieh YC, Chen JR, Yang SC. Folate and vitamin B12 improved alcohol-induced hyperhomocysteinemia in rats. Nutrition. 2011;27(10):1034–1039. doi: 10.1016/j.nut.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010;39(Suppl. 1):I110–I121. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safi J, Joyeux L, Chalouhi GE. Periconceptional folate deficiency and implications in neural tube defects. J. Pregnancy. 2012;2012:295083. doi: 10.1155/2012/295083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanaguita M, Gutierrez C, Ribeiro C, Lima G, Machado H, Peres L. Pregnancy outcome in ethanol-treated mice with folic acid supplementation in saccharose. Childs Nerv. Syst. 2008;24(1):99–104. doi: 10.1007/s00381-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 54.Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22(8):1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22(3):619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res. A Clin. Mol. Teratol. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovacheva VP, Mellott TJ, Davison JM, et al. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by upregulation of Dnmt1 expression. J. Biol. Chem. 2007;282(43):31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- 60.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J. Biol. Chem. 2009;284(4):1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soliman M, Rosenberger T. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011;352(1):173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- 62.Kenyon SH, Nicolaou A, Gibbons WA. The effect of ethanol and its metabolites upon methionine synthase activity in vitro. Alcohol. 1998;15(4):305–309. doi: 10.1016/s0741-8329(97)00134-1. [DOI] [PubMed] [Google Scholar]
- 63.Garro AJ, Mcbeth DL, Lima V, Lieber CS. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin. Exp. Res. 1991;15(3):395–398. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 64.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 65.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 1994;8(1):59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 66.Kaminen-Ahola N, Ahola A, Maga M, et al. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6(1):e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes-implications for fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 2009;33(9):1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 68.Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol. Reprod. 2009;81(4):607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- 69.Marutha Ravindran CR, Ticku MK. Changes in methylation pattern of NMDA receptor NR2B gene in cortical neurons after chronic ethanol treatment in mice. Brain Res. Mol. Brain Res. 2004;121(1-2):19–27. doi: 10.1016/j.molbrainres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 70.Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol. Psychiatry. 2012;72(5):378–388. doi: 10.1016/j.biopsych.2012.04.006. ■ Reported fetal alcohol-induced POMC promoter methylation that persisted across generations in the male germline.
- 71.Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin. Exp. Res. 2012;36(10):1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou F. DNA Methylation program during development. Front. Biol. 2012;7(6):485–494. doi: 10.1007/s11515-012-9246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JS, Shukla SD. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 2006;41(2):126–132. doi: 10.1093/alcalc/agh248. [DOI] [PubMed] [Google Scholar]
- 74.Shukla SD, Lee YJ, Park PH, Aroor AR. Acetaldehyde alters MAP kinase signalling and epigenetic histone modifications in hepatocytes. In: Chadwick DJ, Goode J, editors. Acetaldehyde-Related Pathology: Bridging the Trans-Disciplinary Divide. John Wiley & Sons, Ltd; NJ, USA: 2007. pp. 217–228. [DOI] [PubMed] [Google Scholar]
- 75.Pal-Bhadra M, Bhadra U, Jackson DE, Mamatha L, Park P-H, Shukla SD. Distinct methylation patterns in histone H3 at Lys-4 and Lys-9 correlate with up- & down-regulation of genes by ethanol in hepatocytes. Life Sci. 2007;81(12):979–987. doi: 10.1016/j.lfs.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Addario C, Caputi F, Ekström T, et al. Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex. J. Mol. Neurosci. 2012;49(2):312–319. doi: 10.1007/s12031-012-9829-y. [DOI] [PubMed] [Google Scholar]
- 77.Guo W, Crossey EL, Zhang L, et al. Alcohol exposure decreases CREB binding protein expression and histone acetylation in the developing cerebellum. PLoS ONE. 2011;6(5):e19351. doi: 10.1371/journal.pone.0019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D’addario C, Johansson S, Candeletti S, et al. Ethanol and acetaldehyde exposure induces specific epigenetic modifications in the prodynorphin gene promoter in a human neuroblastoma cell line. FASEB J. 2011;25(3):1069–1075. doi: 10.1096/fj.10-168534. [DOI] [PubMed] [Google Scholar]
- 79.Xiangyuan Wang, Gomutputra P, Wolgemuth DJ, Baxi LV. Acute alcohol exposure induces apoptosis and increases histone H3K9/18 acetylation in the mid-gestation mouse lung. Reprod. Sci. 2010;17(4):384–390. doi: 10.1177/1933719109356984. [DOI] [PubMed] [Google Scholar]
- 80.Jiang X, Yan J, West AA, et al. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 2012;26(8):3563–3574. doi: 10.1096/fj.12-207894. [DOI] [PubMed] [Google Scholar]
- 81.Wang LL, Zhang Z, Li Q, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009;24(3):562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- 82.Miranda R. MicroRNAs and fetal brain development: implications for ethanol teratology during the second trimester period of neurogenesis. Front. Genet. 2012;3:77. doi: 10.3389/fgene.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soares AR, Pereira PM, Ferreira V, et al. Ethanol exposure induces upregulation of specific microRNAs in zebrafish embryos. Toxicol. Sci. 2012;127(1):18–28. doi: 10.1093/toxsci/kfs068. [DOI] [PubMed] [Google Scholar]
- 84.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J. Neurosci. 2011;31(9):3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl Acad. Sci. USA. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baer C, Claus R, Frenzel LP, et al. Extensive promoter DNA hypermethylation and hypomethylation is associated with aberrant microRNA Expression in chronic lymphocytic leukemia. Cancer Res. 2012;72(15):3775–3785. doi: 10.1158/0008-5472.CAN-12-0803. [DOI] [PubMed] [Google Scholar]
- 87.Wilhelm-Benartzi CS, Houseman EA, Maccani MA, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ. Health Perspect. 2012;120(2):296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haycock PC, Ramsay M. Exposure of mouse embryos to ethanol during preimplantation development: effect on DNA methylation in the H19 imprinting control region. Biol. Reprod. 2009;81(4):618–627. doi: 10.1095/biolreprod.108.074682. ■ Proposed a novel mechanism for growth deficits in a fetal alcohol spectrum disorder model that arises from hypomethylation of paternal alleles on the imprinting control region H19/Igf2.
- 89.Downing C, Johnson TE, Larson C, et al. Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure. effects of a methyl-supplemented diet. Alcohol. 2011;45(1):65–71. doi: 10.1016/j.alcohol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubert G, Miñana R, Pascual M, Guerri C. Ethanol exposure during embryogenesis decreases the radial glial progenitor pool and affects the generation of neurons and astrocytes. J. Neurosci. Res. 2006;84(3):483–496. doi: 10.1002/jnr.20963. [DOI] [PubMed] [Google Scholar]
- 91.Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin. Exp. Res. 1993;17(2):304–314. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 92.Roitbak T, Thomas K, Martin A, Allan A, Cunningham LA. Moderate fetal alcohol exposure impairs neurogenic capacity of murine neural stem cells isolated from the adult subventricular zone. Exp. Neurol. 2011;229(2):522–525. doi: 10.1016/j.expneurol.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De La Monte SM, Ganju N, Banerjee K, Brown NV, Luong T, Wands JR. Partial rescue of ethanol-induced neuronal apoptosis by growth factor activation of phosphoinositol-3-kinase. Alcohol Clin. Exp. Res. 2000;24(5):716–726. [PubMed] [Google Scholar]
- 94.Goh JM, Bensley JG, Kenna K, et al. Alcohol exposure during late gestation adversely affects myocardial development with implications for postnatal cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2011;300(2):H645–H651. doi: 10.1152/ajpheart.00689.2010. [DOI] [PubMed] [Google Scholar]
- 95.Parnell SE, Dehart DB, Wills TA, et al. Maternal oral intake mouse model for fetal alcohol spectrum disorders: ocular defects as a measure of effect. Alcohol Clin. Exp. Res. 2006;30(10):1791–1798. doi: 10.1111/j.1530-0277.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 96.Hofer R, Burd L. Review of published studies of kidney, liver, and gastrointestinal birth defects in fetal alcohol spectrum disorders. Birth Defects Res. A Clin. Mol. Teratol. 2009;85(3):179–183. doi: 10.1002/bdra.20562. [DOI] [PubMed] [Google Scholar]
- 97.Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol. Teratol. 2003;25(6):697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 98.Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: a study of two adult samples. Neuropsychologia. 2006;44(5):744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin. Exp. Res. 2002;26(6):875–882. [PubMed] [Google Scholar]
- 100.Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin. Exp. Res. 1999;23(11):1808–1815. [PubMed] [Google Scholar]
- 101.Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry. 1998;155(4):552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- 102.O’Connor M, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. Am. J. Drug Alcohol Abuse. 2002;28(4):743. doi: 10.1081/ada-120015880. [DOI] [PubMed] [Google Scholar]
- 103.Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption – association with epigenome stability and cancer development. FEBS J. 2009;276(8):2175–2191. doi: 10.1111/j.1742-4658.2009.06959.x. [DOI] [PubMed] [Google Scholar]
- 104.Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin. Exp. Res. 2008;32(9):1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 105.Walker AK, Nakamura T, Byrne RJ, et al. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses. Implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34(10):1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 106.Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL. Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics. 2012;4(3):303–315. doi: 10.2217/epi.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sabunciyan S, Aryee MJ, Irizarry RA, et al. Genome-wide DNA methylation scan in major depressive disorder. PLoS ONE. 2012;7(4):e34451. doi: 10.1371/journal.pone.0034451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer. 2007;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 109.Walsh C, Macmillan HL, Jamieson E. The relationship between parental substance abuse and child maltreatment: findings from the Ontario Health Supplement. Child Abuse Negl. 2003;27(12):1409–1425. doi: 10.1016/j.chiabu.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 110.Branchi I, Karpova NN, D’Andrea I, Castrén E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci. Lett. 2011;495(3):168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 111.Anway MD, Rekow SS, Skinner MK. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod. Toxicol. 2008;26(2):100–106. doi: 10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crépin M, Dieu MC, Lejeune S, et al. Evidence of constitutional MLH1 epimutation associated to transgenerational inheritance of cancer susceptibility. Hum Mutat. 2012;33(1):180–188. doi: 10.1002/humu.21617. [DOI] [PubMed] [Google Scholar]
- 113.Bielawski DM, Zaher FM, Svinarich DM, Abel EL. Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcohol Clin. Exp. Res. 2002;26(3):347–351. [PubMed] [Google Scholar]
- 114.Knezovich JG, Ramsay M. The effect of preconception paternal alcohol exposure on epigenetic remodelling of the H19 and Rasgrf1 imprinting control regions in mouse offspring. Front. Genet. 2012;3:10. doi: 10.3389/fgene.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stouder C, Somm E, Paoloni-Giacobino A. Prenatal exposure to ethanol: a specific effect on the H19 gene in sperm. Reprod. Toxicol. 2011;31(4):507–512. doi: 10.1016/j.reprotox.2011.02.009. ■ Reported loci-specific hypomethylation across generations in a model of fetal alcohol spectrum disorder.
- 116.Cisneros FJ, Branch S. Transplacental exposure to the DNA demethylating agent, 5-AZA-CdR, affects the sexual behavior of CD-1 male mice. Neuroyoxicology. 2004;25(3):411–417. doi: 10.1016/j.neuro.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Zimmermann U, Spring K, Wittchen HU, et al. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. J. Psychiatr. Res. 2004;38(4):385–393. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 118.Ueno M, Katayama KI, Nakayama H, Doi K. Mechanisms of 5-azacytidine (5AzC)-induced toxicity in the rat foetal brain. Int. J. Exp. Pathol. 2002;83(3):139–150. doi: 10.1046/j.1365-2613.2002.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Li XM, Caudill M, et al. Betaine feeding prevents the blood alcohol cycle in rats fed alcohol continuously for 1 month using the rat intragastric tube feeding model. Exp. Mol. Pathol. 2011;91(2):540–547. doi: 10.1016/j.yexmp.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.A Report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients, Food and Nutrition Board, Institute of Medicine . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Press; Washington, DC, USA: 1998. [PubMed] [Google Scholar]
- 121.Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299(11):1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- 122.Halsted CH, Villanueva J, Chandler CJ, et al. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23(3):497–505. doi: 10.1002/hep.510230314. [DOI] [PubMed] [Google Scholar]
- 123.Bönsch D, Lenz B, Fiszer R, Frieling H, Kornhuber J, Bleich S. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J. Neural Transm. 2006;113(9):1299–1304. doi: 10.1007/s00702-005-0413-2. [DOI] [PubMed] [Google Scholar]
- 124.Ehrlich D, Pirchl M, Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.