Abstract
Introduction
Previous work has shown that daily skin cleansing with chlorhexidine gluconate (CHG) is effective in preventing infection in the medical intensive care unit (MICU).
Methods
A colorimetric, semi-quantitative indicator was used to measure CHG concentration on skin (neck, antecubital fossae, inguinal areas) of patients bathed daily with CHG during MICU stay and after discharge from MICU, when CHG bathing stopped. Skin sites were also cultured quantitatively. The relationship between CHG concentration and microbial density on skin was explored in a mixed effects model using gram-positive colony forming unit (CFU) counts.
Results
For 20 MICU patients studied (240 measurements), lowest CHG concentrations (0–18.75μg/mL) and highest gram-positive CFUs were on the neck (median 1.07log10CFU, p=0.014). CHG concentration increased post-bath and decreased over 24h(p<0.001). In parallel, median log10CFUs decreased pre- to post-bath (0.78 to 0) and then increased over 24h to baseline of 0.78 (p=0.001). A CHG concentration >18.75μg/mL was associated with decreased gram-positive CFU(p=0.004). In all but 2 instances, CHG was detected on patient skin during entire inter-bath (~24h) period (18/20(90%) patients). In 11 patients studied after MICU discharge (80 measurements), CHG skin concentrations fell below effective levels after 1–3 days.
Conclusion
In MICU patients bathed daily with CHG, CHG concentration was inversely associated with microbial density on skin; residual antimicrobial activity on skin persisted up to 24h. Determination of CHG concentration on patients’ skin may be useful in monitoring adequacy of skin cleansing by healthcare workers.
INTRODUCTION
Healthcare-associated infections cause significant morbidity and mortality in intensive care unit (ICU) patients. Numerous infection control measures have been implemented in an effort to reduce nosocomial infections1, 2. The skin of hospitalized patients can become colonized with microbes that serve as a source of infection, blood culture contamination, and healthcare worker hand contamination. The density of potential pathogens on skin varies according to body site 3. ICU patients are especially vulnerable to skin colonization and infection with multidrug-resistant organisms due to comorbidities, antibiotic exposures, and breaks in skin as a result of devices 4. Infection control practices such as disinfection of patients’ skin before central venous catheter (CVC) insertion and healthcare worker hand hygiene can reduce the number of healthcare-associated infections that originate from skin microbes5.
Daily skin cleansing with the antiseptic agent chlorhexidine gluconate (CHG) is an adjunctive infection control strategy that reduces the density of potential microbial pathogens on patients’ skin and decreases infection6–9. Cleansing the skin of medical intensive care unit (MICU) patients daily with no-rinse, 2% chlorhexidine gluconate (CHG)-impregnated cloths (Sage Inc., Cary, IL) decreases skin colonization with vancomycin-resistant enterococci 6, reduces vancomycin-resistant enterococcal cross-transmission and bacteremia8, and lowers rates of blood culture contamination and CVC-associated bloodstream infections 7, 10.
To gain a better understanding of the effective durability of CHG bathing and of the mechanism by which CHG prevents CVC-associated bloodstream infections, we studied several aspects of CHG skin cleansing in an MICU population using a semi-quantitative, colorimetric CHG indicator test, quantitative skin cultures, and direct observations of CHG bathing.
METHODS
Routine daily bathing with no-rinse 2% CHG-impregnated cloths (Sage) was introduced in November 2005 in the MICU at Rush University Medical Center (RUMC). MICU healthcare personnel received education on proper CHG bathing (according to the manufacturer) prior to implementation; details of this procedure have been published elsewhere6, 7. Nurses and patient care technicians were responsible for daily patient bathing. Products (e.g., certain soaps and lotions) with the potential to interact with CHG and reduce its activity were eliminated from the MICU10.
From February 2007 through May 2007, we examined the usefulness of a semi-quantitative, colorimetric indicator test as a surrogate measure of effective skin cleansing.
Primary Objective
Our primary objective was to determine the relationship between residual CHG concentration on patients’ skin, as determined by the semi-quantitative, colorimetric CHG assay, and colony counts of potentially pathogenic microbes. At predetermined intervals, we collected concurrent and anatomically-proximate samples from the skin of MICU patients who were bathed daily with CHG; one sample was processed to determine CHG concentration and the other to determine microbial colonization. Patients who were in the MICU ≥72 hours and who also had received at least one CHG bath were selected randomly for enrollment. Patients were excluded if (1) the patient’s nurse felt the patient would be discharged from the unit that day, (2) the patient was well enough to be self-bathing, or (3) the patient was undergoing cardiopulmonary resuscitation at the time of planned enrollment.
Swab samples of intact skin surfaces were obtained from patients’ necks (from jaw line to clavicle), antecubital fossae, and inguinal areas 1 hour before and 1, 4, and 23 hours after a CHG bath. Since all patients had been in the ICU for over 72 hours and had been receiving daily CHG skin cleansing before study participation, the 1 hour “pre-bath” and 23 hours post-bath specimens were similar in temporal relation to CHG cleansing. Specimens for CHG concentration determination (6.25 cm2) and culture (10cm2) were collected from adjacent areas of skin. Demographic and clinical information such as mechanical ventilation, presence and location of a CVC, and APACHE II score were recorded.
For MICU and non-ICU ward patients, residual CHG concentration on skin was determined using a sterile dry swab (Arrowhead Forensics, Lenexa, KS) rubbed over the skin surface. Swabs were placed into a test tube (one swab per tube) containing 150 μl of a freshly-prepared solution of 5 parts cetyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO) and 2 parts sodium hypobromite (Sigma) with color change observed immediately. An observer blinded to time of specimen collection determined the CHG concentration by comparison to a defined color scale (no color change and 4 shades from light to dark red). Quality control testing (known CHG concentrations were added to swabs, processed with the prepared reagent and compared to the color scale) was performed daily before analysis of clinical samples11.
For culture, swabs were inoculated onto Columbia CNA agar (Remel, Lenexa, KS) to isolate gram-positive bacteria, MacConkey agar (Remel) to isolate gram-negative bacteria, chromogenic agar (BBL™ CHROMagar™, BD, Franklin Lakes, NJ) specific for Candida spp and Staphylococcus aureus, and bile esculin azide agar (BD) to isolate vancomycin-resistant enterococci. Plates were incubated at 35°C in ambient air for 24–48 hours.
Chi-Square analysis was used for comparisons between categorical variables. A random intercept model to explain the relationship of CHG concentration and density of gram-positive bacteria on skin was developed and a minimum concentration of CHG needed to significantly inhibit microbial growth on skin was determined. Gram-positive bacteria were used in the model as they were isolated most frequently; all patients had at least one measurement with gram-positive bacteria. Contiguous CHG concentrations were combined based on similar levels of effect on CFU density in the model and level of statistical significance. In this model, the repeated measure was the specific measurement episode for each patient, and within each measurement episode, the body site of culture. Colony counts, CHG concentration, time from bath, and body site (i.e., neck, antecubital fossae, and inguinal areas) were variables entered into the model. Appropriate use of the mixed effects model was tested by examining the statistical significance of the variance of the random intercepts, and random effects were retained when significant. Statistical testing was done with SAS v9.2 (Cary, NC), using the Proc Mixed procedure.
Second Objective
Our second objective was to determine whether variability in thoroughness of CHG skin cleansing affected CHG concentrations on skin or density of skin microbes. Research personnel directly observed CHG bathing of enrolled patients and graded baths using a bathing assessment form that included grading of cleansing for the neck, antecubital, and inguinal areas. Parameters assessed included thoroughness of bathing—whether bathers fully wiped over bio-occlusive dressings (as recommended) or, if the patient had a tracheostomy, whether they thoroughly cleaned the area around it; whether bathers dried the patient after the CHG bath (undesirable); whether visible dirt was still present following a bath; and whether patients were bathed up to the jaw line. Results of observations were qualitatively compared with CHG concentration and colony counts at specific body sites.
Third Objective
Our third objective was to evaluate the duration of CHG effect. We studied a cohort of patients discharged from the MICU to a non-ICU ward; CHG cleansing was not performed outside the ICU. Patients on the non-ICU wards bathed daily with soap and water according to standard practice for that general medicine ward. Indicator and culture swabs of the neck and inguinal area were collected from enrolled patients on the final day of the MICU stay and on the first day on the ward. Patients were followed daily after transfer to a non-MICU ward until no or minimal CHG was detected by the colorimetric assay or they were discharged from the hospital.
Fourth Objective
Our fourth objective was to determine on a microbial population level whether decreased susceptibility to CHG was detectable after long-term universal, daily CHG skin cleansing of patients in the MICU. We determined the minimum inhibitory concentration (MIC) of CHG against bacteria and yeast isolated from the skin of randomly selected patients who were cared for in the MICU between February and May 2007, 15–18 months after CHG bathing was introduced. Susceptibility testing was done using a microdilution method12. We also compared the minimum effective concentration of CHG on skin to the median and maximum MICs of CHG for all microbes.
This project was reviewed and approved by the RUMC Institutional Review Board.
RESULTS
CHG concentration and colony counts on MICU patients’ skin
Twenty MICU patients (240 measurements) were enrolled in the study; 12 were men. The mean age of enrolled patients was 61 years (SD±16.3 years); mean body mass index was 31 (SD±8.8) and mean APACHE II score was 23.1 (SD±7.4). Ten (50%) patients had an endotracheal tube or tracheostomy in place; 13 (65%) patients had a CVC in the internal jugular or subclavian vein. The average number of days from hospital or MICU admission until day of enrollment was 12 and 6, respectively.
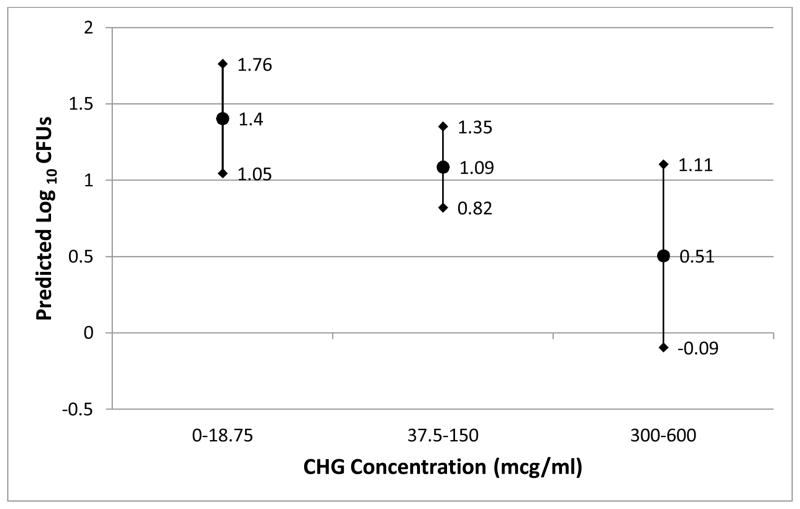
After adjusting for patient and patient body site in the random intercept model, CHG concentration correlated inversely with density of gram-positive bacteria on patient skin (Figure 1). In unadjusted analysis, this relationship was most striking for gram-positive bacteria (Figure 2a).
Figure 1.
The correlation between CHG concentration and predicted gram positive colony counts (± 95% confidence limits) adjusted for patient and patient body site
ABBREVIATIONS:
CFU, colony forming units; CHG, chlorhexidine digluconate
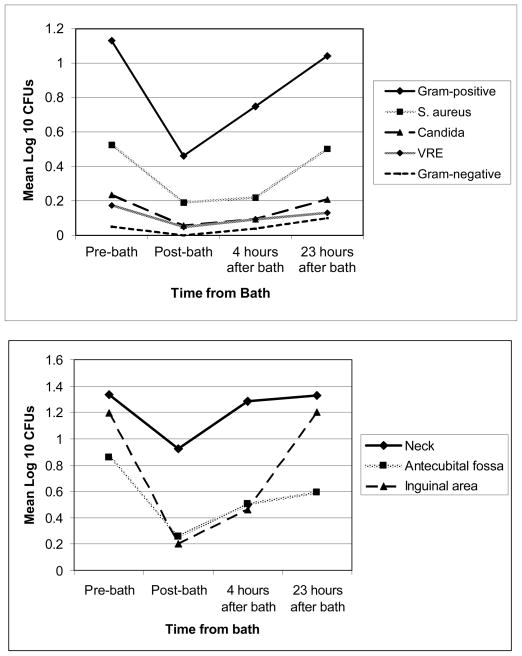
Figure 2.
The relationship of mean log10 colony counts and time from CHG bath. All patients tested had received at least one CHG bath approximately 24 hours before the pre-bath time.
Figure 2a (top): Relationship displayed by category of microbe isolated. Gram-positive organisms included coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus spp., Aerococcus sp., Micrococcus sp., and Enterococcus spp. Gram-negative organisms included Proteus mirabilis, Pseudomonas aeruginosa, and Acinetobacter baumannii.
Figure 2b (bottom): Relationship for gram-positive organisms displayed by body site.
ABBREVIATION: CFUs, colony counts. VRE, vancomycin-resistant enterococcus. CHG, chlorhexidine digluconate.
We found variability in CHG concentration and gram-positive microbial density by body site. The lowest CHG concentration and highest CFU counts were observed on patient necks (median 1.07 log10CFU, p=0.014). Compared to the neck, antecubital CFU counts were 0.61 log10lower (p=0.004) and inguinal CFU counts were 0.37 log10lower (p=0.07).
CHG concentration on skin increased immediately following a CHG bath, with the concentration decreasing to pre-bath levels, but usually still remaining detectable over the 24 hours post-bath (p<0.001). The inguinal area more often had a higher CHG concentration than the neck at all 4 time points measured (Table 1). In parallel, there was a decrease in median log10gram-positive CFUs post-bath from 0.78 to 0 with a subsequent increase over the next 24 hours to pre-bath levels (p=0.001), and with the neck having higher mean CFU counts than other sites (Figure 2b).
Table 1.
The relationship between time of bath and CHG concentration by body site among patients in medical intensive care unit. A CHG concentration of > 18.75 μg/ml was found to be significantly associated with inhibition of gram positive bacterial growth in a mixed effects model.
| Body Site | Pre-Bath (n=19) | Post-Bath (n=20) | 4 hours after (n=20) | 23 hours after (n=20) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent of Patients with Specified CHG Concentration (μg/mL) by Time Pre/Post CHG Cleansing | ||||||||||||||||
| 0 | 4.69–18.75 | 37.5–150 | 300–600 | 0 | 4.69–18.75 | 37.5–150 | 300–600 | 0 | 4.69–18.75 | 37.5–150 | 300–600 | 0 | 4.69–18.75 | 37.5–150 | 300–600 | |
| Neck | 5.3% | 52.6% | 42.1% | 0 | 0 | 25% | 65% | 10% | 0 | 40% | 60% | 0 | 0 | 60% | 40% | 0 |
| Antecubital fossae | 10.5% | 36.8% | 47.4% | 5.3% | 0 | 15% | 85% | 0 | 0 | 15% | 80% | 5% | 5% | 15% | 80% | 0 |
| Inguinal area | 0 | 26.3% | 68.4% | 5.3% | 0 | 5% | 75% | 20% | 0 | 5% | 90% | 5% | 0 | 35% | 65% | 0 |
ABBREVIATION: CHG, chlorhexidine digluconate
A CHG concentration of >0.001875% (18.75 μg/ml) was associated with significantly less gram-positive CFU counts (p=0.004). For 18 (90%) of 20 patients, CHG was detected throughout the entire ~24 hour inter-bath period. A dose response was observed between CHG concentration and CFU decline: CHG concentrations of 0.03% to 0.06% were associated with a 0.90 log10 reduction in CFU (p=0.004) and CHG concentrations between 0.00375% and 0.015% were associated with a 0.32 log10 reduction in CFU (p=0.02) as compared with concentrations at or below 18.75 μg/ml (Figure 1).
Thoroughness of CHG bathing
Bathing observations revealed that inguinal area cleansing received higher bath grades, indicating more thorough bathing, than other body sites; less thorough bathing were observed in the neck area. Eighteen (90%) of 20 patient baths had high bath grades for the inguinal region versus 14 (70%) of 20 for the antecubital region and 2 (11%) of 19 for the neck region. In 7 (37%) of 19 patient baths, healthcare workers failed to cleanse skin with CHG up to the jaw line. In 7 (35%) of 20 patient baths, healthcare workers failed to clean with CHG over bio-occlusive dressings.
CHG concentration and colony counts on patient skin following MICU discharge
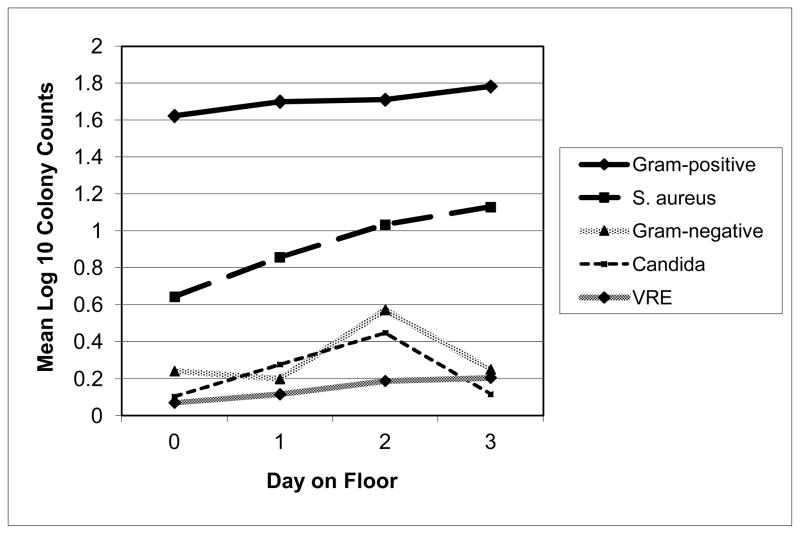
Eleven patients (80 measurements) were followed from the MICU to a non-ICU ward; all patients were tested on day 1, 10 patients were tested through day 2, and 8 patients were tested through day 3. The number of patients with skin CHG concentrations above the level that was significantly associated with decreased gram-positive colony counts (18.75μg/ml) declined over time, with no patients having a CHG concentration above this level for the neck area by Day 2 and for the inguinal area by Day 4. At all time points, the proportion of patients with CHG concentrations in the inguinal area that were above 18.75μg/ml was higher than the proportion of patients with this concentration of CHG in the neck area. Colony counts on skin for all identified microbes increased after patients left the MICU (Figure 3). Colony counts of gram-positive bacteria and of S. aureus on patients’ skin were higher on the day of MICU discharge than earlier in the MICU stay.
Figure 3.
The relationship of mean log10 colony counts and time from CHG bath by category of microbe isolated. Gram-positive organisms included coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus spp., Aerococcus sp., Micrococcus sp., and Enterococcus spp.
ABBREVIATIONS: VRE-vancomycin resistant enterococcus. CHG, chlorhexidine digluconate
NOTE: Day on general ward of “0” signifies day of transfer from the medical intensive care unit to the general ward.
Susceptibility of Skin Isolates from MICU Patients to CHG
The median and maximum MICs of CHG for microbes isolated from the skin of MICU patients 15 to 18 months after introduction of universal, daily CHG bathing to the MICU were low (Table 2). All isolates had MICs below the minimum effective inhibitory concentration of CHG on skin (18.75μg/mL).
Table 2.
Minimum inhibitory concentrations of CHG against microbes isolated from MICU patients’ skin after routine daily skin cleansing of patients with 2% CHG-impregnated cloths was introduced to the unit.
| Microorganism (N= 70) | No. of Isolates | CHG MIC (μg/ml) Median (range) |
|---|---|---|
| Gram-positive | ||
| Coagulase-negative staphylococcia | 14 | 1 (0.5 – 4) |
| Staphylococcus aureus | 2 | 3 (2 – 4) |
| Streptococcus spp.b | 9 | 1 (0.25 – 2) |
| Aerococcus sp. | 1 | 1 (1) |
| Micrococcus sp. | 1 | 0.5 (0.5) |
| Enterococcus spp.c | 18 | 2 (1 – 4) |
| Gram-negative | ||
| Proteus mirabilis | 1 | 1 (1) |
| Pseudomonas aeruginosa | 3 | 8 (4 – 8) |
| Acinetobacter baumannii | 1 | 1 (1) |
| Yeastd | 20 | 4 (4–8) |
Abbreviations: No., number; CHG, chlorhexidine digluconate; MICU, medical intensive care unit; MIC, minimum inhibitory concentration.
Coagulase-negative staphylococci include Staphylococcus auricularis, S. epidermidis, S. haemolyticus, S. xylosus
Streptococcus spp. include Streptococcus agalactiae, S. salivarius, S. uberis
Enterococcus spp. include Enterococcus faecalis, E. faecium, E. avium
Yeast include Candida albicans, C. parapsilosis, C. tropicalis
DISCUSSION
Daily bathing with no-rinse, 2% CHG-impregnated cloths is an effective infection control intervention in the MICU10. Routine use has led to a decrease in CVC-associated bloodstream infections 7, 9, 10, likely due to a durable reduction in potential pathogens on patients’ skin 13, on healthcare worker hands, and in the environment6. The current study explains in part why daily bathing with CHG is effective. Using a semi-quantitative colorimetric CHG indicator test, we demonstrated that CHG concentrations correlated inversely with gram-positive colony counts on the skin of MICU patients; a concentration >18.75μg/mL was associated with significantly decreased colony counts. CHG was detected on the skin for up to 24 hours.
Lower concentrations of CHG and higher colony counts were detected in the neck region, the area noted to receive less thorough cleansing when bathing was directly observed. Patients who were intubated or who had tracheostomy tubes in place—half of the patients studied—may have had increased oropharyngeal and tracheal secretions that contaminated the neck area soon after daily bathing 14. The neck region may require more frequent cleansing in these patients to reduce the higher density of colonizing organisms on skin 15, 16 resulting from secretions. The variation in cleansing may also help explain why some CVC-associated BSIs occur despite daily CHG bathing7. Regardless, effective concentrations of CHG were nearly always detected at all body sites and at all time points tested when daily CHG bathing occurred. While strict adherence to proper bathing technique should be the goal, even imperfect application of CHG appears to have a beneficial impact.
Our data suggest, though, that bathing less frequently than daily provides less benefit; after patients left the MICU and once regular CHG bathing was no longer occurring, CHG concentration on skin declined and microbial growth increased. However, the microbial burden even 1 to 3 days after leaving the MICU was significantly lower than historical values from patients who were bathed with soap and water17, suggesting that CHG may have some residual antiseptic activity once CHG bathing has stopped. CHG concentration was maintained at higher levels in the inguinal area than the neck, perhaps reflecting differential thoroughness of bathing and resultant CHG concentrations observed at those body sites when patients were in the MICU. It is unclear why colony counts for gram-positive bacteria and S. aureus on skin were higher on the day of MICU discharge. We hypothesize that this finding may reflect a change in bathing practice on more stable patients awaiting transfer to the floor; e.g., last MICU bath may have been deferred to the ward nursing staff.
CHG MICs for microorganisms isolated from the skin of MICU patients more than one year after the introduction to the unit of universal, daily CHG bathing were comparable to published values for microbes identified in other clinical settings18, 19. The highest CHG MIC observed in the small sample of microbes tested in this study was 8μg/mL which is below the concentration of CHG on skin that was found to significantly inhibit gram positive bacterial growth. In a related report by Edmiston, et al. of healthy volunteers who cleansed their own skin with 2% CHG-impregnated cloths, CHG skin concentrations measured were consistently above the MIC90 for staphylococcal isolates recovered from study subjects’ skin11. Of note, CHG concentrations measured on patients’ skin in our study were lower than those reported by Edmiston, et al.11. Our study design differed from Edmiston’s in that we studied MICU patients whose skin was cleansed by healthcare workers rather than healthy volunteers who bathed themselves. In addition, calibration of the assay was done independently in each laboratory and may have differed.
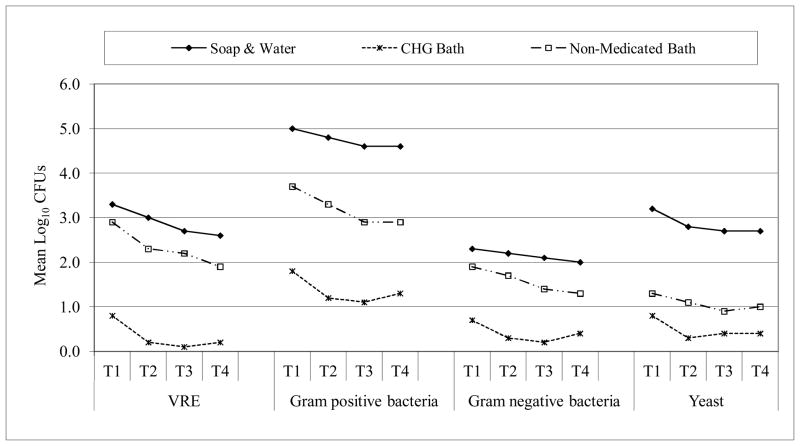
Our study should be viewed in light of the following limitations. The sample of patients tested was small and the period of study relatively short. We did not monitor individual-level outcomes; e.g., we did not assess rates of CVC-associated bloodstream infections among enrolled patients and compare them to CHG concentration or microbial colony counts on patients’ skin. However, in a previous publication, we reported much lower CVC-associated bloodstream infection and blood culture contamination rates after the introduction of CHG in this MICU10. Perhaps most importantly, all patients were studied after their first CHG bath, i.e., neither baseline cultures nor cultures from skin of control patients who were not bathed with CHG were collected. In a prior study17 that used similar swab collection and culture methods, we compared CHG cleansing to soap-and-water bathing and to use of a non-medicated cleansing cloth (Figure 4). CHG cleansing was associated with lower microbial density than the other methods across all microorganism categories.
Figure 4.
Effect of bath procedure on microbial contamination in the inguinal region.
NOTE. T1 = before bath; T2 = 0–2 hours after bath; T3 = 3–5 hours after bath; T4 = 6–8 hours after bath. CHG and non-medicated bath performed with no-rinse cloths that contained CHG (and emollients) or only bland cleansing agents (and emollients), respectively17.
Abbreviation. VRE-vancomycin resistant enterococcus
Understanding deficiencies in implementation of an infection prevention practice are critical to achieving its maximum benefit. The current study suggests that education of nurses and patient care technicians on improved cleansing of the neck region may be needed. The success of infection control practices can be limited by compliance of healthcare personnel 20 and direct observations to assess compliance can be inaccurate due to the Hawthorne effect 21. Simple, minimally intrusive methods such measurement of residual CHG concentration on skin using the semi-quantitative colorimetric CHG assay evaluated in this study may be useful in monitoring quality of, or compliance with, CHG bathing. This approach could help highlight areas of practice that need improvement and provide an opportunity to maximize CHG’s beneficial effect.
Acknowledgments
Funding Source Disclosure Statement:
Centers for Disease Control and Prevention, Atlanta, GA, Cooperative Agreement No. 5 U01 C111000327 (RAW, PI) and U54CK000161 (RAW, PI)
Sage Products, Inc. for MIC determination.
NIAID K23AI085029 (PI: KJP)
We would like to thank Michael Vernon, DrPH for his contributions to the project.
Footnotes
Financial Disclosure: Kyle Popovich, Bala Hota, Rosie Lyles, and Robert Hayes have no conflicts of interest. Mary Hayden and Robert Weinstein received research funding from Sage Products, Inc.
Contributor Information
Kyle J Popovich, Rush University Medical Center/Stroger Hospital Of Cook County.
Rosie Lyles, Stroger Hospital Of Cook County.
Robert Hayes, Rush University Medical Center.
Bala Hota, Rush University Medical Center/Stroger Hospital Of Cook County.
William Trick, Stroger Hospital Of Cook County.
Robert A Weinstein, Rush University Medical Center/Stroger Hospital Of Cook County.
Mary K Hayden, Rush University Medical Center.
References
- 1.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011 May;52(9):e162–193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003 May;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 3.Larson EL, McGinley KJ, Foglia AR, Talbot GH, Leyden JJ. Composition and antimicrobic resistance of skin flora in hospitalized and healthy adults. J Clin Microbiol. 1986 Mar;23(3):604–608. doi: 10.1128/jcm.23.3.604-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med. 1999 Jun;20(2):303–316. viii. doi: 10.1016/s0272-5231(05)70143-x. [DOI] [PubMed] [Google Scholar]
- 5.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002 Dec;23(12):759–769. doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- 6.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006 Feb 13;166(3):306–312. doi: 10.1001/archinte.166.3.306. [DOI] [PubMed] [Google Scholar]
- 7.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007 Oct 22;167(19):2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 8.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009 Jun;37(6):1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 9.Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2011 Mar;145(3):240–246. doi: 10.1001/archsurg.2010.5. [DOI] [PubMed] [Google Scholar]
- 10.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009 Oct;30(10):959–963. doi: 10.1086/605925. [DOI] [PubMed] [Google Scholar]
- 11.Edmiston CE, Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008 Aug;207(2):233–239. doi: 10.1016/j.jamcollsurg.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001 Jun;107(6):1431–1436. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 13.Lowbury EJ, Lilly HA. Use of 4 per cent chlorhexidine detergent solution (Hibiscrub) and other methods of skin disinfection. Br Med J. 1973 Mar 3;1(5852):510–515. doi: 10.1136/bmj.1.5852.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorente L, Henry C, Martin MM, Jimenez A, Mora ML. Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care. 2005;9(6):R631–635. doi: 10.1186/cc3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991 Sep 16;91(3B):197S–205S. doi: 10.1016/0002-9343(91)90369-9. [DOI] [PubMed] [Google Scholar]
- 16.Heard SO, Wagle M, Vijayakumar E, et al. Influence of triple-lumen central venous catheters coated with chlorhexidine and silver sulfadiazine on the incidence of catheter-related bacteremia. Arch Intern Med. 1998 Jan 12;158(1):81–87. doi: 10.1001/archinte.158.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Vernon MO, Blom DW, Hayes RA, Hayden MK, Trick WE, Peterson BJ, Welbel SF, Rice TW, Phillips LJ, Weinstein RA. Efficacy of a chlorhexidine gluconate (CHG) body cleanser for reducing skin contamination with vancomycin-resistant enterococci (VRE) among intensive care unit (ICU) patients. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. September 14–17, 2003; p. abstract no. K-1108.p. 358. [Google Scholar]
- 18.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J Antimicrob Chemother. 2008 Mar;61(3):651–657. doi: 10.1093/jac/dkn006. [DOI] [PubMed] [Google Scholar]
- 19.Koljalg S, Naaber P, Mikelsaar M. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J Hosp Infect. 2002 Jun;51(2):106–113. doi: 10.1053/jhin.2002.1204. [DOI] [PubMed] [Google Scholar]
- 20.Pittet D, Mourouga P, Perneger TV. Compliance with handwashing in a teaching hospital. Infection Control Program. Ann Intern Med. 1999 Jan 19;130(2):126–130. doi: 10.7326/0003-4819-130-2-199901190-00006. [DOI] [PubMed] [Google Scholar]
- 21.Eckmanns T, Bessert J, Behnke M, Gastmeier P, Ruden H. Compliance with antiseptic hand rub use in intensive care units: the Hawthorne effect. Infect Control Hosp Epidemiol. 2006 Sep;27(9):931–934. doi: 10.1086/507294. [DOI] [PubMed] [Google Scholar]