Abstract
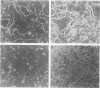
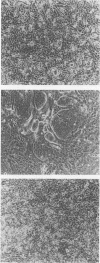
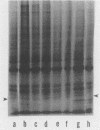
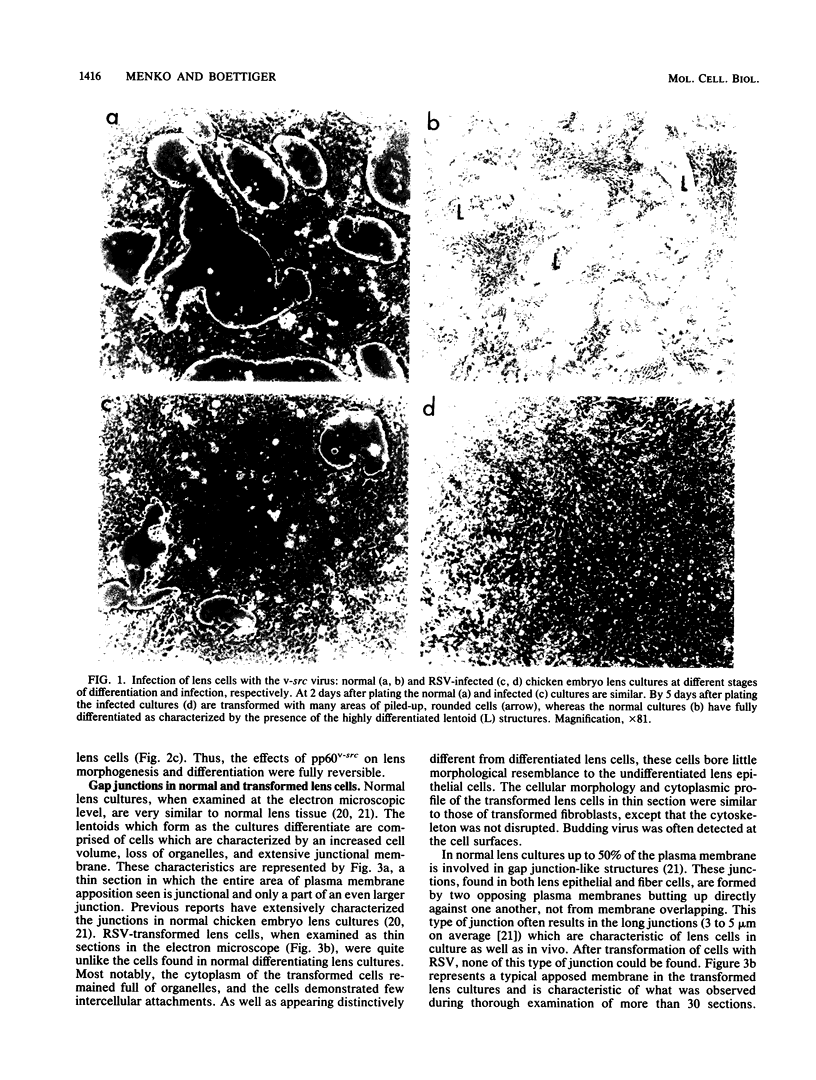
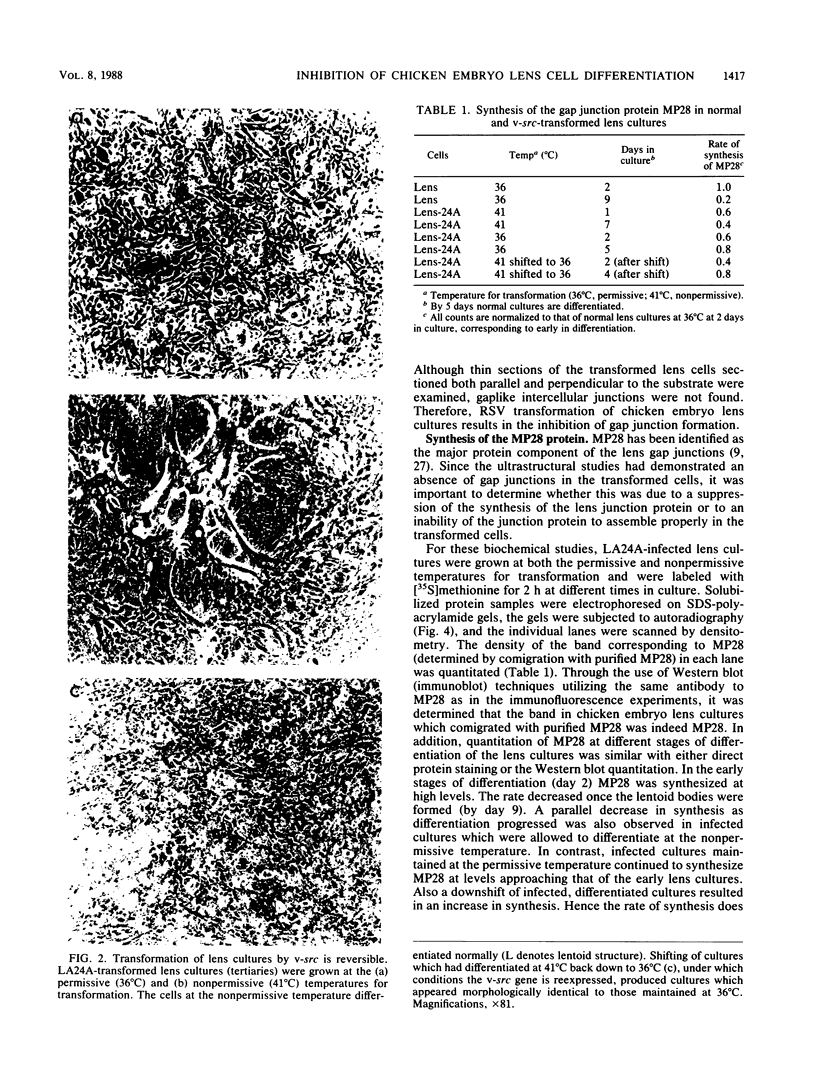
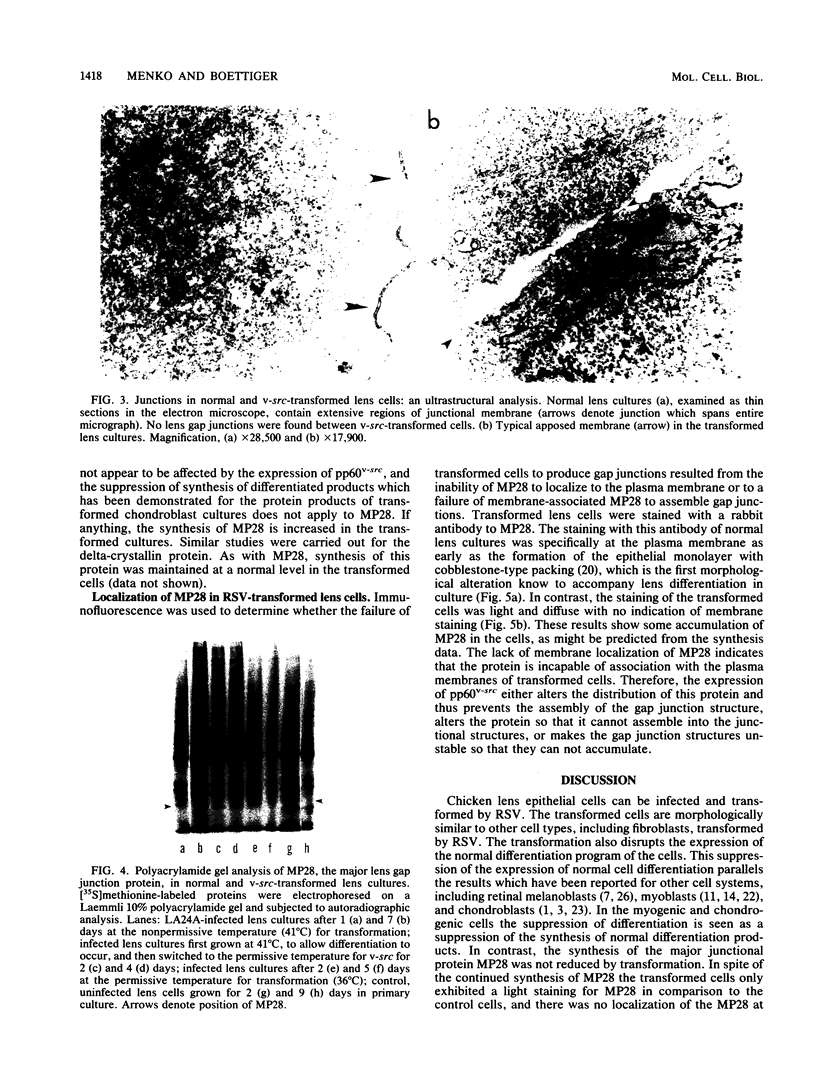
A culture system was developed which permitted the differentiation of chicken lens epithelial cells to lentoid bodies which contained several cell layers, accumulated high levels of delta-crystallin, and produced extensive gap junctions. This differentiation process was prevented when the cells were infected with a temperature-sensitive src mutant of Rous sarcoma virus and maintained at the permissive temperature. These transformed cells continued to proliferate and also synthesized the major lens gap junction protein, MP28, at near-normal rates. However, this MP28 was not assembled to produce gap junctions. Cultures shifted to the nonpermissive temperature formed lentoid bodies similar to those in uninfected lens cultures, including the establishment of gap junctions containing MP28.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Allebach E. S., Boettiger D., Pacifici M., Adams S. L. Control of types I and II collagen and fibronectin gene expression in chondrocytes delineated by viral transformation. Mol Cell Biol. 1985 May;5(5):1002–1008. doi: 10.1128/mcb.5.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Bentzel C. J., Vermorken A. J., Kibbelaar M., Bloemendal H. A portrait of plasma membrane specializations in eye lens epithelium and fibers. Biochim Biophys Acta. 1976 Dec 14;457(3-4):353–384. doi: 10.1016/0304-4157(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Bok D., Dockstader J., Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982 Jan;92(1):213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Stols A. L. Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp Eye Res. 1976 Sep;23(3):365–371. doi: 10.1016/0014-4835(76)90135-4. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1104–1122. [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Lampe P. D., Hur K. C., Louis C. F., Johnson R. G. A lens intercellular junction protein, MP26, is a phosphoprotein. J Cell Biol. 1986 Apr;102(4):1334–1343. doi: 10.1083/jcb.102.4.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986 Jan 17;44(1):187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Klukas K. A., Johnson R. G. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984 May;103(1):129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Klukas K. A., Liu T. F., Quade B., Sas D. F., Preus D. M., Johnson R. G. Junctions between lens cells in differentiating cultures: structure, formation, intercellular permeability, and junctional protein expression. Dev Biol. 1987 Oct;123(2):307–320. doi: 10.1016/0012-1606(87)90389-7. [DOI] [PubMed] [Google Scholar]
- Moss P. S., Honeycutt N., Pawson T., Martin G. S. Viral transformation of chick myogenic cells. The relationship between differentiation and the expression of the SRC gene. Exp Cell Res. 1979 Oct 1;123(1):95–105. doi: 10.1016/0014-4827(79)90425-7. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Paul D. L., Goodenough D. A. Preparation, characterization, and localization of antisera against bovine MP26, an integral protein from lens fiber plasma membrane. J Cell Biol. 1983 Mar;96(3):625–632. doi: 10.1083/jcb.96.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J. L. The electrophysiology of the crystalline lens. Curr Top Eye Res. 1979;1:37–90. [PubMed] [Google Scholar]
- Roby K., Boettiger D., Pacifici M., Holtzer H. Effects of Rous Sarcoma Virus on the synthetic programs of chondroblasts and retinal melanoblasts. Am J Anat. 1976 Nov;147(3):401–405. doi: 10.1002/aja.1001470311. [DOI] [PubMed] [Google Scholar]
- Sas D. F., Sas M. J., Johnson K. R., Menko A. S., Johnson R. G. Junctions between lens fiber cells are labeled with a monoclonal antibody shown to be specific for MP26. J Cell Biol. 1985 Jan;100(1):216–225. doi: 10.1083/jcb.100.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Goodenough D. A. Dye transfer between cells of the embryonic chick lens becomes less sensitive to CO2 treatment with development. J Cell Biol. 1982 Mar;92(3):694–705. doi: 10.1083/jcb.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]