Abstract
Isoflavone-rich diets are associated with reduced menopausal symptoms and lowered risk of cancers of reproductive tissues. Isoflavones may mimic some effects of estrogen by binding to estrogen receptors, and/or altering steroid availability. Despite their potential health benefits, neither the effects, nor mechanisms, of isoflavones are well understood. We hypothesized that isoflavones would alter behavior and physiology of rats in sex and/or gonad-dependent manner. An isoflavone-based, commercially-available, dietary supplement was administered via subcutaneous implantation to female and male, intact and gonadectomized Long–Evans rats. Affective (elevated plus-maze), cognitive (water-maze), and reproductive (sexual) behavior was examined. Weights of reproductive structures were measured, as an index of trophic effects. Steroid levels in circulation and brain regions associated with behavioral measures were evaluated by radioimmunoassay. The supplement increased anti-anxiety behavior of intact, but not gonadectomized, rats. The supplement enhanced visual–spatial performance of all rats, but this effect was most evident among proestrous female rats, which had the poorest spatial performance. There were neither effects of the supplement on sexual behavior, mass of reproductive tissues, nor plasma steroid levels. The supplement increased levels of 5α-androstane,17β-diol-3α-diol (3α-diol) in the hippocampus (but not other brain regions) of gonadectomized females. Thus, the supplement altered anxiety and cognitive behavior and brain production of steroids; however, the anti-anxiety effects were limited to rats with an intact reproductive axis and effects on cognitive performance and neurosteriodogenesis were most evident among intact and gonadectomized, female rats respectively.
Keywords: Soy, Dietary supplement, Estrogen, Estradiol, Non-genomic
1. Introduction
Isoflavones, a class of phytoestrogen, are abundant in the human diet, with soy-based foods being among the highest in isoflavone content (Grun et al., 2001; Reinli and Block, 1996). Diets rich in isoflavones include traditional Asian or vegetarian diets and many infant-formulas (Franke et al., 1995; Reinli and Block, 1996; Setchell et al., 1998). Diets rich in isoflavones have also been associated with less severe psychological and physiological symptoms of menopause, as well as a decreased risk for reproductive cancers and heart disease (Borrelli and Ernst, 2010; Cano et al., 2010; Cassidy and Faughnan, 2000; de Souza et al., 2010; Fitzpatrick, 2003; Limer and Speirs, 2004). Dietary supplements containing isoflavones may therefore have added health benefits (Lethaby et al., 2007). Thus, effects and mechanisms of isoflavone-based supplements need to be better understood.
Isoflavones may have some effects through 17β-estradiol, the primary estrogen secreted by the ovaries. In vivo studies examining physiologically-relevant concentrations of isoflavones show significant estrogen-like or anti-estrogen-like effects, including alterations in animal behavior, sexually-dimorphic changes in brain volumes, and antiproliferative effects in reproductive tissues (Lephart et al., 2003; Lund and Lephart, 2001; Totta et al., 2005). For example, intact rats on an isoflavone diet show reduced anxiety behavior compared to those on an isoflavone-free diet (Lund and Lephart, 2001) and changes in volume of regions of the hypothalamus sensitive to estrogen (Lephart et al., 2003). Thus, these effects of isoflavones may be due in part to actions through estrogen signaling.
Estrogen acts throughout the body to produce a variety of effects in part through actions at intracellular estrogen receptors (ERs) and their many variants, including ERα and ERβ (Pedram et al., 2002; Toran-Allerand, 2004). Estrogen has trophic effects which enhance proliferation of cancer cells, mammary and uterine tissues, and aids in the maintenance of cholesterol and bone density (Li et al., 2008; Marino et al., 2001). Administration of an ER antagonist, such as tamoxifen, can attenuate these effects, demonstrating that some of estrogen’s trophic actions are through ERs (Russo and Russo, 1998; White, 1999). Estrogen has a high affinity for ERα and ERβ (Kuiper et al., 1997), but estrogen’s actions at these substrates may exert different trophic actions. For example, actions at ERα have been linked to proliferative effects in breast, uterus, and/or ovaries (Harris et al., 2002; Koehler et al., 2005; Leygue et al., 1998; Ostlund et al., 2003; Rhodes and Frye, 2006; Walf and Frye, 2005b), whereas actions at ERβ can be antiproliferative in breast and prostate cancer (Acconcia et al., 2005;Mazzucco et al., 2008; Ostlund et al., 2003; Pravettoni et al., 2007; Sotoca et al., 2008; Walf and Frye, 2005b). Many isoflavones bind preferentially to ERβ in competitive binding analyses (Kuiper et al., 1998; Mueller et al., 2004). Both genistin and diadzin, the primary isoflavones in soy and red clover (Fang et al., 2004), bind with greater preferential affinity for ERβ, than does estrogen (Kuiper et al., 1998). Isoflavones, and other ERβ ligands, can inhibit tumor development among rodents administered carcinogens (Jensen et al., 2010; Onozawa et al., 1999; Walf and Frye, 2010). Indeed, isoflavones are potential treatments for cancer (de Souza et al., 2010). Thus, isoflavones may have beneficial effects in peripheral reproductive tissues, in part through actions at ERβ.
In addition to trophic effects in peripheral reproductive tissues, actions at ERs may mediate some pleiotropic effects in the brain. ERα and ERβ are differentially expressed in brain. ERα is greater in the hypothalamus of human brains (Koehler et al., 2005; Leygue et al., 1998; Ostlund et al., 2003). ERβ is more abundant in the hippocampus and cortex (Acconcia et al., 2005; Milner et al., 2005, 2010; Ostlund et al., 2003), brain regions involved in anxiety and cognitive behavior (Engin and Treit, 2007; Save and Poucet, 2009). Estrogen and ERβ agonists reduce anxiety behavior and enhance cognitive performance among rodents unless ERβ is knocked down (Rhodes and Frye, 2006; Walf and Frye, 2005b, 2006, 2008b; Walf et al., 2008). Given that actions at ERβ in the brain and body may be beneficial and isoflavones can have effects through ERβ, we sought to assess both central and peripheral actions of an isoflavone-based supplement.
Evidence suggests that steroidogenesis may underlie some effects of isoflavones. In vivo and in vitro data support the effect of isoflavones on steroidogenesis through several mechanisms, including modulating levels of testosterone, estrogen, and dihydrotestosterone (DHT), and interacting with aromatase, 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-HSD, and 5α-reductase enzymes, which converts their respective prohormones to metabolites (Almstrup et al., 2002; Brueggemeier et al., 2001a,b; Handa et al., 2008; Hiipakka et al., 2002; Lacey et al., 2005; Laurenzana et al., 2002; Le Bail et al., 2000; Thomas et al., 2004; Whitehead and Rice, 2006; Yi et al., 2002). Thus, we investigated effects of isoflavones on levels of steroid hormone and their metabolites peripherally and in brain.
This study investigated central and peripheral effects of isoflavones. A commercially-available, isoflavone-based, dietary supplement was administered to male and female, intact and gonadectomized rats (as gonadal steroids and/or sex differences may be important for some functional effects of isoflavones). The effects on anxiety behavior, spatial function, and steroid biosynthesis in brain were assessed as indices of central trophic effects. The effects on sexual behavior, reproductive tissue growth, and circulating hormones were considered for consequences on peripheral reproductive parameters. We hypothesized that administration of an isoflavone supplement, compared to placebo vehicle control, would alter measures of affect, cognition, sexual behavior, and/or reproductive tissue mass, and steroid levels, in a sex-dependent and/or gonad-dependent manner.
2. Results
Affective, cognitive and sexual behavior was evaluated in proestrous, gonadally-intact male, ovariectomized or castrated rats that have received subscapular implants of a dietary supplement or placebo, two to four weeks prior. Following behavioral evaluation, peripheral tissues were collected and weighed to evaluate trophic effects. Levels of progesterone, testosterone and their 5α-reduced metabolites 3α,5α-THP and 3α-diol were measured in plasma, midbrain, hypothalamus, hippocampus, cortex and cerebellum. The findings, described below in detail, were that the supplement increased anti-anxiety behavior in the elevated plus maze and spatial performance in the Morris water maze. The supplement increased levels of 3α-diol in the hippocampus, but not other regions. There were neither effects on sexual behavior, nor evidence for trophic effects on reproductive tissues of the supplement.
2.1. Among intact, but not gonadectomized rats, supplement increased percentage of time spent on open arms of the elevated plus-maze
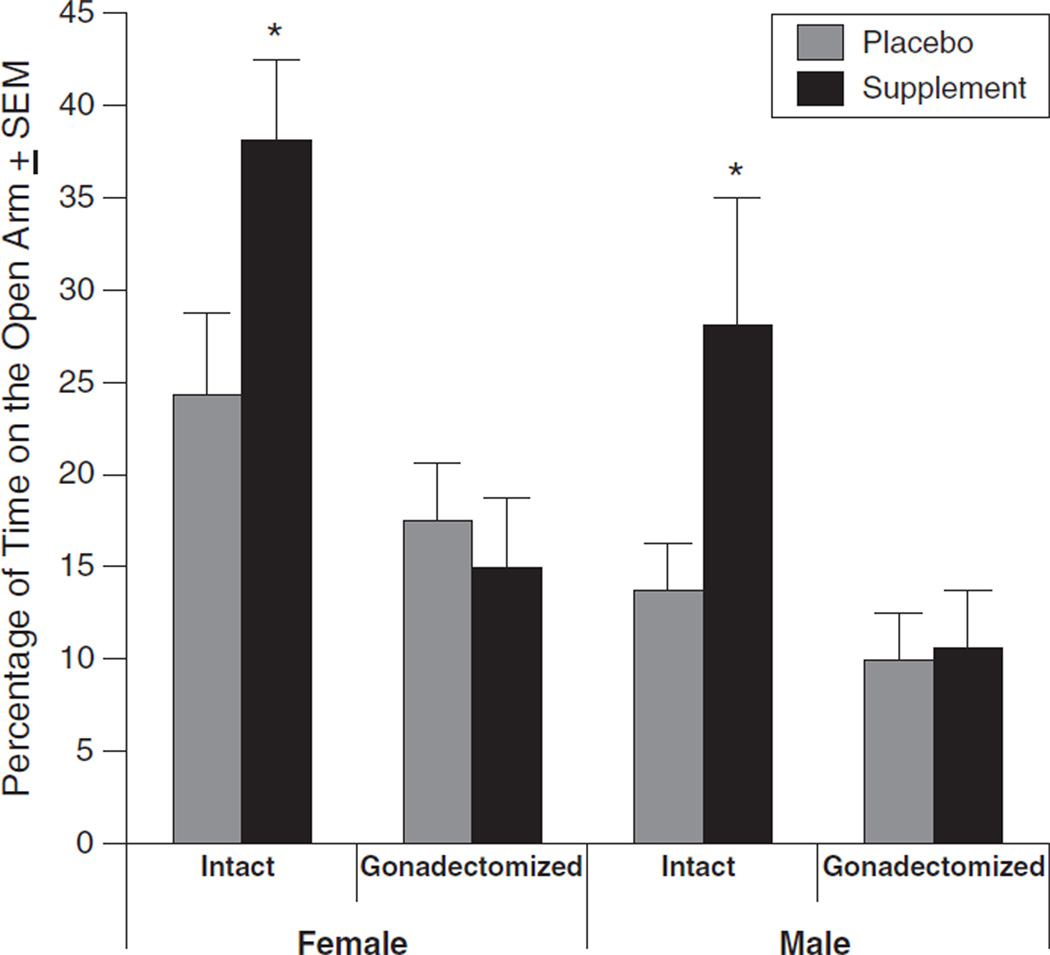
Percentage of time spent on the open arms (mean±SEM) in the elevated plus-maze was altered by sex [F(1, 84)=7.2, p<0.05], endogenous hormonal status [F(1, 84)=19, p<0.05], and supplement condition [F(1, 84)=5.3, p<0.05]. Female rats spent a greater percentage of time on the open arms (24±2%) than did male rate (16±2%). Gonadally-intact rats spent a greater percentage of time on the open arms (26±32%) than did gonadectomized rats (13±2%). Rats that received the supplement spent a greater percentage of time on the open arms (24±3%) than did those that received placebos (26±2%).
Gonadal status and supplement condition significantly interacted to influence percentage of time spent on the open arms [F(1, 84)=6.7, p<0.05]. Intact rats with supplement spent a greater percentage of time on the open arms (33±4%) compared to intact placebos (19±3%) or gonadectomized animals irrespective of supplement condition (supplement: 13±3%; placebo: 14±2%; see Fig. 1).
Fig. 1.
The percentage (mean±SEM) of time(during 300 s task) spent on the open arms of the elevated plus-maze for intact and gonadectomized, female and male rats administered placebo (grey) or supplement (black). A * indicates a significant interaction of gonadal status and supplement condition to be greater for marked groups (p<0.05).
2.2. Among intact>gonadectomized rats, supplement decreased latencies to the hidden platform in the water-maze
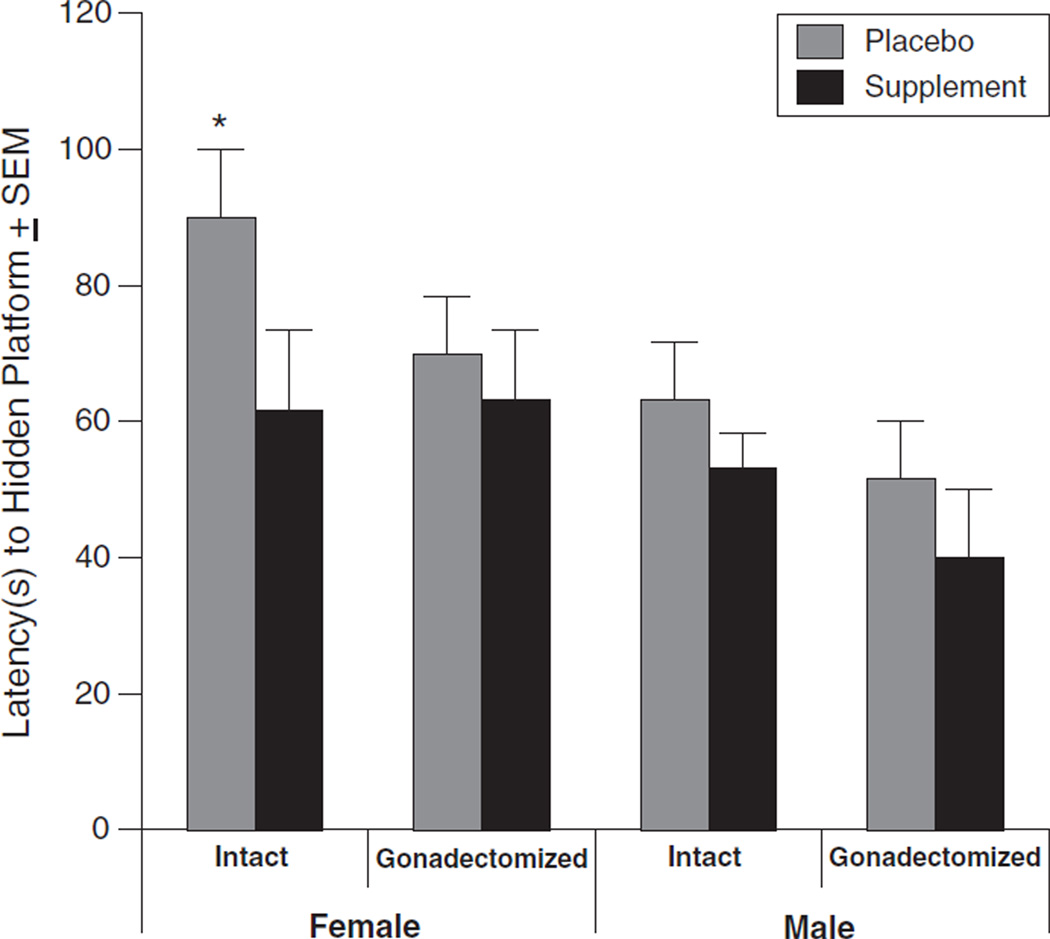
Supplement condition [F(1, 84)=5.6, p<0.05] and sex [F(1, 84)=10, p<0.05] influenced mean latency to the hidden platform in the water-maze. Rats that received supplement (57±4 s) performed better than did those that received placebo (73±5 s). Female rats (73 ± 5) were slower to find the hidden platform than were male rats (55±4 s). Proestrous females had longer latencies to the hidden platform, and showed the greatest improvement, than did rats in all other conditions [t(23)=−2.7, p<0.05; see Fig. 2].
Fig. 2.
The mean (± SEM) latency (s) to the hidden platform in the water-maze for intact and gonadectomized, female and male rats administered placebo (grey) or supplement (black). A * indicates a significant difference for the marked group to be greater compared to all other groups (p<0.05).
2.3. Gonadal status, but not supplement, influence sexual behavior and accessory structures of female rats
Gonadal status, but not supplement, influenced latency to first mount or intromission [F(1, 40)=84, p<0.05], lordosis rating [F(1, 40)=38, p<0.05], lordosis quotient [F(1, 40)=47, p<0.05], proceptive quotient [F(1, 40)=25, p<0.05], aggressive quotient [F(1, 40)=5.1, p<0.05], and percentage of exits [F(1, 40)=25, p<0.05]. Intact females had shorter latencies to receive mounts or intromission, greater lordosis quotient, lordosis rating, and proceptivity quotient, lower aggression quotient, and were made more exits following a sexual contact than did ovariectomized rats (see Table 1).
Table 1.
Sexual behavior. For females, latency to first mount or intromission, lordosis rating, lordosis quotient, proceptive quotient, aggressive quotient, and percentage of exits in the paced mating chamber. Intact females had a reduced latency to first mount or intromission, and higher lordosis rating, lordosis quotient, proceptivity quotient, aggression quotient, and percentage of exits compared to gonadectomized females. For males, latency to mount, intromission, and total number of sexual contacts in standard sex testing. Intact males had a reduced latency to mount and intromission, and a greater number of sexual contacts than did gonadectomized males. Data are represented as mean ± SEM.
| Sexual Behavior of Females | ||||
| Intact | Gonadectomized | |||
| Placebo (n=12) | Supplement (n=11) | Placebo (n=11) | Supplement (n=11) | |
| Contact latency (s) | 160.0±67.0 | 168.0±68.0 | 600.0±0.0 | 600.0±0.0 |
| Lordosis rating (score 0–3) | 2.2±0.4 | 1.6±0.4 | 0.0±0.0 | 0.0±0.0 |
| Lordosis quotient (%) | 85.0±17.0 | 78.0±17.0 | 0.0±0.0 | 0.0±0.0 |
| Proceptivity quotient (%) | 36.0±9.8 | 35.0±10.0 | 0.0±0.0 | 0.0±0.0 |
| Aggressive quotient (%) | 5.6±1.8 | 18.5±10.5 | 0.0±0.0 | 0.0±0.0 |
| Percent exits (%) | 39.0±9.1 | 24.0±8.7 | 0.0±0.0 | 0.0±0.0 |
| Sexual Behavior of males | ||||
| Intact | Gonadectomized | |||
| Placebo (n=13) | Supplement (n=13) | Placebo (n=11) | Supplement (n=10) | |
| Mount latency (s) | 540.0±91.0 | 640.0±86.0 | 900.0±0.0 | 900.0±0.0 |
| Intromission latency (s) | 490.0±40.0 | 460.0±63.0 | 0.0±0.0 | 0.0±0.0 |
| Total sexual contacts (#) | 1.6±0.5 | 2.7±1.6 | 0.0±0.00 | 0.0±0.00 |
Gonadal status, but not supplement, influenced uterine weight [F(1, 40)=76, p<0.05; see Table 2].
Table 2.
Reproductive tissues. For females, mass of the uterus was less in gonadectomized animals, and neither uterus, nor ovary mass, was different due to supplement. For males, mass of the prostate was less in gonadectomized animals, and neither prostate nor testicle mass were different due to supplement. Data are represented as mean ± SEM.
| Female | ||||
| Intact | Gonadectomized | |||
| Placebo (n=12) | Supplement (n=11) | Placebo (n=11) | Supplement (n=11) | |
| Uterus mass (mg) | 162±11 | 168±11 | 120±10 | 120±10 |
| Ovaries mass (mg) | 190±80 | 130±15 | – | – |
| Male | ||||
| Intact | Gonadectomized | |||
| Placebo (n=13) | Supplement (n=13) | Placebo (n=11) | Supplement (n=10) | |
| Prostate Mass (mg) | 290±020 | 260±20 | 170±30 | 130±20 |
| Testicles Mass (mg) | 2900±170 | 2900±130 | – | – |
2.4. Gonadal status, but not supplement, altered sexual behavior, not accessory structures, of male rats
Gonadal status, but not supplement, influenced latency mount [F(1, 43)=72, p<0.05], intromission [F(1, 43)=130, p<0.05], and total number of sexual contacts [F(1, 43)=5.3, p<0.05; see Table 1]. Intact males had lower latencies to mount and intromission, and a greater number of sexual contacts, than did gonadectomized males (see Table 1).
Gonadal status, but not supplement, influenced prostate mass [F(1, 43)=25, p<0.05; see Table 2].
2.5. Radioimmunoassay-supplement alters steroid levels selectively in hippocampus
2.5.1. Sex and gonadal status, but not supplement, influenced plasma levels of steroids
Sex significantly influenced circulating progesterone [F(1, 84)=74, p<0.05], 3α,5α-THP [F(1, 84)=250, p<0.05], corticosterone [F(1, 84)=4.0, p<0.05], testosterone: [F(1, 84)=33, p<0.05] and 3α-diol levels [F(1, 84)=5.2, p<0.05]. Female rats had significantly higher circulating concentrations (M±SEM) of progesterone (females 43±3.0; males 15±4 ng/ml), 3α,5α-THP (females 13±1; males 3±1 ng/ml), and corticosterone (females 2±1; males 1±1 µg/dl), than did males. Males rats had significantly greater testosterone (females 4±1; males 10±1 ng/ml), and 3α-diol (females 2±1; males 3±1 ng/ml) levels compared to females.
Gonadal status significantly influenced progesterone [F(1, 84)=7.5, p<0.05], 3α,5α-THP [F(1, 84)=4.1, p<0.05], corticosterone [F(1, 84)=7.1, p<0.05] and testosterone [F(1, 84)=54, p<0.05] levels. Intact rats had higher circulating concentrations of progesterone (intact 31±3; extirpated 23±4 ng/ml), 3α,5α-THP (intact 8±1; extirpated 7±1 ng/ml), corticosterone (intact 2±1; extirpated 1±1 µg/dl), and testosterone (intact 4±1; extirpated 11±2 ng/ml) than did extirpated rats.
Supplement condition had no effect on plasma steroid levels.
2.5.2. Midbrain. Sex and gonadal status, but not supplement, influenced midbrain steroid levels
Sex significantly influenced midbrain testosterone levels [F(1, 84)=31, p<0.05]. Male rats had significantly higher circulating concentrations of testosterone (2.1±0.6 ng/g) than did females 0.5±0.1 ng/g).
Gonadal status significantly influenced progesterone [F(1, 84)=6.9, p<0.05], 3α,5α-THP [F(1, 84)=8.3, p<0.05], and 3α-diol [F(1, 84)=11, p<0.05] levels. Intact rats had higher midbrain concentrations of progesterone (intact 1.0±0.5; extirpated 0.5±0.3 ng/g), 3α,5α-THP (intact 2.5±0.7; extirpated 1.3±0.5 ng/g), and 3α-diol (intact 0.8±0.3; extirpated 0.5±0.3 ng/g) than did gonadectomized rats.
Supplement condition had no effect on midbrain steroid levels.
2.5.3. Hypothalamus. Sex, gonadal status, not supplement, altered hypothalamic steroid levels
Sex significantly influenced hypothalamic testosterone levels [F(1, 84)=83, p<0.05]. Male rats had significantly higher hypothalamic concentrations of testosterone (1.1±0.3 ng/g) than did females (0.3±0.1 ng/g).
Gonadal status significantly influenced progesterone [F(1, 84)=7.9, p<0.05] and 3α,5α-THP [F(1, 84)=12, p<0.05] levels in the hypothalamus. Intact rats had higher hypothalamic concentrations of progesterone (intact 0.4±0.1; extirpated 0.1±0.1 ng/g) and 3α,5α-THP (intact 1.4±0.2; extirpated 0.6±0.2 ng/g) than did extirpated rats.
Supplement condition had no effect on hypothalamic steroid levels.
2.5.4. Hippocampus. Sex, gonadal status, and supplement, altered hippocampal steroid levels
Sex significantly influenced hippocampal 3α,5α-THP [F(1, 84)=14, p<0.05], testosterone [F(1, 84)=9.6, p<0.05], and 3α-diol [F(1, 84)=26, p<0.05] levels. Male rats had significantly lower hippocampal concentrations of 3α,5α-THP (8.1±1.5 ng/g) than did females (15.5±3.1 ng/g). Levels of testosterone among males (5.2±1.1 ng/g) were greater than for females (2.7±1.6 ng/g). Hippocampal 3α-diol levels were higher among males (3.5±0.6 ng/g) than females (1.4±0.2 ng/g).
Gonadal status effected progesterone [F(1, 84)=9.2, p<0.05], 3α,5α-THP [F(1, 84)=6.1, p<0.05], testosterone [F(1, 84)=4.6, p<0.05], and 3α-diol [F(1, 84)=10, p<0.05] levels in the hippocampus. Intact rats had higher hippocampal concentrations of progesterone (intact 5.1±011; extirpated 1.1±0.4 ng/g), 3α,5α-THP (intact 12.3±1.2; extirpated 8.5±1.1 ng/g), testosterone (intact 5.4±1.1; extirpated 2.3±1.0 ng/g), and 3α-diol (intact 2.5±0.7; extirpated 1.6±0.6 ng/g), than did extirpated rats.
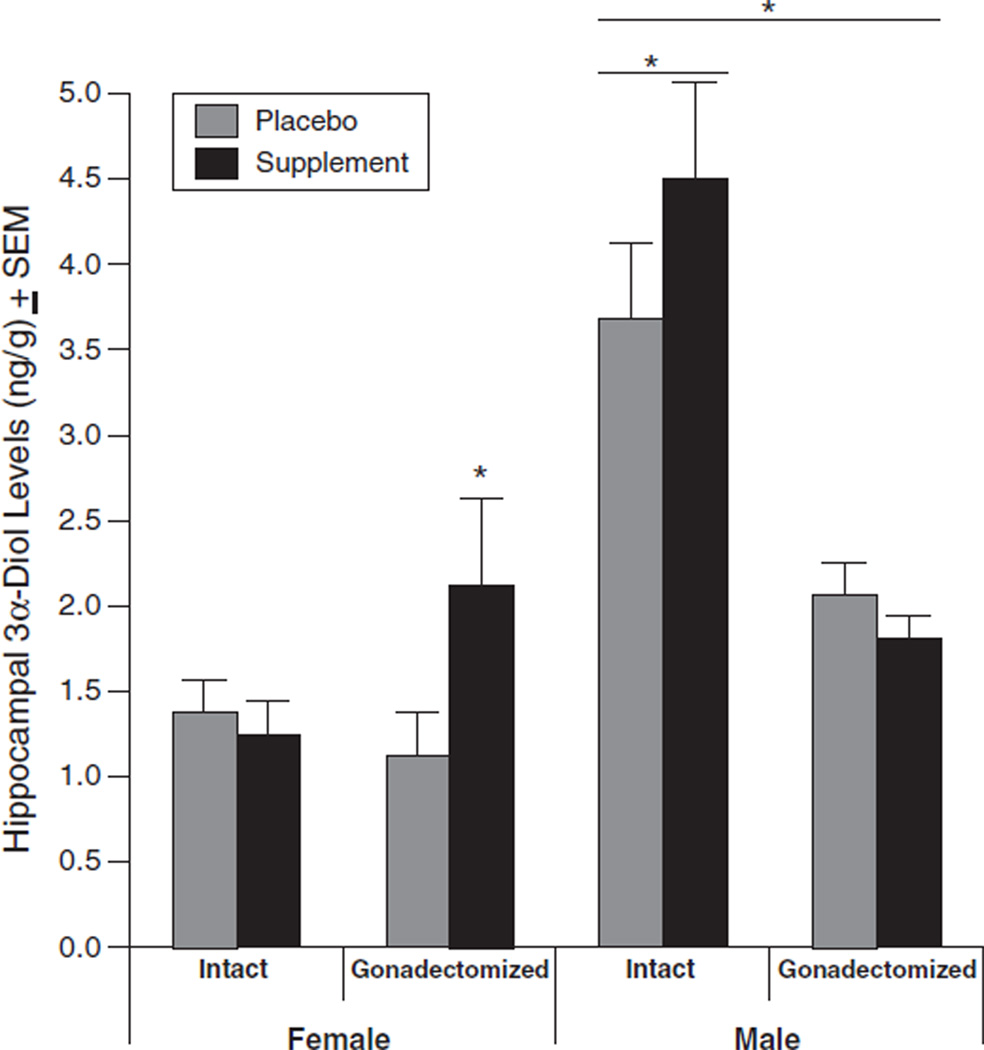
Sex, endogenous hormonal status, and supplement condition interacted to alter hippocampal 3α-diol levels [F(1, 84)=4.1, p<0.05; see Fig. 3]. Supplement had effects contingent upon gonadal status in female and male rats. Gonadectomized female animals that received supplement had higher levels of 3α-diol in the hippocampus compared to gonadectomized females that received placebo or intact females regardless of supplement condition. Whereas intact male rats had higher levels of 3α-diol in the hippocampus than did castrated male rats, an effect that was amplified by the supplement.
Fig. 3.
Levels of 3α-Androstanediol (mean±SEM) in the hippocampus of intact and gonadectomized, female and male rats administered placebo (grey) or supplement (black). A * indicates a significant interaction of sex, gonadal status and supplement condition to be greater for marked groups (p<0.05).
2.5.5. Cortex. Sex and gonadal status, but not supplement, altered cortical steroid levels
Sex significantly influenced cortical 3α,5α-THP [F(1, 84)=7.6, p<0.05], testosterone [F(1, 84)=170, p<0.05], and 3α-diol [F(1, 84) =8.4, p<0.05] levels. Female rats had significantly higher cortical concentrations of 3α,5α-THP (10.1±1.3 ng/g) than did males (5.5±1.3 ng/g). Levels of cortical testosterone among males (4.5 ± 1.1 ng/g) were greater than for females (1.3±0.6 ng/g). Cortical 3α-diol levels were higher among males (1.8±0.4 ng/g) than females (1.2±0.2 ng/g).
Gonadal status effected 3α,5α-THP [F(1, 84)=8.4, p<0.05] levels in the cortex. Intact rats had higher cortical concentrations of 3α,5α-THP (intact 11.3±1.4; extirpated 5.8±1.8 ng/g than did extirpated rats.
Supplement condition had no effect on cortex steroid levels.
2.5.6. Cerebellum sex and gonadal status, but not supplement, altered cerebellar steroid levels
Sex significantly influenced cerebellar 3α,5α-THP F(1, 84)=5.8, p<0.05, testosterone F(1, 84)=31, p<0.05, and3α-diol F(1, 84)=4.6, p<0.05 levels. Female rats had significantly higher cerebellar concentrations of 3α,5α-THP (21.1±3.4 ng/g) than did males (14.5±1.3 ng/g). Levels of cerebellar testosterone among males (10.2±21.1 ng/g) were greater than for females (4.2±1.1 ng/g). Cortical 3α-diol levels were higher among males (2.0±0.6 ng/g) than females (1.2±0.2 ng/g).
Gonadal status effected progesterone [F(1, 84)=4.5, p<0.05] levels in the cortex. Intact rats had higher cerebellar concentrations of progesterone (intact 4.9±2.1; extirpated 2.4±0.9 ng/g than did extirpated rats.
Supplement condition had no effect on cortex steroid levels.
3. Discussion
These data support the idea that an isoflavone supplement can improve anxiety-like and cognitive behavior, with an absence of deleterious effects on sexual behavior or reproductive tissue mass, albeit sex and endogenous hormonal status may be important for these effects. Intact rats that received the supplement had reduced anxiety-like behavior compared to intact controls, and this effect was not seen in gonadectomized animals. The supplement also enhanced measures of visual–spatial memory. This effect was most evident among intact females. When administered placebo, intact, proestrous female rats had the longest latencies to find the hidden platform compared to all other groups. We have previously shown that proestrous rats perform more poorly in the water maze than do diestrous or male rats (Frye, 1995). Notably, supplement administration improved performance in the water maze of all rats, but this effect was most evident in the proestrous rats. Furthermore, the supplement did not alter measures of sexual behavior, reproductive tissue mass, or plasma steroid levels. For brain steroid measures, the level of 3α-diol in the hippocampus was affected by the supplement. Ovariectomized rats that received the supplement had higher levels of hippocampal 3α-diol than did ovariectomized rats administered vehicle; however, the supplement did not alter hippocampal 3α-diol levels among intact female rats, intact male rats, or gonadectomized rats.
3.1. Anxiety behavior
The supplement’s effect on anxiety-like behavior confirms and extends previous reports. Intact animals that received supplement had reduced anxiety-like behavior in the elevated plus-maze, while gonadectomized animals did not have increased anti-anxiety behavior. Previous studies revealed anti-anxiety-like effects with soy consumption for intact animals of both sexes (Lund and Lephart, 2001; Patisaul et al., 2005). Although in one study anxiety-like behavior for intact males in the elevated plus-maze were greater with a low-dose, soy diet (Patisaul et al., 2005) another study that used a higher dose shows anti-anxiety-like effects in intact male animals (Lund and Lephart, 2001). Thus, our finding, that an isoflavone supplement exerted anti-anxiety-like effects in male and female rats, extends the previous reports that have demonstrated different effects on anxiety of isoflavone-based dietary interventions.
In the elevated plus maze, gonadectomy abrogated the anti-anxiety-like effect of the supplement. The gonads are the primary source of steroid hormones for intact animals, and behavioral tasks that measure anxiety-like behavior are sensitive to endogenous hormone levels that are modified by gonadectomy. The decline in steroid hormones due to gonadectomy in males (androgens) and females (estrogens, progestogens), and the natural fluctuation in the estrogen cycle of female animals can influence anxiety-like behavior (Frye, 2001; Walf and Frye, 2006, 2008a). However, plasma steroid levels did not differ between supplement and placebo groups. One explanation for gonadectomy attenuating anti-anxiety effects of the supplement irrespective of steroid hormone levels may be that isoflavones modulate factors that can alter steroid sensitivity or availability.
3.2. Visual–spatial memory
The present study found a significant effect of the isoflavone supplement to enhance spatial cognitive performance in the water-maze task. The supplement’s effect on visual–spatial memory in this experiment confirms others’ reports for intact males and females. Previous studies show an enhancement to cognitive performance in the radial arm-maze task for intact females fed isoflavones (Lund et al., 2001). Intact male rats fed a long-term diet enriched with a high-dose of isoflavones resulted in no effect on visual–spatial memory, while a low-dose diet resulted in enhancement to visual–spatial memory (Lee et al., 2004). Thus, isoflavones effects on visual–spatial performance may be dose-dependent, and our data are in concordance with studies that use higher doses of isoflavones in the diet.
Contrary to the present data, two studies found cognitive enhancing effects in the radial arm-maze and water-maze for ovariectomized female rats given isoflavones (Monteiro et al., 2008; Pan et al., 2000). However, both of these studies used soy protein isolate (SPI) in the experimental animal diets to investigate the effects of isoflavones. Administration of SPI, rather thanpurified isoflavones, may result in behavioral effects that are not due to isoflavones, but rather other constituents found in SPI (Kang et al., 2010). While SPI is rich in isoflavones genistein and daidzein, it also contains 134 other chemicals with mostly unknown metabolic actions (Fang et al., 2004). Indeed, SPI has been shown to have effects dissimilar to pure isoflavone extracts. A human study found that SPI, regardless of isoflavone content, lowered serum levels of DHT, DHT/testosterone, and testosterone, and that SPI with little-to-no isoflavone present also increased levels of estrogen and dehydroepiandrosterone sulfate (Dillingham et al., 2005). SPI stripped of isoflavones can induce cytochrome P450 enzymes CYP3A1 and CYP3A2, which are important for the biosynthesis and metabolism of steroid hormones (Ronis et al., 2006). Meanwhile, the current experiment used a supplement containing pure soy isoflavone extracts devoid of SPI; as such, differences in effects between studies among gonadectomized rats may be due in part to SPIs, and not isoflavones.
3.3. Sexual behavior
These data confirm and extend previous findings. Intact males fed soy for 40 days did not have different sexual behavior compared to that of rats fed soy-free diet (Cicero et al., 2004). Another study assessing the effects of the isoflavone, genistein, on sexual behavior of ovariectomized rats also found no differences between genistein and control (Patisaul et al., 2002). One study on ovariectomized rats sexual behavior using soy protein isolate (SPI) revealed negative effects on sexual behavior due to SPI in hormone-primed rats compared to SPI-free controls (Patisaul et al., 2001, 2004). These negative effects of isoflavones on sexual behavior may be due to properties that are unique to SPI and not specifically to isoflavones.
3.4. Tissue mass
The supplement in the present experiment also had no trophic effect on reproductive tissue mass. This is commensurate with previous research that suggests isoflavones do not increase risk of reproductive cancers. A majority of data supporting isoflavones as having antiproliferative effects comes fromin vitro and animal models (Banerjee et al., 2008; Sarkar et al., 2010). In vitro studies reveal that isoflavones may act through several mechanisms to halt cell cycle progression or induce apoptosis of cancer cell lines (Davis et al., 1998; Li et al., 1999; Matsukawa et al., 1993; Peterson and Barnes, 1993; Sarkar et al., 2010). Animal models also show that isoflavones are preventative against reproductive cancers. Compared to isoflavone-free controls, isoflavone fed rats show a reduced incidence of chemically induced ovarian, breast, uterine, and prostate cancers (Cabanes et al., 2004; Eason et al., 2005; Onozawa et al., 1999; Tanaka et al., 2002). Isoflavones bind and activate ERβ (Kuiper et al., 1998), in part through action involving the estrogen response element (Koohi et al., 2007). ERβ agonism may be a potential mechanism for antiproliferative effects of isoflavones in these tissues, as estrogen can also induce apoptosis to cancer cell lines through similar activation of ERβ (Acconcia et al., 2005). As such, we are presently investigating isoflavones in ERβ knock-out mice and their wildtype counterparts.
3.5. Endocrine measures
The present experiment is the first to show that a supplement containing isoflavones alters levels of 3α-diol in the hippocampus, and does so in a sex- and gonad-dependent manner. Gonadectomized female animals that received supplement had higher levels of hippocampal 3α-diol than gonadectomized controls or intact females regardless of supplement condition. Furthermore, this effect was not seen in males. Previous reports indicate that isoflavones can inhibit 3β-HSD and 17β-HSD, two enzymes necessary for the irreversible reduction of DHT to 3β-diol (Lacey et al., 2005; Whitehead and Rice, 2006). By inhibiting this reaction, DHT may be forced to equilibrate and form additional 3α-diol in the hippocampus. While isoflavones may increase 3α-diol synthesis, the ability of isoflavones to bind to ERβ may block 3α-diol from exerting previously reported behavioral effects (Edinger and Frye, 2007; Osborne et al., 2009). Thus, elevated levels of 3α-diol in the hippocampus of gonadectomized females may represent one of the numerous endocrine disrupting effects reported with isoflavones. Yet the role 3α-diol plays in mediating the behavioral effects of isoflavones, and how this interacts with other possible mechanisms, requires further research.
3.6. Caveats, considerations, and conclusions
The present study was an empirical investigation of a commercially-available supplement that has the benefits of face validity, but is not without limitations. One criticism of this study is that the effects of a dietary supplement, which is isoflavone-based, but has other components, was investigated. Isoflavone supplements, infant-formulas, and diets rich in isoflavones, such as traditional Asian or vegetarian diets, all contain an array of isoflavones (Franke et al., 1995; Reinli and Block, 1996; Setchell et al., 1998). Indeed, in addition to some rats being administered a supplement composed of various isoflavones, all animals in this study consumed a Purina Rat Chow diet. This diet contains isoflavones, which may have influenced the outcome of these studies. Multiple studies show that human plasma, urine, and breast milk contain a variety of isoflavones as well, making the study of the combined effects of isoflavones an important focus for research. Another weakness of this study was that isoflavones were not measured in diet, blood, or brain. Indeed, it would have been better to ascertain the absorption rate(s) of the supplement to ensure that the circulating levels of isoflavones were increased above baseline concentrations. However, comparing the findings of this experiment to that of others’, the data appear to be in accordance with those studies that used high (but not supraphysiological) dose regime of isoflavones in their treatment, as seen by the reduced anxiety-like behavior and cognitive effects in intact males (Lee et al., 2004; Lund and Lephart, 2001). Indeed, supraphysiological levels of isoflavones increase ovary mass after four weeks of daily administration (McClain et al., 2006). Our data did not show any significant change in ovary mass. Despite these limitations, the present study demonstrates that isoflavone administration need not be via diet, nor long-term, to produce salient behavioral and neuroendocrine effects that are sex- and/or gonad-dependent.
The present study revealed that a commercially available, isoflavone-based supplement, selectively enhanced neurosteroidogenesis in the hippocampus and improved spatial performance of rats. These findings are relevant for the special “Window of Opportunity” concept regarding hormone-replacement therapies, as many people look to over-the-counter supplements as a means to manage physical, psychological, or cognitive changes they experience at midlife. In this study, supplements improved spatial performance of all rats, but most notably that of rats in proestrus. Whether differences in learning and memory may have been observed with other behavioral tasks, or if the rats were tested in other stages of the estrous cycle, is an intriguing possibility that was not addressed. We also saw that the supplement enhanced neurosteroidogenesis in the hippocampus of rats within weeks of extirpation. Whether the magnitude of the effects would have been different at later timepoints following ovariectomy/gonadectomy is an intriguing possibility to consider. Despite these limitations, there were beneficial effects of this commercially available, isoflavone-based, dietary supplement. The supplement reduced anxiety-like behavior, improved visual–spatial memory, and enhanced neurosteroidogenesis. Whether the latter mediates the former was not ascertained; however, these effects occurred independent of trophic effects on peripheral reproductive tissues. Concerns regarding susceptibility to cancer are the primary reasons that some individuals forego hormone replacement therapies. Thus, effects of an isoflavone based supplement to enhance spatial performance and selectively increase hippocampal steroidogenesis are of interest and worthy of further investigation.
4. Experimental procedures
These methods were approved by The Institutional Animal Care and Use Committee at the University of Albany—State University of New York.
4.1. Subjects and housing
Experimental rats were adult Long–Evans male and female rats (n=92), bred in the Laboratory Animal Care Facility at The University at Albany (original stock from Taconic Farms, Germantown, NY). At the start of the experiment, all rats were within a few days age of each other (50 days±4). The rats were group housed (3–4 per cage) in polycarbonate cages (45 cm × 24 cm × 21 cm), and kept in a room that was temperature controlled (21 °C±1) on a 12 h reverse light/dark cycle (8 am/pm switch). Rats had constant access to Purina Rat Chow and tap water.
4.2. Herbal supplement
A commercially-available herbal supplement, Women’s Iso-Formula (AmeriSciences; Houston, TX), was used for this experiment, as the manufacturer ensured quality control validation of the components in their product to ensure uniformity. The supplement contains a combination of β-sitosterol extract, black cohosh extract (root), soy isoflavone extract (bean), kudzu (root), and red clover extract (flower) totaling 470mg. The soy isoflavone extract used in this product contains ~40% total isoflavones with 19% being daidzein, 15% being genistein, 3% being daidzin, 2% being genestin, and 1% glycitein.
4.3. Surgery
Rats were gonadectomized, ovariectomized, or left intact under xylazine (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and ketamine (60 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia. In females, a small incision was made on the flank of the animal and then through the abdominal wall on each side. The ovary, oviduct, and top of the fallopian tube were ligated and removed, and the abdominal wall was sutured. In males, an incision was made in the scrotum. The testicles were ligated and removed, and the scrotum was sutured.
One week later, all subjects were assigned to two conditions (supplement or placebo) and surgically implanted with either one tablet of the supplement or a silastic capsule placebo of approximately the same size, subcutaneously. Implant surgery was performed under the same anesthesia as gonadectomy. In brief, an incision was made in the skin on the back. The supplement or placebo was inserted into the subscapular space. The skin was then sutured closed. Following all surgery, rats were monitored for a healthy recovery.
4.4. Behavioral testing
Four days post implant-surgery all rats underwent behavioral testing. The order of tasks was not counterbalanced because stressful tasks that may alter subsequent behavior (i.e. water maze may alter sex testing and elevated plus maze behavior) were carried out last. Behavioral data were collected by using an observer who was blind to the experimental condition of the rats, and with the ANY-Maze video-tracking system (Stoelting Co., Wheat Dale, IL, USA).
Estrous cycle phase was determined by daily examination of vaginal smear cytology (between 0700 and 0800 h), per previous methods (Cooke et al., 1999). All female rats were sex tested on proestrus, which is characterized by the presence of many nucleated epithelial cells, when estrogen levels are declining, but progestin levels are high (Feder, 1984; Frye and Bayon, 1999).
4.4.1. Elevated plus-maze
The elevated plus-maze was utilized as per previous methods (Walf and Frye, 2007). The maze consisted of four evenly sized arms (49 cm × 10 cm) arranged in a plus shape, and elevated 50 cm off the ground. Two of the arms are surrounded by 30 cm high walls, while the other two arms are left open and exposed. Rats were placed in the center of the maze and left to freely explore the novel environment for 5 min. The total percentage of time spent on either open arm is utilized as an index of decreased anxiety-like behavior.
4.4.2. Water-maze
A version of the Morris water-maze was used as a measure of spatial ability as per previous methods (Osborne et al., 2009). A pool (circumference 555 cm, 71 cm deep) of water (24 °C) was colored white with non-toxic tempura paint, and a hidden platform (clear plexiglass, 5.3 cm × 5.3 cm) was placed roughly 2.5 cm below the surface of the water, approximately 60 cm from the side of the pool. Habituation and training in the water maze occurred on day five of our testing regiment, following all previous testing for that day. Rats were given one minute to freely explore the maze without the hidden platform as habituation. Sixty seconds after habituation was completed, rats were trained by placing them in the pool with the platform, for two 120 s trials, with a 120 s latency between trials. If rats found the platform before the trial was complete, they were held on the platform for 45 s, and the trial was ended. If they did not find the platform in 120 s, rats were led to the platform and held there for 45 s, allowing time to take in visual cues from around the room as to the location of the platform. There were many visual cues in the room that could aid the rat in learning the position of the platform, including shelving, a computer, desk, door, and pipes. Testing was performed 24 h post training, and consisted of four 120 s trials, with 3 min between trials. Rats began the maze from different quadrants in the pool at the start of each trial to maximize exposure to spatial cues; the order of placement in quadrants was alternated to counter-balance for effects of starting placement. Average latency to finding the platform was recorded for the testing trials.
4.4.3. Standard sex testing
Standard sex testing was used for male rats as per previous methods (Frye, 2001). Male subjects were placed in a chamber (37.5 cm × 75 cm × 30 cm) along with a female hormonally primed stimulus rat. Rats were allowed to freely interact. The test lasted 10 min, or one ejaculatory series. Sex behaviors recorded were the latency to mounts and intromissions, and the total number of sexual contacts.
4.4.4. Paced mating
Paced mating was utilized as per previous methods (Frye et al., 2007). Paced mating was carried out in a chamber (37.5 cm × 75 cm × 30 cm) partitioned into two equal sections. The partition had a small hole (5 cm diameter) that the female rat could crawl through, but the stimulus male rat could not due to his larger size. Experimental females were vaginally-masked using a 1.5 cm × 1.5 cm square of masking tape (to prevent pregnancy), then placed in the partition opposite that of the male and left in the box for 15 min or until the male ejaculated. Measures recorded include: latency to first mount or intromission, the frequency and intensity of lordosis (quantified by rating of dorsiflexion on a scale of 0–3), as well as frequency of proceptive (hopping, darting, ear-wiggling) and aggressive (vocalizations, defensive posture) behavior. The number of times the female left the chamber containing the male following copulatory behavior (percent exits) was also recorded.
4.5. Tissue collection
Male and ovariectomized female subjects were killed the day after completion of behavioral testing. Intact females were killed 1–7 days after behavioral testing. Ovaries, uterus, dorsal and ventral prostate, and testes were collected. Total wet mass by weight of ovaries, uterus, prostate, and testes was determined. Whole brains and trunk blood were collected for later measurement of estrogen, testosterone, 3α-diol, progesterone, 3α,5α-THP, and corticosterone. Trunk blood was centrifuged at 3000g for 10min, plasma was stored in Eppendorfs at −80 °C. Brains were rapidly frozen on dry ice and stored at −80 °C prior to RIA.
4.6. Radioimmunoassay
4.6.1. Extraction and dissection
Standard steroid extraction and radioimmunoassay techniques used by our laboratory, and others, for measurement of these steroids in plasma were utilized (Frye et al., 2006; Walf and Frye, 2005a). Briefly, brains were thawed, and the midbrain, hypothalamus, hippocampus, cortex, and cerebellum were grossly dissected. Following dissection, steroids were extracted from the midbrain, hypothalamus, hippocampus, cortex, cerebellum, and serum as described below. Testosterone, 3α-diol, progesterone, 3α,5α-THP, and corticosterone were extracted from serum with ether. After snap-freezing twice, test tubes containing steroid and ether were evaporated to dryness in a speed drier. Samples were reconstituted in 500 µl assay buffer.
Testosterone, 3α-diol, progesterone, and 3α,5α-THP were extracted from brain tissues following homogenization with a glass/Teflon homogenizer in a PBS solution. Tissues were centrifuged at 3000g and the supernatant was chromatographed on Sepak-cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH). Solvents were removed using a speed drier. Samples were reconstituted in 500 µl assay buffer and standard curves were set up for each steroid. These procedures result in recovery of ~50–60% of steroids from original concentrations.
4.6.2. Standard curves
The range of the standard curves was 0–2000 pg/ml for testosterone and 3α-diol, 0–8000 pg/ml for progesterone and 3α,5α-THP, and 0–4 µg/dl corticosterone. Standards were added to assay buffer followed by addition of the appropriate antibody and 3H steroid.
4.6.3. Antibodies
The testosterone antibody (T3-125; Endocrine Sciences, Calabasas Hills, CA) typically binds between 60% and 65% of [3H] testosterone, and was used in a ratio of 1:20,000 dilution. These antibodies have modest cross reactivity with DHT and negligible binding to other androgens. The 3α-diol antibody (X-144;Dr. P.N. Rao, Southwest Foundation for Biomedical Research, San Antonio, TX), is highly specific for 3α-diol, typically binds ~96% of [3H] 3α-diol, and does not cross-react with other steroids. The progesterone antibody (P#337 from Dr G D Niswender, Colorado State University) typically binds between 30% and 50% of [3H]progesterone, and was used in a ratio of 1 : 30000 dilution. This P antibody has very low levels (<4%) of cross-reactivity with DHP and 3α,5α-THP (Niswender 1973). The 3α,5α-THP antibodies (#921412-5, Dr Robert Purdy, Veterans Medical Affairs, La Jolla, CA, USA) binds between 40%and 60%of [3H]3α,5α-THP, and was used in a ratio of 1 : 5000 dilution. The 3α,5α-THP antibody cross-reacts with 3α-hydroxypregn-4en-20-one (84%) and DHP (11%), and its β-isomer (7%), P (6%), and pregnenolone (<2%; Purdy et al. 1990; Finn and Gee 1994). The corticosterone antibody (Endocrine Sciences: #B3-163), used in a 1: 20,000 dilution, has little cross-reactivity with deoxycorticosterone (4%), but negligible (<1%) cross-reactivity with other steroid hormones, including cortisol, aldosterone, and P (McCormick et al., 2005).
4.6.4. Measurement
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000g and the supernatant was pipetted into a glass scintillation vial with 5ml scintillation cocktail. Sample tube concentrations were calculated using the logit–log method and interpolation of the standards with Assay Zap.
4.7. Statistical analyses
To determine the interactive effects of sex (male, female), endogenous hormonal status (intact, gonadectomized), and supplement condition (supplement, placebo) on measures of anxiety-like behavior, cognitive behavior, and endocrine measures, data were analyzed using three-way analyses of variance (ANOVAs). Two-way ANOVAs including endogenous hormonal status and supplement condition were used for measures of sexual behavior, as different testing paradigms were used for males and females in this measure. One and two-way ANOVAs of supplement condition, or of endogenous hormonal status and supplement condition, were used for analyses of tissue masses. Group differences were determined with Fisher’s PLSD comparisons when significant main or interactive effects were found (p<0.05). Descriptive data are presented as the mean (M)±standard error of the mean (SEM).
Acknowledgments
This research was supported in part by grants from The National Science Foundation (IBN98-96263, IBN03-16083), The National Institute of Mental Health (MH06769801) and the State University of New York at Albany. This work was in partial fulfillment of JAF’s undergraduate honor’s thesis. Although the authors have received travel support to present these research findings, the research was neither solicited nor supported from the company making the dietary supplement, and the authors have no commercial interest in this dietary supplement. The insight and support provided by Dr. Elizabeth Gath (Primary for Women Health, Albany, NY) in the development and implementation of these studies is greatly appreciated.
REFERENCES
- Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J. Cell. Physiol. 2005;203:193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H. Dual effects of phytoestrogens result in u-shaped dose–response curves. Environ. Health Perspect. 2002;110:743–748. doi: 10.1289/ehp.02110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli F, Ernst E. Alternative and complementary therapies for the menopause. Maturitas. 2010;66:333–343. doi: 10.1016/j.maturitas.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Gu X, Mobley JA, Joomprabutra S, Bhat AS, Whetstone JL. Effects of phytoestrogens and synthetic combinatorial libraries on aromatase, estrogen biosynthesis, and metabolism. Ann. NY Acad. Sci. 2001a;948:51–66. doi: 10.1111/j.1749-6632.2001.tb03986.x. [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Richards JA, Joomprabutra S, Bhat AS, Whetstone JL. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J. Steroid Biochem. Mol. Biol. 2001b;79:75–84. doi: 10.1016/s0960-0760(01)00127-3. [DOI] [PubMed] [Google Scholar]
- Cabanes A, Wang M, Olivo S, DeAssis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- Cano A, Garcia-Perez MA, Tarin JJ. Isoflavones and cardiovascular disease. Maturitas. 2010;67:219–226. doi: 10.1016/j.maturitas.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Faughnan M. Phyto-oestrogens through the life cycle. Proc. Nutr. Soc. 2000;59:489–496. doi: 10.1017/s0029665100000719. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Derosa G, Arletti R. Effect of oral chronic isoflavones supplementation on male rat sexual performances and sexual hormone plasma levels. Phytother. Res. 2004;18:849–852. doi: 10.1002/ptr.1561. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc. Natl Acad. Sci. USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr. Cancer. 1998;32:123–131. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- de Souza PL, Russell PJ, Kearsley JH, Howes LG. Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr. Rev. 2010;68:542–555. doi: 10.1111/j.1753-4887.2010.00314.x. [DOI] [PubMed] [Google Scholar]
- Dillingham BL, McVeigh BL, Lampe JW, Duncan AM. Soy protein isolates of varying isoflavone content exert minor effects on serum reproductive hormones in healthy young men. J. Nutr. 2005;135:584–591. doi: 10.1093/jn/135.3.584. [DOI] [PubMed] [Google Scholar]
- Eason RR, Till SR, Velarde MC, Geng Y, Chatman L, Jr, Gu L, Badger TM, Simmen FA, Simmen RC. Uterine phenotype of young adult rats exposed to dietary soy or genistein during development. J. Nutr. Biochem. 2005;16:625–632. doi: 10.1016/j.jnutbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol. Behav. 2007;92:443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav. Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J. Agric. Food Chem. 2004;52:4012–4020. doi: 10.1021/jf049842y. [DOI] [PubMed] [Google Scholar]
- Feder HH. Hormones and sexual behavior. Annu. Rev. Psychol. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J. Pharmacol. Exp. Ther. 1994;27:164–170. [PubMed] [Google Scholar]
- Fitzpatrick LA. Alternatives to estrogen. Med. Clin. North Am. 2003;87:1091–1113. doi: 10.1016/s0025-7125(03)00116-0. x. [DOI] [PubMed] [Google Scholar]
- Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc. Soc. Exp. Biol. Med. 1995;208:18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol. Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res. Brain Res. Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol. Biochem. Behav. 1999;62:315–321. doi: 10.1016/s0091-3057(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Zimmerberg B, Brunelli SA. Rats bred for high versus low anxiety responses neonatally demonstrate increases in lordosis, pacing behavior, and midbrain 3 alpha, 5 alpha-THP levels as adults. Behav. Neurosci. 2006;120:281–289. doi: 10.1037/0735-7044.120.2.281. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun IU, Adhikari K, Li C, Li Y, Lin B, Zhang J, Fernando LN. Changes in the profile of genistein, daidzein, and their conjugates during thermal processing of tofu. J. Agric. Food Chem. 2001;49:2839–2843. doi: 10.1021/jf010028+. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta, 17beta-diol. Horm. Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem. Pharmacol. 2002;63:1165–1176. doi: 10.1016/s0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol. Behav. 2010;99:151–162. doi: 10.1016/j.physbeh.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Badger TM, Ronis MJ, Wu X. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010;58:8119–8133. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr. Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- Koohi MK, Walther N, Ivelli R. A novel molecular assay to discriminate transcriptional effects caused by xenoestrogens. Mol. Cell. Endocrinol. 2007;276:45–54. doi: 10.1016/j.mce.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. Dose-response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J. Steroid Biochem. Mol. Biol. 2005;96:279–286. doi: 10.1016/j.jsbmb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Laurenzana EM, Weis CC, Bryant CW, Newbold R, Delclos KB. Effect of dietary administration of genistein, nonylphenol or ethinyl estradiol on hepatic testosterone metabolism, cytochrome P-450 enzymes, and estrogen receptor alpha expression. Food Chem. Toxicol. 2002;40:53–63. doi: 10.1016/s0278-6915(01)00095-3. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000;66:1281–1291. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- Lee YB, Lee HJ, Won MH, Hwang IK, Kang TC, Lee JY, Nam SY, Kim KS, Kim E, Cheon SH, Sohn HS. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J. Nutr. 2004;134:1827–1831. doi: 10.1093/jn/134.7.1827. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Rhees RW, Setchell KD, Bu LH, Lund TD. Estrogens and phytoestrogens: brain plasticity of sexually dimorphic brain volumes. J. Steroid Biochem. Mol. Biol. 2003;85:299–309. doi: 10.1016/s0960-0760(03)00210-3. [DOI] [PubMed] [Google Scholar]
- Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD001395.pub3. CD001395. [DOI] [PubMed] [Google Scholar]
- Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–519. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Limer JL, Speirs V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004;6:119–127. doi: 10.1186/bcr781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Distefano E, Pallottini V, Caporali S, Bruscalupi G, Trentalance A. Activation of IP(3)-protein kinase C-alpha signal transduction pathway precedes the changes of plasma cholesterol, hepatic lipid metabolism and induction of low-density lipoprotein receptor expression in 17-beta-oestradiol-treated rats. Exp. Physiol. 2001;86:39–45. doi: 10.1113/eph8602069. [DOI] [PubMed] [Google Scholar]
- Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Matsumoto K, Nishino H, Aoike A. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993;53:1328–1331. [PubMed] [Google Scholar]
- Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LA. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behav. Brain Res. 2008;191:111–117. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm. Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Michael McClain R, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem. Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, McEwen BS, Pfaff DW, Waters EM. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro SC, de Mattos CB, Ben J, Netto CA, Wyse AT. Ovariectomy impairs spatial memory: prevention and reversal by a soy isoflavone diet. Metab. Brain Dis. 2008;23:243–253. doi: 10.1007/s11011-008-9093-6. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Niswender GD. Influence of the site of conjugation on the specificity of antibodies to progesterone. Steroids. 1973;22:413–424. doi: 10.1016/0039-128x(73)90104-9. [DOI] [PubMed] [Google Scholar]
- Onozawa M, Kawamori T, Baba M, Fukuda K, Toda T, Sato H, Ohtani M, Akaza H, Sugimura T, Wakabayashi K. Effects of a soybean isoflavone mixture on carcinogenesis in prostate and seminal vesicles of F344 rats. Jpn J. Cancer Res. 1999;90:393–398. doi: 10.1111/j.1349-7006.1999.tb00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr) 2009;31:191–198. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. NY Acad. Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Watson S, Clarkson TB. Soy phytoestrogens improve radial arm maze performance in ovariectomized retired breeder rats and do not attenuate benefits of 17beta-estradiol treatment. Menopause. 2000;7:230–235. doi: 10.1097/00042192-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor alpha- and beta-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ER beta- but not ER alpha-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm. Behav. 2004;45:270–277. doi: 10.1016/j.yhbeh.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Blum A, Luskin JR, Wilson ME. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav. Neurosci. 2005;119:587–594. doi: 10.1037/0735-7044.119.2.587. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 2002;277:50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- Pravettoni A, Mornati O, Martini PG, Marino M, Colciago A, Celotti F, Motta M, Negri-Cesi P. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol. Cell. Endocrinol. 2007;263:46–54. doi: 10.1016/j.mce.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3 alpha-hydroxy-5 alpha-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Reinli K, Block G. Phytoestrogen content of foods—a compendium of literature values. Nutr. Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol. Learn. Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Chen Y, Badeaux J, Laurenzana E, Badger TM. Soy protein isolate induces CYP3A1 and CYP3A2 in prepubertal rats. Exp. Biol. Med. (Maywood) 2006;231:60–69. doi: 10.1177/153537020623100107. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J. Mammary Gland Biol. Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y, Wang Z, Kong D. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–394. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Poucet B. Role of the parietal cortex in long-term representation of spatial information in the rat. Neurobiol. Learn. Mem. 2009;91:172–178. doi: 10.1016/j.nlm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- Sotoca AM, van den Berg H, Vervoort J, van der Saag P, Strom A, Gustafsson JA, Rietjens I, Murk AJ. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol. Sci. 2008;105:303–311. doi: 10.1093/toxsci/kfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Tanino M, Yanaida Y. Inhibitory effects of estrogenic compounds, 4-nonylphenol and genistein, on 7, 12-dimethylbenz[a]anthracene-induced ovarian carcinogenesis in rats. Ecotoxicol. Environ. Saf. 2002;52:38–45. doi: 10.1006/eesa.2002.2159. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Duax WL, Addlagatta A, Scaccia LA, Frizzell KA, Carloni SB. Serine 124 completes the Tyr, Lys and Ser triad responsible for the catalysis of human type 1 3beta-hydroxysteroid dehydrogenase. J. Mol. Endocrinol. 2004;33:253–261. doi: 10.1677/jme.0.0330253. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Totta P, Acconcia F, Virgili F, Cassidy A, Weinberg PD, Rimbach G, Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J. Nutr. 2005;135:2687–2693. doi: 10.1093/jn/135.11.2687. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic–pituitary–adrenal axis activity. Neuropsychopharmacology. 2005a;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005b;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Parity and estrogen-administration alter affective behavior of ovariectomized rats. Physiol. Behav. 2008a;93:351–356. doi: 10.1016/j.physbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008b;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Raloxifene and/or estradiol decrease anxiety-like and depressive-like behavior, whereas only estradiol increases carcinogen-induced tumorigenesis and uterine proliferation among ovariectomized rats. Behav. Pharmacol. 2010;21:231–240. doi: 10.1097/fbp.0b013e32833a5cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol. Learn. Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IN. The tamoxifen dilemma. Carcinogenesis. 1999;20:1153–1160. doi: 10.1093/carcin/20.7.1153. [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Rice S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:45–61. doi: 10.1016/j.beem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Yi MA, Son HM, Lee JS, Kwon CS, Lim JK, Yeo YK, Park YS, Kim JS. Regulation of male sex hormone levels by soy isoflavones in rats. Nutr. Cancer. 2002;42:206–210. doi: 10.1207/S15327914NC422_9. [DOI] [PubMed] [Google Scholar]