Abstract
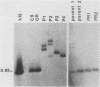
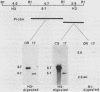
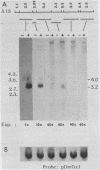
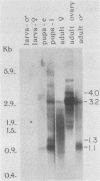
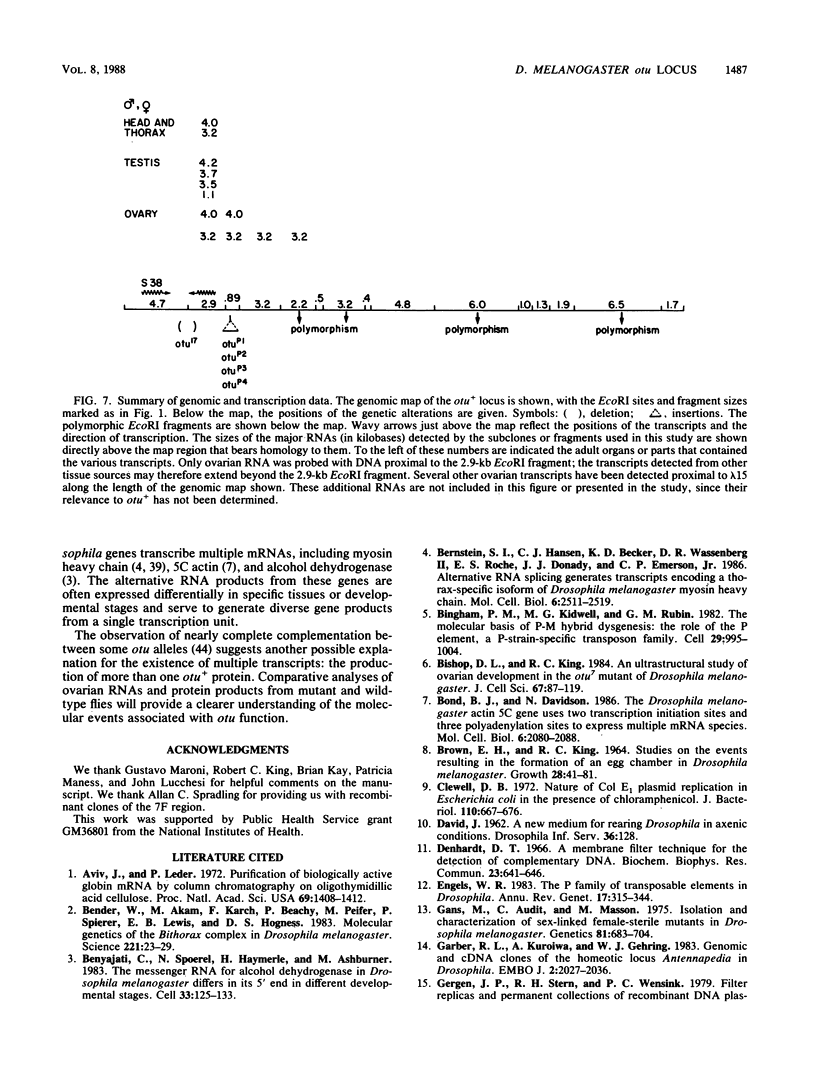
The female-sterile ovarian tumor gene, otu, is located in cytological region 7F1 on the Drosophila melanogaster chromosome map. We have mapped the gene at the molecular level by using four dysgenic alleles and two revertant derivatives of these alleles as well as an ethyl methanesulfonate-induced allele. The insertional (dysgenic) changes were all associated with one restriction fragment, and its size was restored after phenotypic reversion. One ethyl methanesulfonate-induced allele had a deletion in the restriction fragment adjacent (distal) to the fragment altered in the insertional alleles. These two restriction fragments were immediately adjacent to the s38 chorion gene. Associated with the two altered restriction fragments were two RNA species, an abundant 3.2-kilobase (kb) poly(A)+ RNA and a minor 4.0-kb RNA. Several other less-abundant RNA species were detectable with more-sensitive single-stranded RNA probes. The otu gene was transcribed proximal to distal relative to the centromere; this was opposite to the direction of transcription of the adjacent s38 gene. During development, the 3.2-kb RNA was absent in larvae, first appeared in the pupal stages, and persisted in adult females, in which it was most prevalent in the ovaries. The DNA that hybridized to the 3.2-kb ovarian RNA hybridized to four different RNAs found in the testes but not in the rest of the adult male. These testis-enriched RNAs were transcribed from the same strand of DNA as the ovarian transcripts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. H., KING R. C. STUDIES ON THE EVENTS RESULTING IN THE FORMATION OF AN EGG CHAMBER IN DROSOPHILA MELANOGASTER. Growth. 1964 Mar;28:41–81. [PubMed] [Google Scholar]
- Bender W., Akam M., Karch F., Beachy P. A., Peifer M., Spierer P., Lewis E. B., Hogness D. S. Molecular Genetics of the Bithorax Complex in Drosophila melanogaster. Science. 1983 Jul 1;221(4605):23–29. doi: 10.1126/science.221.4605.23. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bernstein S. I., Hansen C. J., Becker K. D., Wassenberg D. R., 2nd, Roche E. S., Donady J. J., Emerson C. P., Jr Alternative RNA splicing generates transcripts encoding a thorax-specific isoform of Drosophila melanogaster myosin heavy chain. Mol Cell Biol. 1986 Jul;6(7):2511–2519. doi: 10.1128/mcb.6.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Kidwell M. G., Rubin G. M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell. 1982 Jul;29(3):995–1004. doi: 10.1016/0092-8674(82)90463-9. [DOI] [PubMed] [Google Scholar]
- Bishop D. L., King R. C. An ultrastructural study of ovarian development in the otu7 mutant of Drosophila melanogaster. J Cell Sci. 1984 Apr;67:87–119. doi: 10.1242/jcs.67.1.87. [DOI] [PubMed] [Google Scholar]
- Bond B. J., Davidson N. The Drosophila melanogaster actin 5C gene uses two transcription initiation sites and three polyadenylation sites to express multiple mRNA species. Mol Cell Biol. 1986 Jun;6(6):2080–2088. doi: 10.1128/mcb.6.6.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Engels W. R. The P family of transposable elements in Drosophila. Annu Rev Genet. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Gans M., Audit C., Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975 Dec;81(4):683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber R. L., Kuroiwa A., Gehring W. J. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO J. 1983;2(11):2027–2036. doi: 10.1002/j.1460-2075.1983.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. alpha-Tubulin genes of Drosophila. Cell. 1981 Apr;24(1):97–106. doi: 10.1016/0092-8674(81)90505-5. [DOI] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- King R. C., Mohler D., Riley S. F., Storto P. D., Nicolazzo P. S. Complementation between alleles at the ovarian tumor locus of Drosophila melanogaster. Dev Genet. 1986;7(1):1–20. doi: 10.1002/dvg.1020070102. [DOI] [PubMed] [Google Scholar]
- King R. C., Rasch E. M., Riley S. F., O'Grady P. M., Storto P. D. Cytophotometric evidence for the transformation of oocytes into nurse cells in Drosophila melanogaster. Histochemistry. 1985;82(2):131–134. doi: 10.1007/BF00708196. [DOI] [PubMed] [Google Scholar]
- King R. C., Riley S. F., Cassidy J. D., White P. E., Paik Y. K. Giant polytene chromosomes from the ovaries of a Drosophila mutant. Science. 1981 Apr 24;212(4493):441–443. doi: 10.1126/science.6782674. [DOI] [PubMed] [Google Scholar]
- Koch E. A., King R. C. Further studies on the ring canal system of the ovarian cystocytes of Drosophila melanogaster. Z Zellforsch Mikrosk Anat. 1969;102(1):129–152. doi: 10.1007/BF00336421. [DOI] [PubMed] [Google Scholar]
- Koch E. A., King R. C. The origin and early differentiation of the egg chamber of Drosophila melanogaster. J Morphol. 1966 Jul;119(3):283–303. doi: 10.1002/jmor.1051190303. [DOI] [PubMed] [Google Scholar]
- Koch E. A., Smith P. A., King R. C. The division and differentiation of Drosophila cystocytes. J Morphol. 1967 Jan;121(1):55–70. doi: 10.1002/jmor.1051210106. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S., Wakimoto B., Spradling A. Replication and expression of an X-linked cluster of Drosophila chorion genes. Dev Biol. 1986 Sep;117(1):294–305. doi: 10.1016/0012-1606(86)90372-6. [DOI] [PubMed] [Google Scholar]
- Rasch E. M., King R. C., Rasch R. W. Cytophotometric studies on cells from the ovaries of otu mutants of Drosophila melanogaster. Histochemistry. 1984;81(2):105–110. doi: 10.1007/BF00490101. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J., Hazelrigg T. I., Polisky B. A., Pirrotta V., Scalenghe F., Kaufman T. C. The molecular organization of the Antennapedia locus of Drosophila. Cell. 1983 Dec;35(3 Pt 2):763–776. doi: 10.1016/0092-8674(83)90109-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981 Nov;27(1 Pt 2):193–201. doi: 10.1016/0092-8674(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B. T., Kalfayan L. J., Spradling A. C. Developmentally regulated expression of Drosophila chorion genes introduced at diverse chromosomal positions. J Mol Biol. 1986 Jan 5;187(1):33–45. doi: 10.1016/0022-2836(86)90404-3. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Audit C., Masson M. A clonal analysis of the roles of somatic cells and germ line during oogenesis in Drosophila. Dev Biol. 1981 Nov;88(1):92–103. doi: 10.1016/0012-1606(81)90221-9. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]