Abstract
We demonstrated a significant inverse correlation between vancomycin and beta-lactam susceptibilities in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA (hVISA) isolates. Using time-kill assays, vancomycin plus oxacillin or ceftaroline was synergistic against 3 of 5 VISA and 1 of 5 hVISA isolates or 5 of 5 VISA and 4 of 5 hVISA isolates, respectively. Beta-lactam exposure reduced overall vancomycin-Bodipy (dipyrromethene boron difluoride [4,4-difluoro-4-bora-3a,4a-diaza-s-indacene] fluorescent dye) binding but may have improved vancomycin-cell wall interactions to improve vancomycin activity. Further research is warranted to elucidate the mechanism behind vancomycin and beta-lactam synergy.
TEXT
The high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) has prompted an increase in the utilization of glycopeptides, such as vancomycin, leading to the emergence of vancomycin-intermediate S. aureus (VISA) and heterogeneous VISA (hVISA) (1). Isolates with reduced susceptibility to vancomycin are being reported more frequently (1, 2). The mechanisms responsible for this phenotype involve multiple, stepwise and inducible changes affecting cell wall metabolism and resulting in increased cell wall thickness, reduced virulence properties, decreased susceptibility to cationic antimicrobial peptides, and reduced growth rate (3, 4). Interestingly, an increased susceptibility to traditional antistaphylococcal beta-lactams despite the presence of the mecA gene has been described among MRSA with reduced susceptibility to lipopeptides and glycopeptides (5–7). The mechanism of this phenomenon, referred to as the “seesaw effect,” remains unclear. Previous studies have suggested that vancomycin may be synergistic with beta-lactams against MRSA, but results are inconsistent (8–13). Data are currently limited regarding potential synergy between vancomycin and ceftaroline (12). In this study, we performed susceptibility testing and time-kill studies using beta-lactams, daptomycin, and vancomycin against mecA-positive hVISA and VISA isolates to look for possible synergy between these antibiotics against these clinically difficult pathogens.
Isolates of MRSA, including 61 VISA and 93 hVISA strains, previously characterized for staphylococcal cassette chromosome mec element (SCCmec) type and accessory gene regulator (agr) group and function, were selected from the Anti-Infective Research Laboratory collection (ARL, Detroit, MI). Five VISA and 5 hVISA strains were selected for time-kill synergy studies.
Vancomycin, daptomycin, cefoxitin, and oxacillin powders were obtained from commercial sources (Sigma Chemical Company, St. Louis, MO, and Cubist Pharmaceuticals, Lexington, MA). Ceftaroline was provided by its manufacturer (Forest Laboratories Inc., New York, NY).
In vitro experiments were performed in Mueller-Hinton broth (MHB; Difco, Detroit, MI) and MHB supplemented appropriately with calcium or sodium chloride for daptomycin and oxacillin susceptibility testing. Time-kill experiments were performed in MHB containing 50% sterile, heat-inactivated human serum at an inoculum of ∼106 CFU/ml. Colony counts were determined using tryptic soy agar (TSA; Difco, Detroit, MI) plates. Brain heart infusion agar (BHIA, Difco, Detroit, MI) supplemented with 1 μg/ml of vancomycin was used to subculture VISA strains in order to maintain this phenotype (14).
MICs were determined in duplicate by broth microdilution per CLSI guidelines (15). Minimum bactericidal concentrations (MBCs) were determined as previously described (16). Oxacillin MICs were repeated on VISA subpopulations of 78 hVISA strains, which were selected by plating 108-CFU/ml suspensions onto BHIA plates containing 4 μg/ml vancomycin and incubating for 48 h at 35°C.
Time-kill studies were performed in duplicate to evaluate the potential for synergy and bactericidal activity of vancomycin plus oxacillin or ceftaroline at sub-MICs (one-half MIC) as described previously (16). Synergy and bactericidal activity were defined according to AAC guidelines (24).
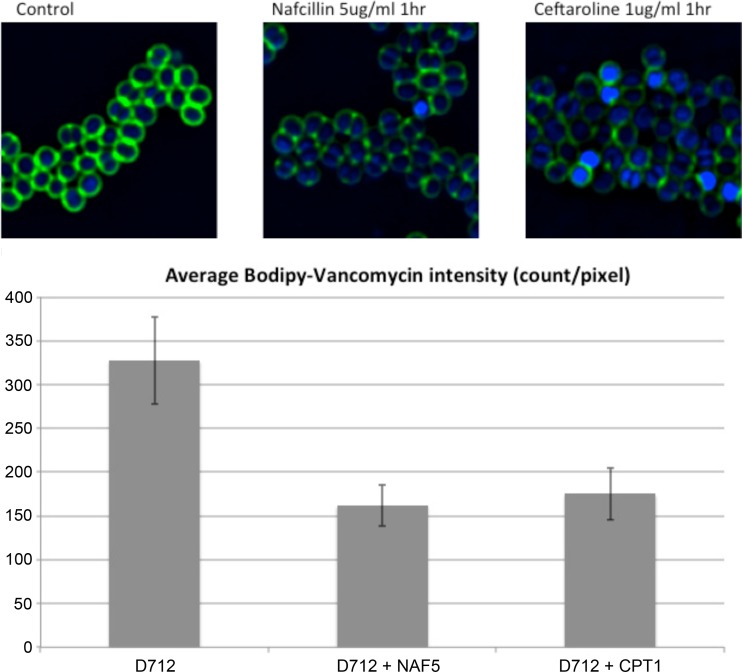
The well-characterized daptomycin-nonsusceptible VISA strain D712 was selected for vancomycin-Bodipy (dipyrromethene boron difluoride [4,4-difluoro-4-bora-3a,4a-diaza-s-indacene] fluorescent dye) binding studies (6). Cells were grown to an optical density at 600 nm (OD600) of 0.6, grown for an additional 1 h with or without 1 μg/ml ceftaroline or 5 μg/ml nafcillin, and then incubated with 2 μg/ml vancomycin-Bodipy for 15 min. Cells were then washed, stained, and imaged before fluorescence quantification as described previously (17).
Pearson's rank correlation coefficient test was used to assess the association between antimicrobial MICs. A P value of ≤0.05 was considered significant. Statistical analyses were performed using SPSS statistical software (release 20.0, SPSS, Inc., Chicago, Illinois).
MIC distributions are summarized in Table 1. VISA strains tended to have higher daptomycin MICs than hVISA strains, which is consistent with the results of prior studies (18, 19). This phenomenon of reduced daptomycin susceptibility in VISA isolates has been attributed to changes in cell wall thickness and membrane surface charge that lead to repulsion of the cationic daptomycin-calcium complex (20). In contrast, a significant inverse correlation was noted between the MIC values of vancomycin and oxacillin (P = 0.003), cefoxitin (P < 0.001), and ceftaroline (P < 0.05), supporting the “seesaw” hypothesis documented in the literature (7). Of interest, 10 of the 61 VISA strains (16.4%) were susceptible to oxacillin (MIC ≤ 2 μg/ml), despite the presence of mecA. Two- to 8-fold decreases in oxacillin MICs were observed in 36% of the VISA subpopulations selected from the hVISA strains, while 41% had 2- to 16-fold increases in oxacillin MICs. This finding was unexpected but exemplifies the inherent heterogeneity in the development of VISA strains. No correlation was observed between agr type, mec type, Panton-Valentine leukocidin (PVL) carriage, and vancomycin or daptomycin MIC values.
Table 1.
MIC distribution of various antibiotics against collections of hVISA and VISA isolates of MRSA
| Phenotype | Level of inhibition | MIC (μg/ml) ofa: |
||||
|---|---|---|---|---|---|---|
| VAN | DAP | OXA | FOX | CPT | ||
| hVISA (n = 93) | MIC50 | 1 | 0.25 | 128 | 128 | 0.5 |
| MIC90 | 2 | 0.5 | 512 | 256 | 1 | |
| Range | 0.5–2 | 0.125–1 | 8–512 | 8–512 | 0.125–2 | |
| VISA (n = 60) | MIC50 | 4 | 1 | 64 | 64 | 0.5 |
| MIC90 | 8 | 2 | 256 | 256 | 1 | |
| Range | 4–8 | 0.125–2 | 0.5–512 | 8–512 | 0.125–1 | |
VAN, vancomycin; DAP, daptomycin; OXA, oxacillin; FOX, cefoxitin; CPT, ceftaroline.
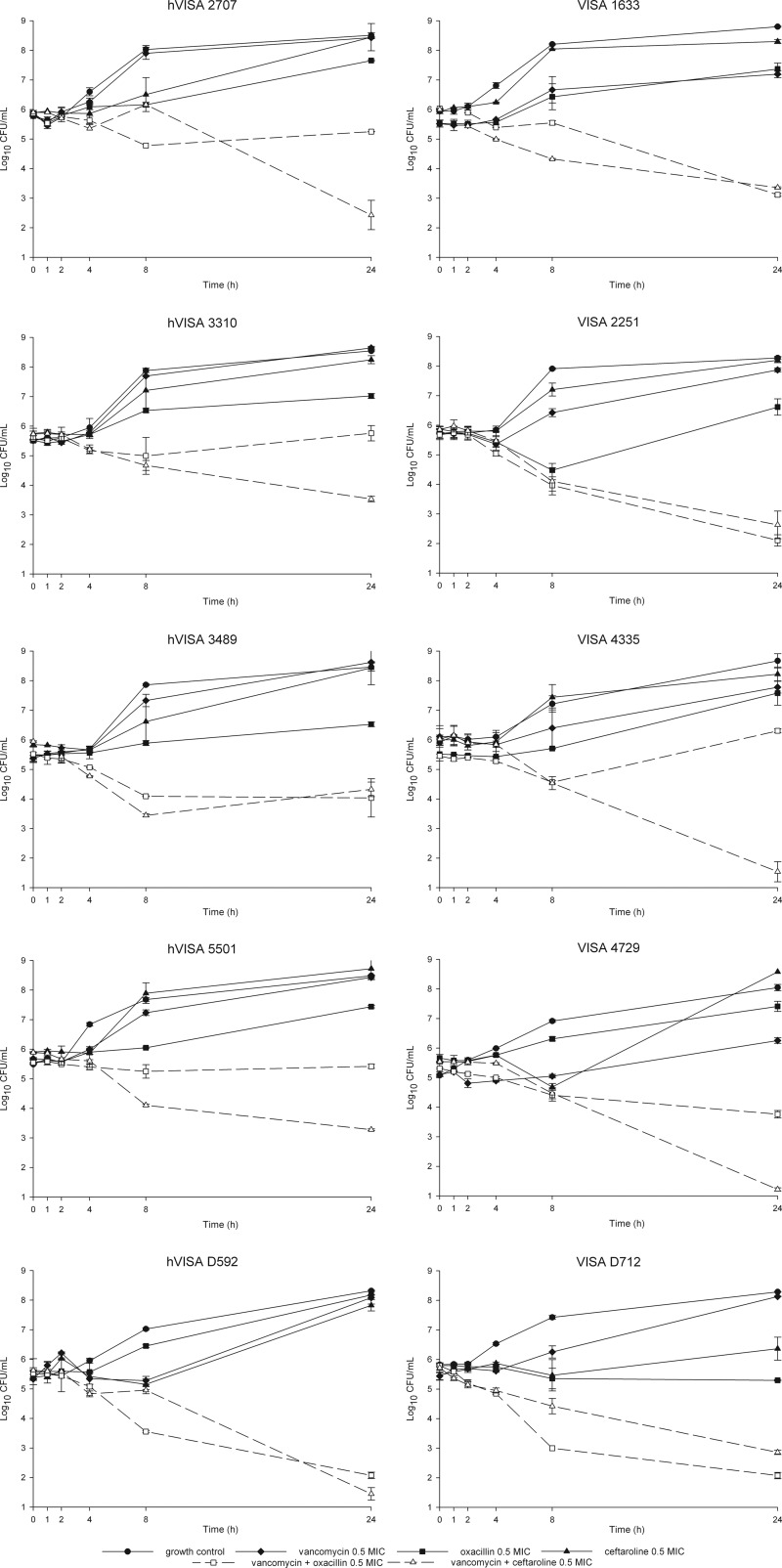
The combination of vancomycin plus ceftaroline was more active than the combination of vancomycin plus oxacillin, demonstrating synergy against 5 of 5 VISA and 4 of 5 hVISA strains tested. Vancomycin plus ceftaroline was bactericidal against 3 of 5 VISA strains and 2 of 5 hVISA strains at 0.5× MICs (Fig. 1). The combination of vancomycin plus oxacillin demonstrated synergy against 3 of 5 VISA isolates and 1 of 5 hVISA strains.
Fig 1.
Activities of vancomycin, oxacillin, ceftaroline, and combinations against VISA and hVISA strains.
Vancomycin-Bodipy binding was reduced by ∼50% in ceftaroline- or nafcillin-treated cells compared to that in untreated cells (Fig. 2). It is well established that vancomycin resistance in VISA arises from changes in cell wall metabolism that lead to vancomycin “sequestering” at nonactive cell wall targets, which allows for continued cell wall synthesis (1, 21). We have previously demonstrated that ceftaroline exposure causes a significant reduction in cell wall thickness (8.94 nm) in the strain D712 (12). A similar relationship has been noted between cell wall thickness and nafcillin exposure (22). Collectively, these data have led us to hypothesize that this cell wall thinning may prevent vancomycin sequestration, improving vancomycin penetration into the septa of dividing cells where it can interact with critical membrane-bound precursors of the nascent cell wall, leading to improved bactericidal action (23).
Fig 2.
VISA strain D712 was treated with 2 μg/ml vancomycin-Bodipy for 15 min after treatment with nafcillin (5 μg/ml) or ceftaroline (1 μg/ml) for 1 h. Relative vancomycin binding to VISA D712 (mean ± standard deviation) in the presence and absence of nafcillin 5 μg/ml (NAF5) or ceftaroline 1 μg/ml (CPT1) is shown.
Beta-lactam–vancomycin combinations have been previously studied in vitro and have yielded conflicting results (8–13). Our results and prior data suggest that beta-lactam–vancomycin combinations are not universally synergistic against MRSA, but the synergistic effect appears to be more pronounced against less-vancomycin-susceptible MRSA. Our results also suggest that ceftaroline may be more consistently synergistic than traditional antistaphylococcal beta-lactams with vancomycin and may yield bactericidal activity. Beta-lactams appear to chemically and morphologically alter the surface of VISA, allowing for reduced quantity but potentially increased target-specific vancomycin binding. Further research is warranted to elucidate the mechanisms of synergy between these agents and clarify the potential role of vancomycin–beta-lactam combinations in clinical settings.
ACKNOWLEDGMENTS
We thank Forest Laboratories for providing ceftaroline analytical powder.
M.J.R. has received grant support, consulted for, or provided lectures for Astellas, Cubist, Forest, Pfizer, Novartis, and Rib-X. M.J.R. is funded in part by NIH grant R21A1092055-01. G.S. has received grant support, consulted for, or provided lectures for Cubist, Astellas, Pfizer, and Ortho-McNeil Pharmaceuticals. J.P. was supported in part by NIH grant GM073898 and has received research grants and consulting fees from Cubist Pharmaceuticals. B.J.W., C.V., K.P.M., P.N., and K.L.N. have nothing to declare.
Footnotes
Published ahead of print 19 February 2013
REFERENCES
- 1. Appelbaum PC. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl 1):16–23 [DOI] [PubMed] [Google Scholar]
- 2. Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J. Clin. Microbiol. 46:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfeltz RF, Wilkinson BJ. 2004. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets Infect. Disord. 4:273–294 [DOI] [PubMed] [Google Scholar]
- 4. Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23:99–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sieradzki K, Tomasz A. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J. Bacteriol. 181:7566–7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin. Infect. Dis. 53:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vignaroli C, Rinaldi C, Varaldo PE. 2011. Striking “seesaw effect” between daptomycin nonsusceptibility and beta-lactam susceptibility in Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 55:2495–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Climo MW, Patron RL, Archer GL. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aritaka N, Hanaki H, Cui L, Hiramatsu K. 2001. Combination effect of vancomycin and beta-lactams against a Staphylococcus aureus strain, Mu3, with heterogeneous resistance to vancomycin. Antimicrob. Agents Chemother. 45:1292–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56:202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leonard SN. 2012. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. PLoS One 7:e42103 doi:10.1371/journal.pone.0042103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 57:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein FW, Atoui R, Ben Ali A, Nguyen JC, Ly A, Kitzis MD. 2004. False synergy between vancomycin and beta-lactams against glycopeptide-intermediate Staphylococcus aureus (GISA) caused by inappropriate testing methods. Clin. Microbiol. Infect. 10:342–345 [DOI] [PubMed] [Google Scholar]
- 14. Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399–403 [DOI] [PubMed] [Google Scholar]
- 15. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. M100-S19. CLSI, Wayne, PA [Google Scholar]
- 16. Leonard SN, Cheung CM, Rybak MJ. 2008. Activities of ceftobiprole, linezolid, vancomycin, and daptomycin against community-associated and hospital-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2974–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pogliano J, Osborne N, Sharp MD, Abanes-De Mello A, Perez A, Sun YL, Pogliano K. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelley PG, Gao W, Ward PB, Howden BP. 2011. Daptomycin non-susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J. Antimicrob. Chemother. 66:1057–1060 [DOI] [PubMed] [Google Scholar]
- 19. van Hal SJ, Paterson DL, Gosbell IB. 2011. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naive patient—a review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 30:603–610 [DOI] [PubMed] [Google Scholar]
- 20. Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. 2009. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin. Infect. Dis. 49:1169–1174 [DOI] [PubMed] [Google Scholar]
- 21. Liu C, Chambers HF. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 47:3040–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakoulas G, Rose WE. 2011. Effect of beta-lactam (BL) exposure in vitro on daptomycin (DAP) activity and cell wall thickness in MRSA, poster E-1332. 51st Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC) American Society for Microbiology, Washington, DC [Google Scholar]
- 23. Lunde CS, Rexer CH, Hartouni SR, Axt S, Benton BM. 2010. Fluorescence microscopy demonstrates enhanced targeting of telavancin to the division septum of Staphylococcus aureus. Antimicrob. Agents Chemother. 54:2198–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Society for Microbiology 2013. Instructions to authors. American Society for Microbiology, Washington, DC: http://aac.asm.org/site/misc/ifora.xhtml [Google Scholar]