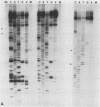
Abstract
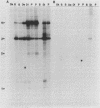
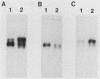
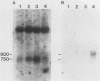
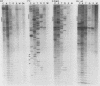
The mitochondrial respiratory system is absent in slender bloodstream forms of Trypanosoma brucei, incomplete in stumpy bloodstream forms, and complete in procyclic (insect) forms. The steady-state abundance of transcripts of some mitochondrially encoded components of the respiratory system correlates with its differential expression in different life cycle stages. Recently, it was reported that uridines which are not encoded in the genome are added to cytochrome b and cytochrome oxidase II transcripts. We now report that the (U)+ transcripts of both genes are found in procyclic forms and to some degree in stumpy forms but are absent in slender forms. The uridine additions to cytochrome oxidase II correct a frameshift in the gene and presumably allow production of a full-length protein, whereas those added to cytochrome b create an in-frame AUG which extends the N terminus of the predicted protein by 20 amino acids. The stage specificity of uridine additions to these transcripts thus reflects the life cycle stage during which the protein products would be used. Transcripts of MURF2, a gene of unknown function, have additional uridines in both slender and procyclic forms which create two in-frame AUGs. MURF2 transcripts additionally differ from the DNA sequence in ways which cannot be explained by uridine addition alone, implying that other processes alter these transcripts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Garofalo J., Mockenhaupt D., McCann P. P., Diekema K. A., Pegg A. E., Nathan H. C., Mullaney E. A., Chunosoff L., Sjoerdsma A. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983 Mar;7(3):209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Janssen J. W., Hoeijmakers J. H., Borst P. The major transcripts of the kinetoplast DNA of Trypanosoma brucei are very small ribosomal RNAs. Nucleic Acids Res. 1983 Jan 11;11(1):105–125. doi: 10.1093/nar/11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Apocytochrome b and other mitochondrial DNA sequences are differentially expressed during the life cycle of Trypanosoma brucei. Nucleic Acids Res. 1985 Jun 25;13(12):4577–4596. doi: 10.1093/nar/13.12.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Developmentally regulated addition of nucleotides within apocytochrome b transcripts in Trypanosoma brucei. Cell. 1987 May 8;49(3):337–345. doi: 10.1016/0092-8674(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Differential mitochondrial gene expression between slender and stumpy bloodforms of Trypanosoma brucei. Mol Biochem Parasitol. 1986 Sep;20(3):207–214. doi: 10.1016/0166-6851(86)90100-3. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Shaw J. M., Simpson L., Stuart K. Creation of AUG initiation codons by addition of uridines within cytochrome b transcripts of kinetoplastids. Proc Natl Acad Sci U S A. 1988 Jan;85(2):539–543. doi: 10.1073/pnas.85.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Stuart K. Differential expression of mitochondrial genes between life cycle stages of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1985 May;82(10):3380–3384. doi: 10.1073/pnas.82.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin B. F., McCann P. P., Bitonti A. J., Bacchi C. J. Polyamine depletion following exposure to DL-alpha-difluoromethylornithine both in vivo and in vitro initiates morphological alterations and mitochondrial activation in a monomorphic strain of Trypanosoma brucei brucei. J Protozool. 1986 May;33(2):238–243. doi: 10.1111/j.1550-7408.1986.tb05599.x. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Opperdoes F. R. The occurrence of glycosomes (microbodies) in the promastigote stage of four major Leishmania species. Mol Biochem Parasitol. 1984 Oct;13(2):159–172. doi: 10.1016/0166-6851(84)90110-5. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Brakenhoff J., De Vries B. F., Sloof P., Tromp M. C., Van Boom J. H., Benne R. The sequence of the gene for cytochrome c oxidase subunit I, a frameshift containing gene for cytochrome c oxidase subunit II and seven unassigned reading frames in Trypanosoma brucei mitochondrial maxi-circle DNA. Nucleic Acids Res. 1984 Oct 11;12(19):7327–7344. doi: 10.1093/nar/12.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer D. P., Feagin J. E., Stuart K. Diverse patterns of expression of the cytochrome c oxidase subunit I gene and unassigned reading frames 4 and 5 during the life cycle of Trypanosoma brucei. Mol Cell Biol. 1985 Nov;5(11):3041–3047. doi: 10.1128/mcb.5.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Payne M., Rothwell V., Jasmer D. P., Feagin J. E., Stuart K. Identification of mitochondrial genes in Trypanosoma brucei and homology to cytochrome c oxidase II in two different reading frames. Mol Biochem Parasitol. 1985 May;15(2):159–170. doi: 10.1016/0166-6851(85)90117-3. [DOI] [PubMed] [Google Scholar]
- Simpson L., Neckelmann N., de la Cruz V. F., Simpson A. M., Feagin J. E., Jasmer D. P., Stuart K. Comparison of the maxicircle (mitochondrial) genomes of Leishmania tarentolae and Trypanosoma brucei at the level of nucleotide sequence. J Biol Chem. 1987 May 5;262(13):6182–6196. [PubMed] [Google Scholar]
- Stuart K., Gobright E., Jenni L., Milhausen M., Thomashow L., Agabian N. The IsTaR 1 serodeme of Trypanosoma brucei: development of a new serodeme. J Parasitol. 1984 Oct;70(5):747–754. [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]
- Stuart K. Mitochondrial DNA of an African trypanosome. J Cell Biochem. 1983;23(1-4):13–26. doi: 10.1002/jcb.240230103. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985 Apr;41(2):105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- de Gee A. L., Carstens P. H., McCann P. P., Mansfield J. M. Morphological changes in Trypanosoma brucei rhodesiense following inhibition of polyamine biosynthesis in vivo. Tissue Cell. 1984;16(5):731–738. doi: 10.1016/0040-8166(84)90006-5. [DOI] [PubMed] [Google Scholar]