Abstract
Biofilms formed by Candida albicans bloodstream isolates on catheters are an important clinical problem. Devising chemotherapeutic strategies to treat these in situ is an attractive option. We report here that liposomal amphotericin effectively kills C. albicans biofilms rapidly (12 h) and effectively (>90%) in a dose-dependent manner, whereas caspofungin displays an inverse concentration-dependent effect. This study has implications for considering the effective doses of antifungal agents used for catheter lock therapy.
TEXT
While there is no question that the use of various medical devices has greatly facilitated the management of serious medical and surgical conditions, the introduction of artificial materials into various anatomical locations has been accompanied by the ability of Candida albicans to colonize and form biofilms on devices such as shunts, stents, endotracheal tubes and various types of catheters (1). In fact, it has been reported that biofilm-forming C. albicans bloodstream isolates were significantly correlated with increased mortality (2).
Bloodstream infections due to C. albicans remain an important cause of morbidity and mortality worldwide. It was reported that at least 1 in 4 hospitalized patients who contract a candidemia die before discharge (3). The intensive care unit (ICU) is of pivotal importance in terms of developing a candidemia, where the use of central venous catheters is extremely common and associated with candidal sepsis (4). It was reported that 45.4% cases of candidemia were associated with the ICU, with an overall 30-day mortality of 26.4%. Removal of the central venous catheter was associated with a significant reduction in mortality (5). There are currently no guidelines for treating C. albicans-associated biomaterial infections with chemotherapeutic agents, other than physical removal of the catheter (6). However, limited anecdotal evidence exists for in situ use of antifungal lock therapy (ALT); nevertheless, the use of amphotericin B deoxycholate to resolve a catheter-related infection has been reported to be successful (7–9).
This study aims to investigate and compare the use of key antifungal agents classes (azole, polyene, and echinocandin) against a range of C. albicans bloodstream isolates growing as biofilms, with the objective of advocating their use in ALT. One hundred C. albicans bloodstream strains obtained from a Scottish candidemia study were selected for testing (10). All isolates were maintained on Sabouraud agar (SAB) at 30°C and propagated in yeast-peptone-dextrose (Sigma, Poole, United Kingdom) medium in an orbital shaker (100 rpm) at 30°C overnight. Cells were harvested, washed in sterile phosphate-buffered saline (PBS; Sigma, Poole, United Kingdom), then resuspended in RPMI 1640 buffered with morpholinepropanesulfonic acid (MOPS; Sigma-Aldrich, Dorset, United Kingdom), counted, and standardized using an improved Neuber hemocytometer.
Initially, antifungal testing to determine planktonic MICs (PMICs) was performed using the CLSI M-27A broth microdilution methodology (11). The following antifungal agents, prepared in double-distilled water (ddH2O), were used in the course of this study against 100 C. albicans isolates: liposomal amphotericin B (AMB) (AmBisome; Gilead Sciences, Cambridge, United Kingdom), caspofungin (CSP) (Cancidas; Merck Sharp & Dohme, Hertfordshire, United Kingdom), and voriconazole (VRZ) (Vfend; Pfizer Pharmaceuticals). AMB, CSP, and VRZ were highly effective against these isolates, exhibiting PMIC50 values of 0.125, 0.0625, and 0.125 mg/liter, respectively (Table 1). The PMIC90 for all three antifungal agents was 0.125 mg/liter for all 100 isolates. These data are in agreement with previous literature (12).
Table 1.
Planktonic and sessile sensitivities of Candida albicans biofilms treated with three antifungal classes
| Parameter | MIC value (mg/liter) witha: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| VRZ |
AMB |
CSP |
|||||||
| PMIC | SMFC 50% | SMFC 80% | PMIC | SMFC 50% | SMFC 80% | PMIC | SMFC 50% | SMFC 80% | |
| MIC range | ≤0.0625–2 | >64 | >64 | ≤0.0625–0.25 | <0.5–32 | 2–64 | 0.0625–0.25 | ≤0.0625–0.5 | ≤0.0625–8 |
| MIC50 | 0.125 | ≥64 | >64 | 0.0625 | 0.5 | 4 | 0.0625 | ≤0.0625 | 0.25 |
| MIC90 | 0.125 | >64 | >64 | 0.125 | 2 | 16 | 0.125 | 0.5 | 1 |
SMFC 50% and SMFC 80%, sessile minimum fungicidal concentration at a 50% and 80% reduction of XTT metabolism, respectively, in comparison to an untreated control.
Next, sessile susceptibility testing was performed as described previously (13). Biofilms were formed using standardized cell suspensions (200 μl of 1 × 106 cells/ml), added to selected microtiter wells, and incubated for 48 h at 37°C. After washing the biofilms, each antifungal agent was then added in serially double-diluted concentrations and incubated for a further 24 h at 37°C. A semiquantitative measure of biofilm killing was assessed using a formazan salt-based XTT (2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-caboxanilide) reduction assay (13). Sessile MICs (SMICs) were determined at a ≥80% XTT reduction. Testing of these isolates was performed in triplicate. All C. albicans isolates formed robust biofilms, as assessed by XTT and crystal violet (data not shown). AMB exhibited good overall activity, with SMIC50/90 of 4 and 16 mg/liter, respectively, ranging from 2 to 64 mg/liter. For CSP, the SMIC50/90 was 0.25 mg/liter and 1 mg/liter, respectively, ranging from ≤0.0625 to 8 mg/liter. VRZ sessile activity was highly ineffective, with no notable activity against any strain tested (SMIC50/90 of ≥64 mg/liter).
We next undertook a comparative time-kill evaluation of C. albicans biofilms (n = 10) (Fig. 1). These isolates exhibiting good biofilm formation were selected based on high biomass and metabolic activity. Biofilms were washed prior to addition of AMB, CSP, and VRZ at concentrations of 1×, 2×, 4×, and 8× the MIC50. Biofilms were incubated in the presence of each antifungal compound for 2, 4, 8, 12, 24, 48, and 72 h, and their metabolic activities were assessed using an XTT reduction assay. Untreated biofilms containing RPMI 1640 served as appropriate comparative controls for each isolate at each time point. Ten replicate biofilms were included for each condition tested, with testing performed on two separate occasions. A two-way analysis of variance (ANOVA) was performed on transformed data using SPSS Software (Chicago, IL) and a Bonferroni posttest to allow different drug concentrations or different time points to be compared. AMB challenge was shown to be rapid and dose-dependent for all concentrations tested, with a >90% kill observed for all concentrations after 12 h (Fig. 1A). Significant differences were observed between each concentration, except between 2× and 4× SMIC50, at each time point up until 12 h (P < 0.0001). No significant differences were observed thereafter. At 8× SMIC50, the activity was significantly more rapid after 4 and 8 h (P < 0.0001), reducing metabolism by 60% and 94%, respectively. CSP challenge showed significant differences between concentrations tested in a time-dependent manner (P < 0.0001), but with an inverse relationship between time-kill characteristics and the concentration tested, i.e., the most effective and rapid concentration of CSP was 1× SMIC50, resulting in a 99% kill after 24 h (Fig. 1B). This was followed by 2×, 4×, and 8× SMIC50, which caused 98, 90, and 85% reductions after 24 h, respectively. Both 1× and 2× SMIC50 were significantly superior compared to 4× and 8× SMIC50 from 12 h onwards (P < 0.0001). VRZ time-kill studies showed dose-dependent characteristics, but with only an 18% kill after 72 h at 8× MIC50 (Fig. 1C). No significant differences were observed between any of the concentrations tested (P > 0.05).
Fig 1.
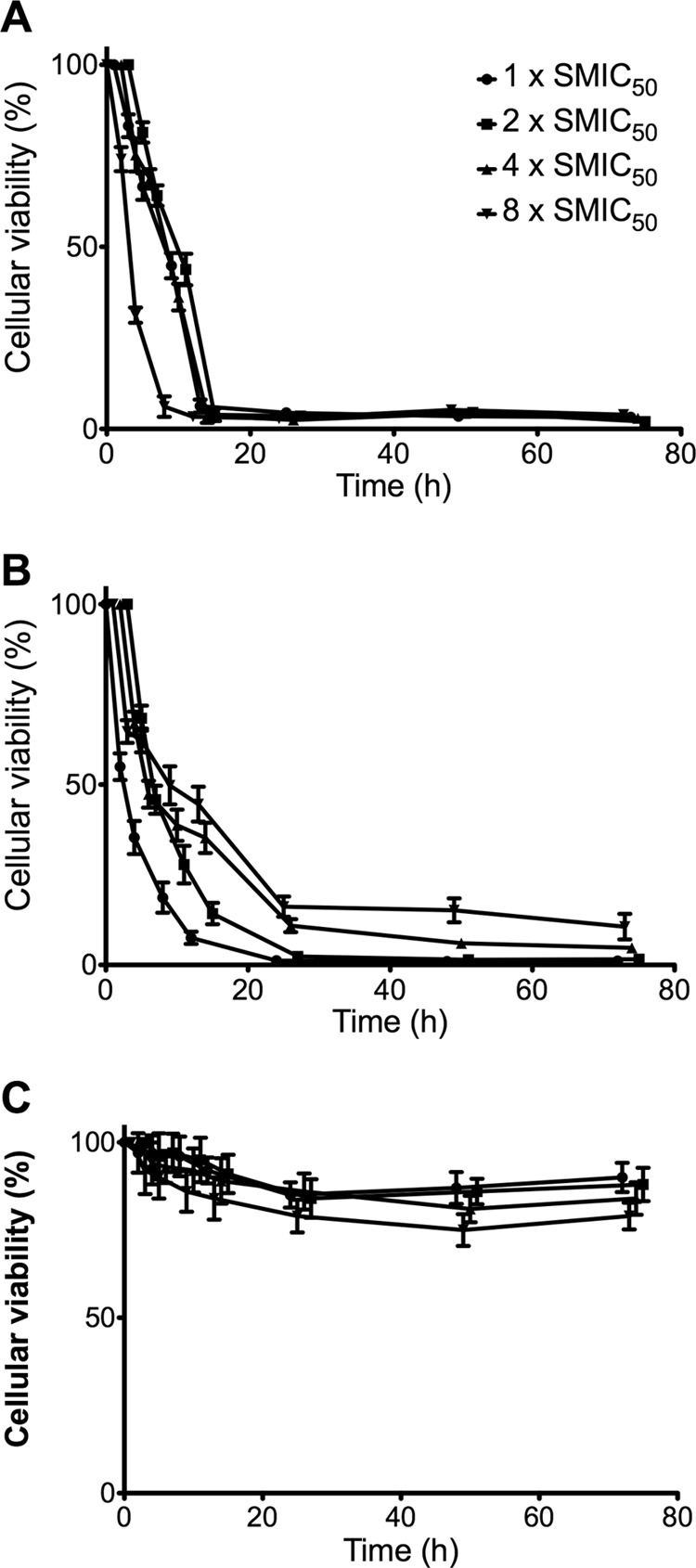
Time-kill kinetics of Candida albicans biofilms challenged with liposomal amphotericin B (A), caspofungin (B), and voriconazole (C) at 1×, 2×, 4×, and 8× SMIC50 and measured by the XTT reduction assay. Data points represent 10 individual clinical isolates in replicate (n = 10). This was analyzed by a two-way ANOVA, with error bars representing the standard deviations.
Collectively, these data demonstrate that both AMB and CSP have the potential for direct in situ treatment of C. albicans biofilms on infected catheters, indwelling biomaterials, or tissue. While AMB did not achieve a 99% kill like that of CSP after 24 h, its kill was rapidly effective (∼95%) after only 12 h of treatment. Moreover, at 1× MIC50, the activity was not significantly different from that of the other concentrations tested at this time, suggesting saturation of the drug within the biofilm. This may be because of the liposomal formulation of AMB that permits greater diffusion through the biofilm. It has been shown that liposomal formulations have the potential for treatment of catheters in vivo (14). In vitro investigations have also reported the potential benefit of this approach, either as a single agent or in combination with EDTA (15–17), though the range of concentrations tested is highly variable. In contrast, CSP showed paradoxical activity against the C. albicans biofilms (18). Recent literature suggests adaptive resistance to echinocandins through cell wall remodelling, specifically increased chitin content (19). It is plausible that higher concentrations of CSP induce these adaptive changes, accounting for this effect.
Recent clinical data have shown that patients with defined biofilm-forming bloodstream C. albicans isolates experienced a short length of hospital stay and low mortality rates when treated with echinocandins and liposomal amphotericin B (20). These observations generally support our findings, yet clinically not all patients respond to antifungal treatment equally. This may be partly because of the level of biofilm formation by individual strains and/or differential biofilm response to different antifungal classes. Interestingly, none of the antifungal agents tested killed the biofilms in their entirety, suggesting adjunctive therapy is required (15). Further studies are required to understand how to maximize antifungal activity against C. albicans biofilms and improve their clinical management.
ACKNOWLEDGMENT
We thank Gilead Sciences for financial support to G.R. through an unrestricted educational research grant.
Footnotes
Published ahead of print 19 February 2013
REFERENCES
- 1. Ramage G, Martinez JP, Lopez-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 6:979–986 [DOI] [PubMed] [Google Scholar]
- 2. Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gagne JJ, Goldfarb NI. 2007. Candidemia in the in-patient setting: treatment options and economics. Expert Opin. Pharmacother. 8:1643–1650 [DOI] [PubMed] [Google Scholar]
- 4. Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, Kauffman CA, Kett D, Larsen RA, Morrison V, Nucci M, Pappas PG, Bradley ME, Major S, Zimmer L, Wallace D, Dismukes WE, Rex JH. 2007. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 26:271–276 [DOI] [PubMed] [Google Scholar]
- 5. Kibbler CC, Seaton S, Barnes RA, Gransden WR, Holliman RE, Johnson EM, Perry JD, Sullivan DJ, Wilson JA. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54:18–24 [DOI] [PubMed] [Google Scholar]
- 6. O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. 2011. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 52:e162–e193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angel-Moreno A, Boronat M, Bolanos M, Carrillo A, Gonzalez S, Perez Arellano JL. 2005. Candida glabrata fungemia cured by antibiotic-lock therapy: case report and short review. J. Infect. 51:e85–e87 doi:10.1016/j.jinf.2004.08.034 [DOI] [PubMed] [Google Scholar]
- 8. Viale P, Petrosillo N, Signorini L, Puoti M, Carosi G. 2001. Should lock therapy always be avoided for central venous catheter-associated fungal bloodstream infections? Clin. Infect. Dis. 33:1947–1948 [DOI] [PubMed] [Google Scholar]
- 9. Wu CY, Lee PI. 2007. Antibiotic-lock therapy and erythromycin for treatment of catheter-related Candida parapsilosis and Staphylococcus aureus infections. J. Antimicrob. Chemother. 60:706–707 [DOI] [PubMed] [Google Scholar]
- 10. Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NA, Jones BL. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56:1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard—third edition. CLSI document M27-A3. CLSI, Wayne, PA [Google Scholar]
- 12. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raad II, Hachem RY, Hanna HA, Fang X, Jiang Y, Dvorak T, Sherertz RJ, Kontoyiannis DP. 2008. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int. J. Antimicrob. Agents 32:515–518 [DOI] [PubMed] [Google Scholar]
- 16. Seidler M, Salvenmoser S, Muller FM. 2010. Liposomal amphotericin B eradicates Candida albicans biofilm in a continuous catheter flow model. FEMS Yeast Res. 10:492–495 [DOI] [PubMed] [Google Scholar]
- 17. Toulet D, Debarre C, Imbert C. 2012. Could liposomal amphotericin B (L-AMB) lock solutions be useful to inhibit Candida spp. biofilms on silicone biomaterials? J. Antimicrob. Chemother. 67:430–432 [DOI] [PubMed] [Google Scholar]
- 18. Melo AS, Colombo AL, Arthington-Skaggs BA. 2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker LA, Gow NAR, Munro CA. 2013. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 57:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, Sanguinetti M, Fadda G, Cauda R, Posteraro B. 2012. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 7:e33705 doi:10.1371/journal.pone.0033705 [DOI] [PMC free article] [PubMed] [Google Scholar]


