Abstract
Background
Generalized Anxiety Disorder (GAD) in older adults is associated with neuropsychological impairment.
Aims
We examined neuropsychological functioning in older adults with GAD in comparison to psychiatrically healthy older adults and we examined changes during a 12-week, placebo controlled trial of escitalopram.
Method
One hundred-sixty non-demented participants aged ≥60 with current GAD and 37 comparison subjects without psychiatric history underwent neuropsychological assessment. One hundred twenty-nine GAD participants were re-assessed post-treatment.
Results
GAD participants performed worse than comparison subjects in information processing speed, working memory, inhibition, problem-solving (including concept formation and mental flexibility), and immediate and delayed memory. Neuropsychological functioning was correlated with everyday functioning. Low cognitive scorers experienced working memory, delayed memory and visuospatial ability improvement and those who reported clinical improvement in anxiety exhibited improvement in the ability to engage inhibition and episodic recall. These improvements were modest and of similar magnitude in both treatment conditions.
Conclusion
GAD in older adults is associated with neuropsychological impairments, which are associated with functional impairment. Those with GAD who either have low cognitive performance or report clinical improvement in anxiety post-treatment, show improvement in multiple cognitive domains. These findings underscore the importance of treatments that aid cognition as well as anxiety symptoms.
Keywords: generalized anxiety disorder, neuropsychological function
Introduction
Generalized Anxiety Disorder (GAD) is a serious and chronic illness, defined by excessive and difficult-to-control worry. Somatic and mental symptoms include poor concentration, restlessness; sleep disturbance, fatigue, and muscle tension. The prevalence of geriatric GAD is as high as 7.3% in community-dwelling older adults (1–3). Older adults with GAD worry about medical problems, family, and finances-typical concerns in this age group but greatly amplified in duration, severity, and distress (4). The disorder tends to be chronic in the absence of effective treatment, with average duration of 20 years or more (5–8) and strongly associated with functional disability (Porensky et al, 2009).
Most studies of cognitive function and anxiety in older adults examine community-based populations with symptomatic measures of anxiety and report that higher levels of anxiety symptoms are associated with poorer fluid intelligence (9), complex visuospatial skills (10), learning and memory (10–13), information processing speed (11, 14, 15), and executive functions (11, 13) including inhibition (14). By contrast, some community-based studies report no association between anxiety symptoms and cognitive function in late-life (16, 17). Three clinic-based studies have examined cognitive functioning in older adults who met specific clinical criteria for GAD. We recently reported that relative to comparison subjects, individuals with late-life GAD exhibited impairment on measures of immediate and delayed recall as well as mental set shifting (18). Caudle and colleagues (19) found that greater GAD symptom severity was associated with poorer working memory and Price and Mohlman (20) found an association with better inhibitory control (as measured with the Stroop).
The causal relationship between generalized anxiety and cognitive impairment is unclear. One prevalent model posits that worry is a cognitive process designed to avoid the anxiogenic images that induce somatic activation through increased noradrenergic discharge (9). However, it is not clear whether the affective (increased anxiety) or cognitive (avoidance) component of GAD plays a more prominent role in the interplay between GAD and cognitive impairment. Affective interference (e.g., selective processing of anxious information at the expense of other cognitive tasks (10)) seems to be related to a particular aspect of anxiety, namely ruminative worry. Rumination might mitigate the ability to shift resources between emotional and cognitive tasks, thus reducing task performance (11). In a recent report, our group has shown that depressed older adults with high, comorbid anxiety have elevated activation of several brain areas involved in cognitive performance, including dorsal prefrontal cortex and dorsal anterior cingulate cortex (12).
GAD may be particularly detrimental to cognitive function in older adults (13), who have less cognitive reserve against CNS insults than do younger adults. Cognitive reserve refers to the degree to which an individual is able to maintain cognitive function in the face of mounting neuropathology. Moreover, worry may compete for cognitive resources necessary for working memory thereby interfering with execution of some cognitive functions (14). Additionally, aging increases vulnerability to cognitive impairment because homeostatic mechanisms that prevent an excessive biological stress response are diminished (7, 15). Consequently, some deleterious effects of excessive stress response – such as neurotoxic hypercortisolemia – worsen with age. Despite these threats to cognition, few studies have examined neuropsychological functioning in late-life anxiety disorders, and there are very few reports on late-life GAD in particular.
While observational studies provide some support for a cross-sectional association between anxiety and cognitive impairment in older adults, better-designed cross-sectional and longitudinal studies may be the more appropriate approach. Experimental research designs that involve manipulating anxiety levels, rather than simply assessing them repeatedly, could also provide informative data about the possible causal impact of anxiety on cognitive change.
To our knowledge, no single study has provided a comprehensive examination of neuropsychological functioning in late-life GAD. Moreover, there is very little available data on whether neuropsychological function changes with treatment. Thus, we carried out a neuropsychological evaluation pre- and post-treatment, in a large group of older adults with GAD in a randomized placebo-controlled trial of the SSRI escitalopram. We also compared GAD participants at baseline, with psychiatrically healthy older adults, equated on gender, race, years of education and medical burden. The goal of this study was two-fold: (1) to characterize neuropsychological function among a large group of older adults with a principal diagnosis of GAD and (2) to identify any cognitive changes related to treatment of anxiety. Based on the published literature (including our own work) with the rationale that worry takes up cognitive capacity and leaves less attentional resources for the tasks at hand, we hypothesized that GAD participants would perform worse than the comparison subjects on measures of attention (Digit Span), information processing speed (Coding), and executive functions including working memory (Letter-Number Sequencing) and problem-solving, conceptual ability, and mental flexibility (Sorting Test), as well as multiple measures of immediate and delayed recall; we also examined measures of visuospatial function and language, on which we did not expect to find differences. We also hypothesized that impairments would improve with successful treatment of anxiety and that there would be a significant relationship between disability and cognitive performance, particularly in the executive domain.
Method
Study Design
This was a National Institute of Mental Health-sponsored 12-week double-blind randomized controlled trial of escitalopram vs. placebo in older adults with a principal diagnosis of GAD, conducted in primary care practices and a specialty academic mental health center in Pittsburgh, Pennsylvania, from 2005–2008.
Subjects
This clinical trial recruited 177 individuals age 60 or older meeting DSM-IV criteria for GAD. We excluded a total of 17 participants, due to CNS disease (n=13), physical impairment that precluded neuropsychological assessment (n=3) or refusal to undergo neuropsychological assessment (n=1). Thus in the present analysis we examined 160 GAD participants whose mean age of onset was 39.6 (26.89) years with a mean duration of 32.01 (26.6) years. Participants were assessed at baseline (pre-treatment) and after the 12 week trial. For comparison, we also assessed 37 non-demented older adults without psychiatric history equated to the GAD participants on gender and race as well as years of education and medical (including vascular disease) burden with the same neuropsychological battery, at a single time point.
Details on subject recruitment, retention and evaluation have been described elsewhere (21, 22). During screening, all potential participants were evaluated by a board certified geriatric psychiatrist (EL). Psychiatric diagnosis was established with a Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID-IV; (23)) interview administered by formally trained master’s and doctoral degree level clinicians and a consensus diagnostic conference attended by the raters and at least two geriatric psychiatrists. Potential participants with an established diagnosis of dementia were excluded from the study; anyone with suspected dementia based on the screening evaluation was excluded. Moreover, the MMSE was administered in this study and all data on anyone with a score <26 were closely reviewed by a geriatric psychiatrist (EL).
Measures
Participants underwent a broad-based pre-treatment assessment that included clinical, psychosocial, and biologic measures (described previously, (21)) as well as neuropsychological assessment.
We used a brief but comprehensive battery of neuropsychological measures that is widely used with older adults. The neuropsychological measures included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); (24). Forms A and B were administered in a counterbalanced manner to minimize practice effects. The RBANS provides a Total Index Score and subscale Index Scores measuring Language (confrontation naming and category fluency), Visuospatial Construction Skills (figure copy and line orientation tasks), Attention (forward digit span and coding tasks), Immediate Memory (list and story recall), and Delayed Memory (list, story, and figure recall). Because the RBANS does not assess executive functioning and other higher level abilities such as working memory, we also administered the Delis-Kaplan Executive Function System (D-KEFS; (25) Sorting Test and used the confirmed correct sorts which measures problem-solving, conceptual ability, and mental flexibility. We also used the color-word interference score of the Stroop Neuropsychological Screening Test (26) to measure the ability to inhibit automatic responses and the Wechsler Adult Intelligence Scale-III (27) Letter Number Sequencing subtest as a measure of working memory.
Among individuals with anxiety disorders, depression and anxiety symptoms are confounded making it extremely difficult to tease the two apart. Moreover, many of the most widely used mood scales (e.g., the Hamilton Rating Scale for Depression (HRSD)) contain items that measure symptoms common to both depression and anxiety. In order to help tease apart the effects of depression vs. anxiety, we examined depressive symptoms with the least confounded measure, the HRSD core depression items (q1+q2+q3 +q7); (28).
We assessed disability with the Function and Disability Instrument (FDI) limitations and frequency subscales (29). These subscales measure a person’s self-reported performance of socially defined life tasks expected of an individual within a typical environment, along two domains: how much difficulty he or she has performing activities (activity limitations subscale) and how often they perform activities (frequency subscale). The limitation and frequency subscales of the FDI correspond to the disability domains of activity limitation and participation restriction, consistent with the International Classification of Function, Disability and Health (30). Both of these domains of function are impaired in late-life GAD (31).
Statistical Analysis
Prior to statistical analysis, we examined the data for normality and used transformations where necessary. If the distribution could not be normalized a non-parametric test was used. We generated descriptive statistics to characterize the GAD and comparison group on key demographic and clinical characteristics.
Neuropsychological functioning of GAD and control participants at baseline
To characterize neuropsychological function among older adults with a principal diagnosis of GAD, we compared neuropsychological functioning of the GAD and comparison groups at baseline using analysis of variance. Seven main neuropsychological measures were chosen a priori to represent the domains of attention (Digit Span forward), information processing speed (Coding), and the specific executive functions of inhibition (Stroop), working memory (Letter-Number Sequencing), and problem-solving, conceptual ability, and mental flexibility (Sorting Test), as well as immediate memory (RBANS Immediate Memory Index) and delayed memory (RBANS Delayed Memory Index). Age and education were used as covariates in these models except for those involving the RBANS Memory scores, because these scores are age-adjusted based on test norms. We also examined baseline differences in index scores of the remaining two RBANS domains, Visuospatial Construction and Language. In these models only education was used as a covariate since the derived scaled scores are adjusted for age. We report the results both with and without correction for multiple comparisons. GAD is frequently accompanied by depressive symptoms, so to examine the effect of depression on neuropsychological functioning without obscuring the effect of GAD, we re-ran the analyses, once excluding the subjects who met criteria for MDD (n=15) and once co-varying for MDD diagnosis.
Functional correlates of neuropsychological performance at baseline
Because group and depression scores are highly multicollinear and could not be entered into the same model, to further examine the potential influence of depression, we examined the correlations of HRSD total score and the core HRSD depression items score with the neuropsychological data. The correlations were not significant and therefore we did not control for either in the analyses comparing cognitive performance between the groups.
To examine the relationship between cognitive function and everyday functioning in late-life GAD, we calculated Spearman correlation coefficients between the nine neuropsychological variables and the FDI frequency and limitations subscales.
Comparing neuropsychological functioning pre- and post-treatment in GAD participants who (a) were low cognitive scorers, and (b) reported improvement in anxiety
Because cognitive function in cognitively normal individuals may not improve with efficacious treatment for anxiety, and to identify any cognitive changes related to treatment of anxiety, we compared neuropsychological functioning pre- and post-treatment in the low-scoring GAD treatment completers group using a repeated measures mixed effect model with random intercept and slope. We examined treatment (drug vs. placebo), time, and treatment-by-time interactions. A participant was considered a low cognitive scorer at baseline if he or she performed in the lower half of a median split of the GAD group’s RBANS Total Index scores.
We also examined neuropsychological change scores among GAD participants whose anxiety symptoms significantly improved, to see which, if any, neuropsychological measures also improved, using the Wilcoxon Signed Rank Exact test. A participant was considered “improved” if he or she reported having “much improved” or “very much improved” on the Clinical Global Impressions (CGI) (32) Scale (CGI score ≤ 2) post-treatment
Results
Descriptive analyses
GAD participants were younger than comparison subjects (71.6 (7.7) vs. 74.9 (6.2) years, p=0.012; see Table 1). Otherwise, the GAD and comparison subjects did not differ in terms of relevant demographic and clinical characteristics, including medical and vascular disease burden, as measured with the Cumulative Illness Rating Scale-Geriatrics (33).
Table 1.
Subjects: Descriptive Information
|
GAD N=160 |
Comparison Subjects N=37 |
Test Statistic | |
|---|---|---|---|
| Age1 (years) | 71.6 (7.7) | 74.9 (6.2) | t = −2.53, df=195, p=0.012 |
| % Female | 68.8 (n=110) | 67.6 (n=25) | χ2 = 0.02, df=1, p=0.89 |
| % Caucasian | 82.5 (n=132) | 91.9 (n=34) | χ2 = 2.00, df=1, p=0.16 |
| Education1 (years) | 13.9 (2.8) | 14.7 (3.0) | t = −1.48, df=195, p=0.14 |
| CIRS total | 8.5 (3.7) | 7.9 (3.1) | t = 0.85, df=195, p=0.40 |
| CIRS Heart + Vascular Scales | 2.3 (1.7) | 2.1 (1.3) | t = 0.70, df=195, p=0.48 |
| HRSA1 | 22.8 (4.5) | 4.8 (3.7) (n=36) | t = 19.89, df=40.4, p<0.0001* |
| PSWQ | 56.3 (12.6) (n=158) | 28.1 (6.8) (n=30) | t = 17.71, df=73.3, p<0.0001* |
| GAD Onset (years) | 39.6 (26.9) (n=159) | -- | -- |
| GAD Duration (years) | 32.01 (26.00) | -- | -- |
| %Co-morbid Anxiety Disorder | 34.0 (n=53/156) | -- | -- |
| % Co-morbid MDD | 15.4 (n=24/156) | -- | -- |
| HRSD | 11.93 (3.89) | 1.78 (2.20) | <0.0001 |
| HRSD-Core Depression Items | 3.01 (1.85) | 0.11 (0.31) | <0.0001 |
| % Prescribed lorazepam | 22.5 | 2.7 | χ2=772, df=1, p=0.006 |
| MMSE | 28.14 (1.72) (n=159) | 29.03 (1.16) | 0.0015 |
Cumulative Illness Rating Scale-Geriatrics (CIRS-G), Hamilton Rating Scale for Anxiety (HRSA), Penn State Worry Questionnaire (PSWQ), Hamilton Rating Scale for Depression (HRSD); Mini-Mental State Exam (MMSE)
SQRT(X) transformation used in the analyses. Means and standard deviations reported in the original units.
Satterthwaite test used due unequal variances.
Participants who dropped out before week 12 (n=31) were not significantly different from completers (n=129) in age, gender, race, years of education age of GAD onset or rates of comorbid MDD. However, participants who dropped out before week 12 were significantly different from completers in terms of baseline severity on Hamilton Rating Scale for Anxiety (25.1; 95% confidence interval [CI], 22.9–27.2; vs 22.6; 95% CI, 21.9–23.2; P=.003) and Hamilton Rating Scale for Depression (14.0; 95% CI, 12.3–15.7; vs 11.6; 95% CI, 11.0–12.2; P=.02), coprescription of benzodiazepine (dropout rate for benzodiazepine users, 33.3% [n=9/27]; and for nonusers, 16.0% [n=24/150]; P=.04), and race (dropout rate for white patients, 15.9% [n=23/145]; and for black patients, 31.2% [n=10/32]; P=.04).
Neuropsychological functioning of GAD and control participants at baseline
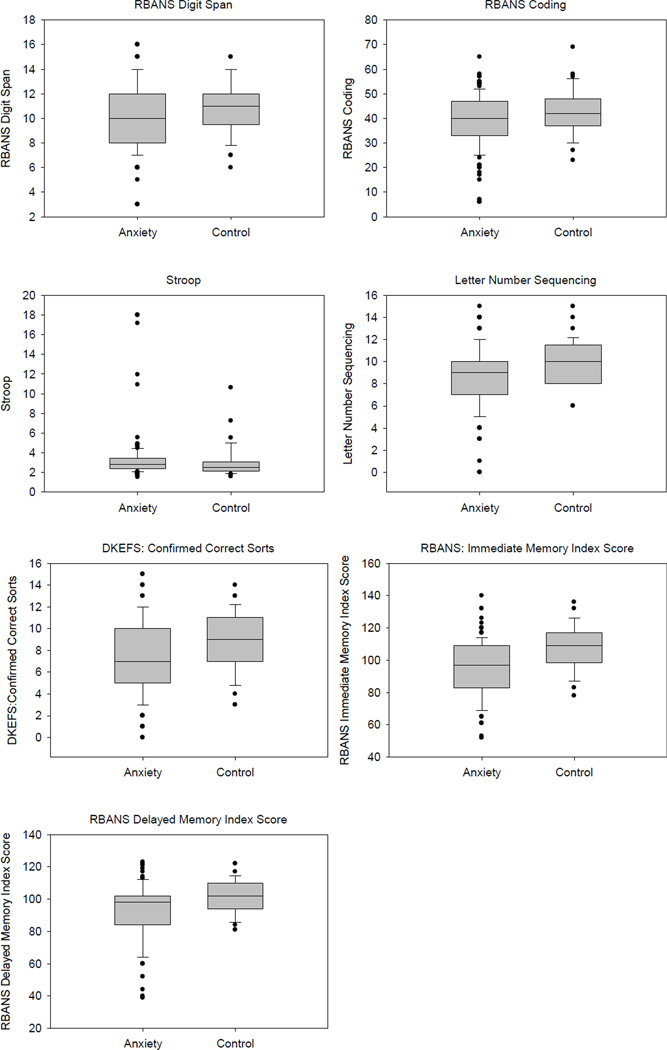
Among the main outcome measures (see Table 2 and Figure 1), after controlling for age and education, GAD participants performed significantly worse than comparison subjects on RBANS Coding (F(1,192)=6.44, p=0.012), the Stroop test (F(1,189)=3.92), p=0.049), Letter-Number Sequencing (F(1,192)=8.37, p=0.004), the DKEFS Sorting test (F(1,192)=10.28, p=0.002) and both RBANS Immediate Memory (F(1,193)=13.38, p=0.0003) and Delayed Memory (F(1,193)=9.03, p=0.003) Indexes. There was no difference between the groups on the RBANS Digit Span (F(1,192)=0.96, p=0.33) or on the other neuropsychological domains assessed, Language (F(1,193)=0.10, p=0.75) and Visuospatial Construction (F(1,193)=2.67, p=0.10). When the comparisons were corrected using the stepwise Bonferroni method, all of the findings remained except that the two groups performed similarly on the Stroop and RBANS Coding (see Table 2). Size and strength of the results did not change when excluding subjects who met criteria for MDD or using MDD as covariate. We also re-ran the analyses using lorazepam co-prescription as a covariate and the results did not significantly change from the previous results (results not reported).
Table 2.
Neuropsychological Measures
| GAD n=160 |
Comparison Subjects n=37 |
F(df), p-value | Partial Eta2 | Stepwise Bonferroni Corrected p-value |
|
|---|---|---|---|---|---|
| RBANS Digit Span | 10.4 (2.7) n=159 |
10.9 (2.3) | Age: F(1,192) = 1.05, p=0.31 Ed: F(1,192)=6.21, p=0.014 Grp: F(1,192)=0.96, p=0.33 |
Age: 0.005 Ed: 0.031 Grp: 0.005 |
Grp: 0.66 |
| RBANS Coding | 39.0 (10.7) n=159 |
42.9 (9.2) | Age: F(1,192) = 17.22, p<0.0001 Ed: F(1,192)=10.15, p=0.002 Grp: F(1,192)=6.44, p=0.012 |
Age: 0.082 Ed: 0.050 Grp: 0.032 |
Grp: 0.06 |
| Stroop* | 3.6 (3.1) n=156 |
3.0 (1.7) | Age: F(1,189) = 14.48, p=0.0002 Ed: F(1,189)=1.57, p=0.21 Grp: F(1,189)=3.92, p=0.049 |
Age: 0.071 Ed: 0.008 Grp: 0.020 |
Grp: 0.20 |
| Letter Number Sequencing | 8.7 (2.7) n=159 |
9.9 (2.1) | Age: F(1,192) = 15.04, p=0.0001 Ed: F(1,192)=15.58, p=0.0001 Grp: F(1,192)=8.37, p=0.004 |
Age: 0.073 Ed: 0.075 Grp: 0.042 |
Grp: 0.026 |
| DKEFS: Confirmed Correct Sorts | 7.2 (3.4) n=159 |
9.0 (2.8) | Age: F(1,192) = 8.67, p=0.004 Ed: F(1,192)=45.13, p<0.0001 Grp: F(1,192)=10.28, p=0.002 |
Age: 0.043 Ed: 0.190 Grp: 0.051 |
Grp: 0.013 |
| RBANS Immediate Memory Index Score | 95.2 (17.4) n=159 |
107.3 (13.5) | Ed: F(1,193)=13.10, p=0.0004 Grp: F(1,193)=13.38, p=0.0003 |
Ed: 0.064 Grp: 0.065 |
Grp: 0.003 |
| RBANS Delayed Memory Index Score | 92.5 (17.4) n=159 |
102.2 (10.8) | Ed: F(1,193)=7.16, p=0.008 Grp: F(1,193)=9.03, p=0.003 |
Ed: 0.036 Grp: 0.045 |
Grp: 0.021 |
| Visuospatial Construction Index Score | 89.4 (18.5) n=159 |
95.7 (14.7) | Ed: F(1,193)=11.07, p=0.001 Grp: F(1,193)=2.67, p=0.10 |
Ed: 0.05 Grp: 0.014 |
Grp: 0.31 |
| Language Index Score | 98.7 (14.7) n=159 |
100.6 (10.5) | Ed: F(1,193)=20.71, p<0.0001 Grp: F(1,193)=0.10, p=0.75 |
Ed: 0.10 Grp: 0.001 |
Grp: 0.75 |
LN(X) transformation used in the analyses. Means and standard deviations reported in the original units
Figure 1.
Boxplots depicting performance of GAD and comparison subjects on the neuropsychological variables
Functional correlates of neuropsychological performance at baseline
Table 3 displays correlations between the two FDI subscales and nine neuropsychological variables. Nine of the 18 correlations were statistically significant at p<0.05 suggesting that in late-life GAD, neuropsychological impairments are correlated with functional disability and lending credence to the importance of neuropsychological functioning in late-life GAD. Significant correlations included: (1) the FDI frequency subscale with RBANS Coding, Letter-Number Sequencing. DKEFS Sorting, and RBANS Immediate and Delayed Memory Index Scores as well as the Language Index Score (2) the FDI limitations subscale with RBANS Coding and Letter-Number Sequencing and the RBANS Language Index Score.
Table 3.
Functional correlates of neuropsychological performance at baseline (N=155)
| Frequency Rho |
Limitation Rho |
|
|---|---|---|
| RBANS Digit Span | 0.08 | 0.04 |
| RBANS Coding | 0.29** | 0.23** |
| Stroop* | −0.11 | −0.09 |
| Letter Number Sequencing | 0.29** | 0.17* |
| DKEFS: Confirmed Correct Sorts | 0.20* | 0.07 |
| RBANS Immediate Memory Index Score | 0.26** | 0.14 |
| RBANS Delayed Memory Index Score | 0.17* | 0.07 |
| RBANS Visuospatial Construction Index Score | 0.08 | 0.03 |
| RBANS Language Index Score | 0.26** | 0.17* |
p<0.05,
p<0.01
Comparing neuropsychological functioning pre- and post-treatment in GAD participants who (a) were low cognitive scorers, and (b) reported improvement in anxiety
Sixty-six participants scored below the group median score (Total Index Score ≤ 94) on RBANS (30 in active and 36 in the placebo) and thus were considered low cognitive scorers for the purpose of these analyses. The scores of the 66 participants who performed below the median RBANS Total Score ranged from the low end of the average range (RBANS Digit Span and the RBANS Language Index Score) to mildly impaired (approximately −1 SD below the age-corrected mean: Letter Number Sequencing, RBANS Immediate and Delayed Memory Index Scores) to moderately impaired (−1 to −2 SDs below the age-corrected mean: DKEFS Sorting, RBANS Coding Task, RBANS Visuospatial Construction Index) to moderately to severely impaired (−2 to −3 SDs: the Stroop test). There was no difference between the lower and upper halves in age of GAD onset (39 years for both groups; p=0.99) or percent who had comorbid major depression (20 vs. 9.7; p=0.10), but the lower half did have slightly higher anxiety as measured by the HRS-A (23.42 vs. 21.37; p=0.05).
The repeated measures ANOVA revealed no significant main effects for treatment group, time, or treatment-by-time interactions for the Stroop Test, RBANS Digit Span, Coding or Immediate Memory or Language Index Scores. There were significant main effects for time but not for treatment group or treatment-by-time interactions on Letter-Number Sequencing (F(1,64)=4.82, p=0.032) and RBANS Delayed Memory (F(1,64)=6.65, p=0.01) and Visuospatial Construction Index Scores, suggesting that working memory, delayed memory and visuospatial ability improved in both treatment conditions over the course of the study. There was a significant treatment by time interaction on the DKEFS Sorting Task (F(1,62)=4.06, p=0.048) indicating that the group receiving escitalopram improved in problem-solving, conceptual ability and mental flexibility more than did the group receiving placebo.
Forty-two participants reported “improvement” in anxiety (CGI score ≤ 2) over the course of the study. These participants experienced significant improvement on the Stroop (change score(SD) 0.60 (5.38), p=0.0063), and both RBANS immediate (change score (SD) 3.74 (10.08), p=0.0114), and delayed memory (change score (SD) 5.41 (10.08), p=0.0019), indicating an association between improved anxiety and improved ability to engage in inhibition and episodic recall in both treatment conditions over the course of the study.
Discussion
To our knowledge, this is the first large-scale study to comprehensively evaluate neuropsychological function in late-life GAD and its response to treatment in a systematic manner. We found broad based, but not global impairments. Older GAD participants performed worse than comparison subjects on measures of information processing speed, working memory, inhibition, and problem-solving (including concept formation and mental flexibility), as well as immediate and delayed memory. These findings are consistent with published literature describing decrements in memory and executive functions in young and middle-aged adults (34) and memory and working memory in older adults with GAD (18, 19). The impairments of highest magnitude were in memory, as immediate and delayed memory in the GAD group were about one standard deviation below healthy comparison subjects. This finding fits well with the literature review of Beaudreau and O'Hara (14), who noted that memory impairment tended to be the most consistent cognitive deficits in late-life anxiety. Our findings also suggest that worry may compete for cognitive resources thereby interfering with execution of some especially vulnerable cognitive functions, including speed, some aspects of executive function and episodic memory.
We also found that neuropsychological functioning was significantly, but relatively weakly correlated with everyday functioning, consistent with the hypothesis that impaired cognitive function plays a significant role in the functional disability associated with late-life GAD (7, 35).
Finally, we found that among GAD participants with poor cognitive performance at baseline, multiple domains showed improvement during the course of the clinical trial, suggesting that either clinical improvement in anxiety led to improvements in poor cognitive performance, or clinical improvement in anxiety led to improvements in poor cognitive performance. Further, those who received escitalopram had greater improvements compared to placebo, on the DKEFS sorting task, which measures problem-solving, concept formation and mental flexibility. In addition, those anxious participants who reported improved anxiety over the course of the trial experienced significant improvement in inhibition and both immediate and delayed recall.
Our study has some limitations. Although the RBANS has parallel forms to reduce practice effects, improved performance over time may be attributable to repetition rather than true treatment effects. Also, the clinical trial was only 12 weeks long; longer protocolized evaluations may be more robust for finding differences in the course of cognitive decline and its amelioration with effective treatment. Comparing individuals with late-life GAD with non-psychiatric controls leaves open the possibility that the impairments that were identified could be due to having a psychiatric disorder in general and may not be specific to late-life GAD. Although future studies should employ this approach, in the case of GAD, the most appropriate psychiatric control group is unclear. It is possible that IQ differences could explain our findings. However, all of our participants were recruited from the same sources and the average years of education was similar in both the GAD and control groups, minimizing the likelihood that there were substantial IQ differences. We did not measure sleep quality or quantity, and it is possible that sleep disturbance may account for some or all of the effects on memory. Finally, our understanding of the neurobiology of late-life GAD is lacking, and better understanding of the structural or functional changes leading to cognitive impairment in this disorder may yield more informative neuropsychological hypotheses and, eventually, better treatments. The greatest strength of this study is that it examines the relationship between anxiety and cognition in the context of an experimental manipulation (i.e., treatment). In the context of a 12 week clinical trial, it is more likely that reductions in anxiety were driving improvements in cognition rather than the reverse.
In sum, GAD in older adults is associated with widespread neuropsychological impairments, including information processing speed, working memory, inhibition, problem-solving and both immediate and delayed memory. While there was specific improvement in problem-solving, concept formation and mental flexibility attributable to SSRI treatment, many of these cognitive domains showed improvement during the course of treatment regardless of treatment assignment. While medication-specific benefits are minimal in this analysis, we found that improvement in anxiety symptoms was associated with neuropsychological improvements. Such findings are not a treatment effect per se but could be interpreted as showing that the clinical fluctuation of anxiety symptoms also affects cognitive performance. Future treatment research might usefully focus on providing long-term remission stability, both clinically and cognitively and should examine whether such improvements translate to meaningful gains in function and quality of life. These findings underscore the importance of cognitive functioning as a potential treatment target to reduce the impairment associated with anxiety disorders in older adults.
Acknowledgments
This research was supported by United States Public Health Service grants MH070547, MH072947 and MH52247, as well as a 2009 NARSAD Young Investigator Award to Carmen Andreescu. We would like to thank Michelle Zmuda, Program Coordinator of the Geriatric Neuropsychology Research Program for overseeing the neuropsychological assessments.
References
- 1.Beekman AT, Bremmer MA, Deeg DJ, van Balkom AJ, Smit JH, de Beurs E, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998 Oct;13(10):717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Bryant C, Jackson H, Ames D. The prevalence of anxiety in older adults: methodological issues and a review of the literature. J Affect Disord. 2008 Aug;109(3):233–250. doi: 10.1016/j.jad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009 Sep;17(9):769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach GJ, Stanley MA, Beck JG. Worry content reported by older adults with and without generalized anxiety disorder. Aging Ment Health. 2001 Aug;5(3):269–274. doi: 10.1080/13607860120065069. [DOI] [PubMed] [Google Scholar]
- 5.Chou KL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2009 Jun;17(6):455–464. doi: 10.1097/jgp.0b013e31818f3a93. [DOI] [PubMed] [Google Scholar]
- 6.Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005 Jan;13(1):23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Lenze EJ, Rollman BL, Shear MK, Dew MA, Pollock BG, Ciliberti C, et al. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009 Jan 21;301(3):295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley MA, Beck JG, Novy DM, Averill PM, Swann AC, Diefenbach GJ, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003 Apr;71(2):309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D, Eisdorfer C, Vitaliano PP, Bloom V. The relationship of age, anxiety, and serum immunoglobulins with crystallized and fluid intelligence. Biol Psychiatry. 1980 Oct;15(5):699–709. [PubMed] [Google Scholar]
- 10.Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci. 2002 May;57(3):P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- 11.Bierman EJ, Comijs HC, Jonker C, Beekman AT. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005 Aug;13(8):686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- 12.Deptula D, Singh R, Pomara N. Aging, emotional states, and memory. Am J Psychiatry. 1993 Mar;150(3):429–434. doi: 10.1176/ajp.150.3.429. [DOI] [PubMed] [Google Scholar]
- 13.Paterniti S, Dufouil C, Bisserbe JC, Alperovitch A. Anxiety, depression, psychotropic drug use and cognitive impairment. Psychol Med. 1999 Mar;29(2):421–428. doi: 10.1017/s0033291798008010. [DOI] [PubMed] [Google Scholar]
- 14.Beaudreau SA, O'Hara R. The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychol Aging. 2009 Jun;24(2):507–512. doi: 10.1037/a0016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan MJ. Divided attention in older but not younger adults is impaired by anxiety. Exp Aging Res. 2003 Apr-Jun;29(2):111–136. doi: 10.1080/03610730303712. [DOI] [PubMed] [Google Scholar]
- 16.Biringer E, Mykletun A, Dahl AA, Smith AD, Engedal K, Nygaard HA, et al. The association between depression, anxiety, and cognitive function in the elderly general population--the Hordaland Health Study. Int J Geriatr Psychiatry. 2005 Oct;20(10):989–997. doi: 10.1002/gps.1390. [DOI] [PubMed] [Google Scholar]
- 17.Lindesay J, Briggs K, Murphy E. The Guy's/Age Concern survey. Prevalence rates of cognitive impairment, depression and anxiety in an urban elderly community. Br J Psychiatry. 1989 Sep;155:317–329. [PubMed] [Google Scholar]
- 18.Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey B, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007 Aug;15(8):673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 19.Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007 Aug;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 20.Price RB, Mohlman J. Inhibitory control and symptom severity in late life generalized anxiety disorder. Behav Res Ther. 2007 Nov;45(11):2628–2639. doi: 10.1016/j.brat.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009 Jan;24(1):1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008 Jul;33(6):773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—nonpatient edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 24.Randolph C. In: The Repeatable Battery for the Assessment of Neuropsychological Status. Corporation TP, editor. San Antonio, TX: 1998. [Google Scholar]
- 25.Delis D, Kaplan E, Kramer J. In: Delis-Kaplan Executive Function Scale. Corporation TP, editor. San Antonio, TX: 2001. [Google Scholar]
- 26.Trennery M, Crosson B, DeBoe J, Leber W. Stroop Neuropsychological Screening Test Manual. Odessa, FL: Psychological Assessment Resources (PAR); 1989. [Google Scholar]
- 27.Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 28.Dombrovski AY, Blakesley-Ball RE, Mulsant BH, Mazumdar S, Houck PR, Szanto K, et al. Speed of improvement in sleep disturbance and anxiety compared with core mood symptoms during acute treatment of depression in old age. Am J Geriatr Psychiatry. 2006 Jun;14(6):550–554. doi: 10.1097/01.JGP.0000218325.76196.d1. [DOI] [PubMed] [Google Scholar]
- 29.Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 30.The World Health Report: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; 2002. [Google Scholar]
- 31.Porensky EK, Dew MA, Karp JF, Skidmore E, Rollman BL, Shear MK, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009 Jun;17(6):473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 33.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992 Mar;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 34.Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. 2005 Mar;39(2):207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003 Sep;18(3):622–627. doi: 10.1037/0882-7974.18.3.622. [DOI] [PubMed] [Google Scholar]