Abstract
Background Obstructive jaundice in patients with hilar cholangiocarcinoma is a known risk factor for hepatic failure after liver resection. Plastic stents are most widely used for preoperative drainage. However, plastic stents are known to have limited patency time and therefore, in palliative settings, the self-expanding metal stent (SEMS) is used. This type of stent has been shown to be superior because it allows for rapid biliary decompression and a reduced complication rate after insertion. This study explores the use of the SEMS for biliary decompression in patients with operable hilar cholangiocarcinoma.
Methods A retrospective evaluation of a prospectively maintained database at a tertiary hepatobiliary referral centre was carried out. All patients with resectable cholangiocarcinoma were recorded.
Results Of 260 patients referred to this unit with cholangiocarcinoma between January 2008 and April 2012, 50 patients presented with operable cholangiocarcinoma and 27 of these had obstructive jaundice requiring stenting. Ten patients were initially treated with SEMSs; no stent failure occurred in these patients. Seventeen patients initially received plastic stents, seven of which failed in the interval between stent placement and laparotomy. These stents were replaced by SEMSs in four patients and by plastic stents in three patients. Median time to laparotomy was 45 days and 68 days in patients with SEMSs and plastic stents, respectively.
Conclusions Self-expanding metal stents provide adequate and rapid biliary drainage in patients with obstruction caused by hilar cholangiocarcinoma. No re-interventions were required. This probably reflects the relatively short interval between stent placement and laparotomy.
Introduction
Although cholangiocarcinoma is a relatively rare tumour type, its incidence is rising worldwide.1 Because there are no early symptoms in this disease, patients often present at an advanced stage of disease, which makes their prognosis extremely poor: 5-year survival rates are reported to be <10%.2 For both intrahepatic and hilar tumours, resection is the only curative option, but fewer than 20% of patients are eligible for surgery after staging, usually because of advanced disease. Patients diagnosed with a hilar cholangiocarcinoma that is deemed to be operable often present with obstructive jaundice, which is an established risk factor in liver surgery.3–5 Surgery for hilar cholangiocarcinoma usually involves an extended parenchymal resection, in which liver failure is the most serious complication. It is therefore recommended that preoperative biliary drainage (PBD) is performed in selected patients with jaundice to reduce the risk for postoperative liver failure.6,7
There has been much debate about the optimal technique for PBD. Whereas some authors advocate the percutaneous approach,8 endoscopic biliary drainage is the most commonly used technique.9 In this approach, a plastic stent is usually inserted to achieve biliary drainage. Plastic stents are relatively cheap and are thought to cause less tissue reaction than metal stents. However, plastic stents have a greater tendency to occlude and complications as a result of this occlusion are common.10 In the palliative setting, the median patency time of plastic stents has been identified as only 1.86 months.11 This is a major problem in potentially resectable patients because the time from stenting to explorative laparotomy is usually much longer and was found to exceed 3 months in a recent study.8 For this reason, there is an urgent need for a biliary decompression technique that provides rapid relief of jaundice and has a low complication rate. In the palliative setting, the self-expanding metal stent (SEMS) has been proven superior to the plastic stent in terms of patency.11 This study explores the use of SEMSs for biliary decompression in patients with resectable hilar cholangiocarcinoma.
Materials and methods
Patients
From January 2008 to April 2012, 260 patients with a suspected cholangiocarcinoma were referred to this hospital's hepatobiliary specialist multidisciplinary team meeting. The standard assessment includes multi-slice triple-phase contrast computed tomography (CT) scanning. When an intrahepatic cholangiocarcinoma is suspected, magnetic resonance imaging (MRI) is performed to further characterize the lesion. In the event of a suspected hilar cholangiocarcinoma, endoscopic retrograde cholangiopancreaticography (ERCP) is used to specify the anatomic location of the tumour and to grade it according to the Bismuth classification scheme.12 Moreover, through ERCP a tissue diagnosis of the lesion can often be obtained. In this unit, single-operator cholangioscopy is used in selected cases to obtain detailed information of the distal extent of the lesion and to obtain targeted biopsies. In the jaundiced and malnourished patient, when the future liver remnant is obstructed, a stent is placed across the lesion for biliary decompression. If imaging has confirmed the suspicion of a cholangiocarcinoma confined to the intrahepatic and/or extrahepatic bile ducts, and sufficient decompression of the bile ducts is achieved, the patient then undergoes a formal anaesthetic assessment including cardiopulmonary exercise testing. If the patient is deemed fit for surgery, a staging laparoscopy is performed.13 If no extrahepatic disease is found, the patient is scheduled for laparotomy with a view to resection. The present study focuses on patients who came to explorative laparotomy in the period from January 2008 to April 2012.
Stent technique
All patients were given prophylactic antibiotics to limit the risk for infection associated with hilar disease according to this unit's local policy. An ERCP procedure involving common bile duct cannulation followed by selective wire-guided cannulation with a 0.035-inch hydrophilic wire was performed in the future remnant sector(s) of the liver.
When a satisfactory wire position had been achieved, a limited cholangiogram was obtained both proximally and distally to the stricture to enable cholangiographic staging of the tumour, particularly of its proximal extent, supplementing the pre-procedural Bismuth classification obtained by cross-sectional imaging.12
Stenting was performed using either a 10-Fr plastic stent (Cook Medical, Inc., Bloomington, IN, USA) or a Boston Wallflex uncovered metallic stent with a 10-mm diameter (Boston Scientific Corp., Natick, MA, USA). Metal stents were sited to ensure strictures were crossed with a short intrahepatic segment upstream of the tumour in order not to impede future resection planes (Fig. 1). Care was taken to avoid unnecessary opacification of sectors that did not require drainage. In all cases, the stents traversed the ampulla, as per the standard approach in this unit. Adequate stent positioning was confirmed by X-ray.
Figure 1.
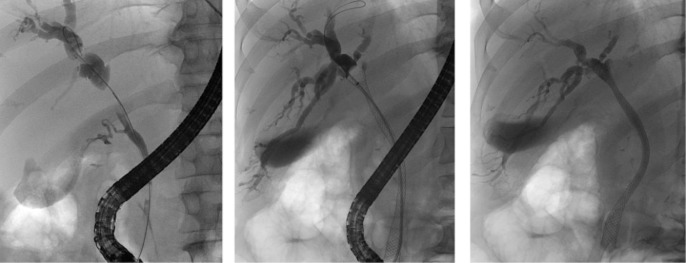
Positioning of a self-expanding metal stent
Definitions of events
The stenting procedure was considered technically successful when the stent was placed across the stricture and initial biliary decompression was obtained. Stent failure was defined as the need for a second intervention to obtain adequate biliary drainage. This might be caused by an insufficient fall in bilirubin levels after the initial procedure or might arise in the context of cholangitis or cholecystitis. A patient was considered fit for laparotomy when hyperbilirubinaemia was largely resolved and the patient's nutritional status was determined to be adequate by a dietician.
Results
During the period under study, 50 patients with suspected cholangiocarcinoma who qualified for explorative laparotomy were identified (Fig. 2). Thirty-five patients had a hilar tumour. Eight patients did not require any preoperative procedure because the future liver remnant was not obstructed and the patients were anabolic. A total of 27 patients presented with a hilar cholangiocarcinoma causing obstructive jaundice. All of these patients underwent stent placement, either at the referring hospital or at the present unit. All procedures were technically successful. A total of 17 patients were given plastic stents initially, but stent failure occurred in seven of these patients at a median of 27 days (range: 10–50 days) after the first stent insertion. In three of these patients, the plastic stent was replaced by a second plastic stent (with subsequent failure in one patient, in whom a third plastic stent was inserted 86 days and 55 days after placement of the first and second stents, respectively). The remaining four patients in whom plastic stents failed each received an SEMS replacement stent.
Figure 2.
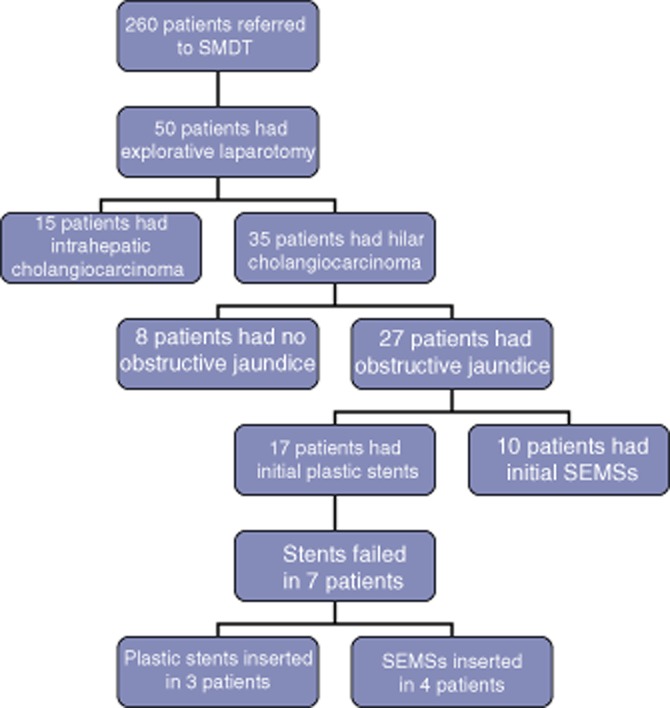
Flow chart of patients. SMDT, specialist multidisciplinary team; SEMS, self-expanding metal stent. The patient with immunoglobulin 4-related sclerosing disease is included
In 10 patients, an SEMS was placed as the initial method of biliary decompression; all of these placements were made at the study unit. At this unit, SEMSs were used from 2010 and the criteria for stenting did not differ between plastic stents and SEMSs. As a result, the present sample included 14 patients with SEMSs and 13 patients with plastic stents at the time of laparotomy. In three patients, stents were inserted using a percutaneous approach (one metal and two plastic stents). The median time between the first stent insertion and laparotomy was 68 days (range: 6–114 days) in patients in whom initial stents were plastic and 45 days (range: 35–69 days) in patients who initially received SEMSs. There were no cases of post-ERCP pancreatitis.
At explorative laparotomy, eight of the 27 stented patients (four with plastic and four with metal stents) were deemed to be inoperable as a result of peritoneal seeding (n = 7) or tumour growth into the duodenum (n = 1); all of these diagnoses were biopsy-proven. In all the resected patients, frozen-section biopsies obtained from the proximal and distal resection margins of the bile duct indicated margins were tumour-free. However, final paraffin-embedded histology of the distal resection margin in one patient, in whom a metal stent had been placed, indicated tumour cells (R1 resection). Final histology in two other patients, of whom one belonged to the plastic stent group and the other to the SEMS group, showed an R1 resection as the intrahepatic portion of the tumour reached the parenchymal resection plane. In one further patient, final histology showed immunoglobulin 4-related sclerosing disease. The extents and final outcomes of resections are shown in Table 1.
Table 1.
Stents and outcomes in 27 patients with hilar cholangiocarcinoma who underwent laparotomy with a stent in situ
| Patient | Bismuth grade | Initial stent | Second stent | Interval between stents, days | Operation | Interval to laparotomy, days | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | IIIb | Plastic | L hemi + caudate + CBD + hep-jej | 95 | IgG-4 | ||
| 2 | IIIa | Plastic | Plastic | 50 | R hemi + CBD + hep-jej | 70 | R1a |
| 3 | Plastic | Open-and-close | 114 | N/A | |||
| 4 | GB cancer | Plastic | Plastic | 10 | GB + IV/V res + CBD | 28 | R0 |
| 5 | IIIb | Plastic | L hemi + caudate + CBD + hep-jej | 66 | R0 | ||
| 6 | IIIa | Ptc – plastic int–ext | R hemi + caudate + CBD + hep-jej | 6 | R0 | ||
| 7 | Plastic | Open-and-close | 78 | N/A | |||
| 8 | IIIb | Metal | L hemi + CBD + hep-jej | 43 | R0 | ||
| 9 | IIIb | Plastic | L hemi + caudate + CBD + hep-jej | 33 | R0 | ||
| 10 | IIIa | Metal | R tri + CBD + hep-jej | 54 | R1b | ||
| 11 | Metal | Open-and-close | 44 | N/A | |||
| 12 | IIIb | Plastic | Ptc – metal | 26 | L hemi + CBD + hep-jej | 101 | R0 |
| 13 | IV | Plastic | R tri + CBD + PV + hep-jej | 24 | R0 | ||
| 14 | Metal | Open-and-close | 35 | N/A | |||
| 15 | Plastic | Metal | 10 | Open-and-close | 88 | N/A | |
| 16 | IIIa | Plastic | Metal | 27 | R tri + CBD + hep-jej | 61 | R0 |
| 17 | Metal | Open-and-close | 69 | N/A | |||
| 18 | Plastic | Plastic | 31c | Open-and-close | 93 | N/A | |
| 19 | II | Plastic | R tri + CBD + PV + hep-jej | 104 | R0 | ||
| 20 | Plastic | Open-and-close | 50 | N/A | |||
| 21 | I | Plastic | Metal | 33 | CBD + part PV + hep-jej | 59 | R0 |
| 22 | IIIb | Metal | L hemi + caudate + CBD + hep-jej | 55 | R0 | ||
| 23 | II | Metal | CBD + hep-jej | 63 | R0 | ||
| 24 | IV | Metal | R tri + caudate + CBD + PV + hep-jej | 37 | R1a | ||
| 25 | IIIb | Metal | L hemi + caudate + CBD + hep-jej | 45 | R0 | ||
| 26 | IV | Ptc – plastic int–ext | R tri + caudate + CBD + PV + hep-jej | 50 | R0 | ||
| 27 | IIIb | Metal | L hemi + caudate + CBD + hep-jej | 53 | R0 |
R1 resection based on the intrahepatic portion of the tumour reaching the parenchymal resection plane.
R1 resection based on the distal resection margin of the bile duct containing tumour cells on final paraffin-embedded histology; frozen-section analysis was negative.
This patient experienced a second failure at 86 days after the first (and 55 days after the second) stent and had a third plastic stent inserted.
GB, gallbladder; Ptc, percutaneous transhepatic cholangiography; int–ext, internal–external; L hemi, left hemihepatectomy; R hemi, right hemihepatectomy; IV/V res, segments IV/V resection; CBD, common bile duct; hep-jej, hepaticojejunostomy; PV, portal vein; R tri, right trisectionectomy; IgG4, immunoglobulin 4-related sclerosing disease; R0, microscopically margin-negative resection; R1, microscopic margin positive for tumour; N/A, not applicable.
Discussion
This study, although it is subject to the potential bias inherent to retrospective studies, shows that the use of SEMSs in operable patients with hilar cholangiocarcinoma is feasible. The wider lumens of the SEMS provide for rapid biliary decompression, making proximal sepsis, which is caused by inadequate antegrade biliary flow, much less likely. In this series, no re-interventions were necessary. Rapid decompression enables the patient to recover quickly and therefore the interval between biliary decompression and explorative laparotomy can be relatively short.
The median time to laparotomy of 45 days following the insertion of an SEMS in this study compares favourably with data in the literature. Many studies on preoperative stenting fail to report the median time interval between stenting and laparotomy, but one recent study cited a median time to laparotomy of 15 weeks.8 This probably reflects the patient selection strategy used: only patients of inadequate nutritional status caused by obstructive jaundice, and patients in whom the future liver remnant was obstructed were considered for preoperative drainage. When the enterohepatic cycle is restored, nutritional status improves and a patient will be considered for surgery once he or she becomes anabolic. Moreover, hepatic regeneration is impaired in the jaundiced patient and the full restoration of hepatic function and regeneration capacity takes 4–6 weeks after biliary decompression.14 Therefore, when major hepatic resection is planned (particularly resections requiring preoperative portal vein embolization), stenting is often required and the interval between stenting and laparotomy is usually prolonged.
The present study was not designed to establish the role of SEMS use in the preoperative setting. The small number of patients, the retrospective setting and the absence of strict and uniform criteria for the insertion of stents and for establishing the optimal timing of the operation according to bilirubin level and nutritional status are all significant shortcomings of the present study. However, this study shows important possible benefits of SEMS use and therefore a prospective comparative study to define the possible role of the SEMS in operable cholangiocarcinoma is proposed.
It is of fundamental importance that the insertion of an SEMS in a patient with potentially operable hilar cholangiocarcinoma takes place in the tertiary setting. Stent placement, especially when metal stents are used, requires the close collaboration of the interventional endoscopist and the hepatobiliary surgeon. Full cross-sectional imaging should be obtained prior to the intervention, which should then be undertaken with a clear plan and objectives. Injudicious intervention or SEMS placement will dramatically compromise the management of the patient by opacifying undrained segments and increasing the attendant risk for sepsis, compromising the further acquisition of tissue if required or rendering resection impossible by excessively proximal stent placement. Health service networks should facilitate the prompt referral of patients with malignant proximal biliary obstruction to enable the early involvement of tertiary specialists in their care.
Many studies conducted in the palliative setting have compared outcomes of the use of plastic and metal stents, respectively. In general, metal stents are superior in terms of patency and are therefore recommended.15,16 In a recent comparative assessment, the median patency of plastic stents was found to be 1.86 months, which is significantly shorter than the patency of 5.56 months identified for metal stents.11 This means that the median patency of plastic stents (in the palliative setting) is shorter than the time between stenting and laparotomy (in preoperatively stented patients). Thus, during the interval between stenting and laparotomy, at least 50% of patients with plastic stents can be expected to require some form of re-intervention. A recent study found that a mean of 2.8 preoperative drainage procedures were required in patients in whom endoscopic plastic stents were used.8 The majority of these re-interventions were for infectious complications, which occurred in 48% of patients.8 This high rate of infectious complications is comparable with rates reported elsewhere in the literature17,18 and therefore it is likely that re-intervention rates will be relatively high across the world.
The precise role of preoperative stenting is still subject to discussion.19 A Cochrane review published in 200820 concluded that preoperative drainage could be neither supported nor refuted because the trials included in the meta-analysis were of insufficient quality. Moreover, most trials investigating preoperative biliary drainage have focused mainly on distal obstructions that do not necessitate liver parenchymal resection.21–24 Although biliary stenting is not recommended as a standard preoperative procedure,25,26 it is recommended in patients with significant biliary obstruction because persistent jaundice is a risk factor for postoperative liver failure.6,7 Patients in a catabolic state will benefit from preoperative drainage when the enterohepatic cycle is restored.27
The preferred method of biliary drainage is also controversial. Some authors advocate the percutaneous approach because it is presumed to carry a lower risk for infectious complications and to enable the targeting of specific sectors for drainage, although improvements in ERCP technique now frequently enable sector-specific drainage at ERCP.8 Others argue that the endoscopic approach is less invasive and enables enteral drainage and hence presumed better nutritional status.27 Endoscopic drainage can be performed by endoscopic stenting or endoscopic nasobiliary drainage, the second of which is presumed to be associated with reduced risk for infectious complications.6,28,29 What is clear is that poorly performed attempts at drainage by either route are worse than no drainage at all.
The preoperative use of metal stents is limited by the fact that intraoperative assessment of the extent of the tumour may be more difficult as the fibrotic reaction is likely to be more pronounced if an SEMS is used than if a plastic stent is inserted. This is believed to lead to technical difficulties during resection and to a higher complication rate.30,31 Self-expanding metal stents have recently been used in distal cholangiocarcinoma, which is subject to the same concerns about technical difficulties, but no stent-related complications occurred either intra- or postoperatively.32 In the present study, no technical difficulties in intraoperative assessment were encountered and the resection planes chosen were all found to be free of tumour on frozen-section analysis. Neither were there any major difficulties in removing the stent from the distal bile duct before its closure. Another possible concern refers to the pathological assessment of the specimen with an SEMS in situ. Again, in the present study this did not appear to be a problem and adequate assessment of the epithelium of the bile ducts was possible in all cases.
Metal stents are considerably more costly than plastic stents. However, the possible costs of further interventions may considerably outweigh the primary stent costs, depending on the number of re-interventions required.33 In the palliative setting, Moss et al.34 performed a meta-analysis of randomized controlled trials comparing the use of plastic and metal stents for obstructions in both proximal and distal biliary tumours. The use of SEMSs was found to be cost-effective when additional procedures carried out in association with the use of plastic stents cost more than US$1820.34 In distal biliary obstructions, initial placement of an SEMS was found to cause a 28% decrease in further endoscopic procedures in a prospective randomized trial.35 The literature does not include any data on the issue of cost-effectiveness in the context of proximal obstructions in a preoperative setting and the present study was not designed to make a cost-effectiveness analysis.
Recently, a study from the MD Anderson Cancer Center showed that preoperative metal stenting in patients with a malignant distal biliary obstruction is feasible.32 The use of neoadjuvant strategies in these patients is gaining in popularity and therefore many patients will require biliary decompression because the time between diagnosis and surgery will be prolonged.36 For proximal biliary tumours, neoadjuvant therapies are not considered to represent a standard of care. However, a recent study by Gruenberger et al.37 has shown promising results of induction chemotherapy using cetuximab, gemcitabine and oxaliplatin. Of the 30 primarily unresectable patients in this study, nine patients underwent intentional curative resection after induction chemotherapy.37 The introduction of neoadjuvant strategies in hilar cholangiocarcinoma will increase interest in biliary stents that provide superior longlasting biliary decompression.
Conclusions
The present study shows that SEMSs provide adequate biliary drainage and do not preclude subsequent curative surgery in selected patients in whom obstruction is caused by hilar cholangiocarcinoma. The reduced need for re-interventions in patients in whom SEMSs have been inserted is promising, but the possible superiority of the SEMS can only be proven in a prospective setting.
Acknowledgments
DJG received a clinical fellowship grant from the Dutch Cancer Society.
Conflicts of interest
None declared.
References
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumours. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845–852. doi: 10.1136/gut.24.9.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta H, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. Bacteraemia after hepatectomy: an analysis of a single-centre, 10-year experience with 407 patients. Langenbecks Arch Surg. 2002;387:117–124. doi: 10.1007/s00423-002-0301-2. [DOI] [PubMed] [Google Scholar]
- Blamey SL, Fearon KC, Gilmour WH, Osborne DH, Carter DC. Prediction of risk in biliary surgery. Br J Surg. 1983;70:535–538. doi: 10.1002/bjs.1800700910. [DOI] [PubMed] [Google Scholar]
- Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro) HPB. 2008;10:130–133. doi: 10.1080/13651820801992666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki S, Chijiiwa K, Komura M, Yamaguchi K, Kuroki S, Tanaka M. Preoperative internal biliary drainage is superior to external biliary drainage in liver regeneration and function after hepatectomy in obstructive jaundiced rats. Ann Surg. 1999;230:655–662. doi: 10.1097/00000658-199911000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloek JJ, van der Gaag NA, Aziz Y, Rauws EA, van Delden OM, Lameris JS, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010;14:119–125. doi: 10.1007/s11605-009-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami H, Kondo S, Kuwatani M, Yamato H, Ehira N, Kudo T, et al. Preoperative biliary drainage for hilar cholangiocarcinoma: which stent should be selected? J Hepatobiliary Pancreat Sci. 2011;18:630–635. doi: 10.1007/s00534-011-0404-7. [DOI] [PubMed] [Google Scholar]
- Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. Am J Gastroenterol. 1996;91:1301–1308. [PubMed] [Google Scholar]
- Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- Joseph S, Connor S, Garden OJ. Staging laparoscopy for cholangiocarcinoma. HPB. 2008;10:116–119. doi: 10.1080/13651820801992690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K, Takagi Y, Ito K, Sato T. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg. 1981;142:293–299. doi: 10.1016/0002-9610(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Peters RA, Williams SG, Lombard M, Karani J, Westaby D. The management of high-grade hilar strictures by endoscopic insertion of self-expanding metal endoprostheses. Endoscopy. 1997;29:10–16. doi: 10.1055/s-2007-1004054. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213–218. doi: 10.1055/s-2007-1010295. [DOI] [PubMed] [Google Scholar]
- dos Santos JS, Junior WS, Modena JL, Brunaldi JE, Ceneviva R. Effect of preoperative endoscopic decompression on malignant biliary obstruction and postoperative infection. Hepatogastroenterology. 2005;52:45–47. [PubMed] [Google Scholar]
- Rerknimitr R, Kladcharoen N, Mahachai V, Kullavanijaya P. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38:518–523. doi: 10.1097/01.mcg.0000123204.36471.be. [DOI] [PubMed] [Google Scholar]
- Rerknimitr R, Kullavanijaya P. Operable malignant jaundice: to stent or not to stent before the operation? World J Gastrointest Endosc. 2010;2:10–14. doi: 10.4253/wjge.v2.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008;(3):CD005444. doi: 10.1002/14651858.CD005444.pub2. [DOI] [PubMed] [Google Scholar]
- Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries-Jones R, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896–899. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Preoperative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371–375. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP., Jr Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–553. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig JD, Kumar H, Suri S, Gupta NM. Usefulness of percutaneous transhepatic biliary drainage in patients with surgical jaundice – a prospective randomized study. J Assoc Physicians India. 1999;47:271–274. [PubMed] [Google Scholar]
- Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci. 2011;56:663–672. doi: 10.1007/s10620-010-1338-7. [DOI] [PubMed] [Google Scholar]
- Maguchi H, Takahashi K, Katanuma A, Osanai M, Nakahara K, Matuzaki S, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:441–446. doi: 10.1007/s00534-006-1192-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakura N, Takayama M, Ozaki Y, Maruyama M, Chou Y, Kodama R, et al. Efficacy of preoperative endoscopic nasobiliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2009;16:473–477. doi: 10.1007/s00534-009-0076-8. [DOI] [PubMed] [Google Scholar]
- Ayaru L, Kurzawinski TR, Shankar A, Webster GJ, Hatfield AR, Pereira SP. Complications and diagnostic difficulties arising from biliary self-expanding metal stent insertion before definitive histological diagnosis. J Gastroenterol Hepatol. 2008;23:315–320. doi: 10.1111/j.1440-1746.2006.04562.x. [DOI] [PubMed] [Google Scholar]
- Lytras D, Olde Damink SW, Amin Z, Imber CJ, Malago M. Radical surgery in the presence of biliary metallic stents: revising the palliative scenario. J Gastrointest Surg. 2011;15:489–495. doi: 10.1007/s11605-010-1389-2. [DOI] [PubMed] [Google Scholar]
- Singal AK, Ross WA, Guturu P, Varadhachary GR, Javle M, Jaganmohan SR, et al. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: single-centre experience with 79 cases. Dig Dis Sci. 2011;12:3678–3684. doi: 10.1007/s10620-011-1815-7. [DOI] [PubMed] [Google Scholar]
- Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49(4 Pt 1):466–471. doi: 10.1016/s0016-5107(99)70044-1. [DOI] [PubMed] [Google Scholar]
- Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119–1124. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]
- Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomized trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142–1148. doi: 10.1016/S1470-2045(10)70247-3. [DOI] [PubMed] [Google Scholar]


