Abstract
Sphere-forming assays have been widely used to retrospectively identify stem cells based on their reported capacity to evaluate self-renewal and differentiation at the single cell level in vitro. The discovery of markers that allow the prospective isolation of stem cells and their progeny from their in vivo niche allows the functional properties of purified populations to be defined. We provide an historical perspective of the evolution of the neurosphere assay, and highlight limitations in the use of sphere-forming assays, in the context of neurospheres. We discuss theoretical and technical considerations of experimental design and interpretation that surround the use of this assay with any tissue.
Stem cells are remarkable cells that are found in many tissues. They exhibit two cardinal properties: the ability to undergo self-renewal and the ability to differentiate. Because of these properties, stem cells are of crucial importance for maintaining tissue homeostasis and for tissue repair after injury. Great excitement has arisen about the therapeutic potential of stem cells, as well as recognition of their contribution to pathological states such as tumours. Changes in stem cell properties and the niches they inhabit may also have profound consequences for understanding aging.
To explore the dynamics, function and regulation of stem cells, and how these may go awry in disease, experimental assays must reliably be able to distinguish stem cells and their progeny. Due to the general lack of unique cell surface markers and the absence of a distinct and discernable morphological phenotype, stem cells have typically been defined and studied on the basis of functional criteria.
With the development of markers to prospectively identify putative stem cells, as well as sophisticated genetic approaches for lineage tracing, it is becoming increasingly feasible to define the dynamics of stem cells in vivo. Moreover, the ability to prospectively purify stem cells and their progeny has allowed their functional properties to be studied in vitro and their potential to be evaluated by transplantation in vivo. In the last few years, exciting discoveries have been made about the existence of quiescent and activated pools of stem cells and their ability to shuttle between these states. Transit amplifying progeny also have the potential to revert back to a stem cell state, at least in some tissues. As we discover more about the biology and behaviour of stem cells within their niches, and novel principles emerge, it is important to re-evaluate the strategies utilized to identify and functionally characterize adult stem cells. In particular, it is crucial to distinguish whether different paradigms evaluate actual in vivo stem cells or reveal stem cell potential, and to have a clear understanding of the strengths and limitations of different assays.
Stem cells from diverse tissues are typically cultured in vitro under non-adherent conditions as spheres, or under adherent conditions in two-dimensional cultures or in three-dimensional matrices. Sphere forming assays are widely used in stem cell biology, as theoretically both self-renewal and differentiation can be evaluated at the single cell level. In this protocol review, we critically assess the utility and the limitations of sphere-forming assays. As they were first used in the neural stem cell field almost twenty years ago, we provide an historical overview of the evolution of the neurosphere assay, which highlights important lessons that have been learned in the neural stem cell field regarding the identity of neurosphere-forming cells. Indeed not all neurospheres arise from stem cells, and this finding critically impacts the broadly held premise that sphere forming assays are a functional assay for uniquely detecting in vivo stem cells. Instead, sphere-forming assays evaluate the potential of a cell to behave as a stem cell when removed from its in vivo niche. We then outline additional important theoretical and technical considerations that incorporate emerging principles in stem cell biology that impact the interpretation of sphere-forming assays when used to evaluate stem cells from any organ.
The neurosphere assay: an historical perspective
The discovery of adult neural stem cells was the result of two coincident and divergent lines of research. The first was the re-investigation of adult neurogenesis and the second was the in vitro study of multipotent precursors from the adult brain.
Neural stem cells present in specialized niches in the adult mammalian brain continuously generate new neurons that are functionally integrated into neural circuits, including in humans. Adult neurogenesis occurs in two regions of the mammalian brain, the subventricular zone (SVZ), which is a thin layer of dividing cells adjacent to the lateral ventricles that generates olfactory bulb interneurons, and the subgranular zone (SGZ) in the hippocampal formation. These areas of continuous neurogenesis harbor stem cells that retain the capacity to proliferate, self-renew over an extended period of time, and differentiate into the three primary cell types of the brain (neurons, astrocytes and oligodendrocytes). As the neurosphere assay is almost exclusively used in the SVZ and not the SGZ, from which cells are predominantly cultured as adherent cells, the rest of this review is focused on the SVZ.
In the late 1960’s, Joseph Altman first showed that new neurons are generated in the adult mammalian brain, yet this finding was largely ignored (Altman, 1969). In the 1980’s the group of Fernando Nottebohm showed that new neurons functionally integrate into the adult songbird brain (reviewed in Nottebohm, 2004). However, it was not until the early 1990’s, when new technical approaches were utilized, combining in vivo labeling and in vitro culture, that it was shown that precursors capable of giving rise to neurons were present in the adult mammalian brain and that neurogenesis and long distance migration occur in vivo (Lois and Alvarez-Buylla, 1993; Kirschenbaum and Goldman 1995; Lois and Alvarez-Buylla, 1994).
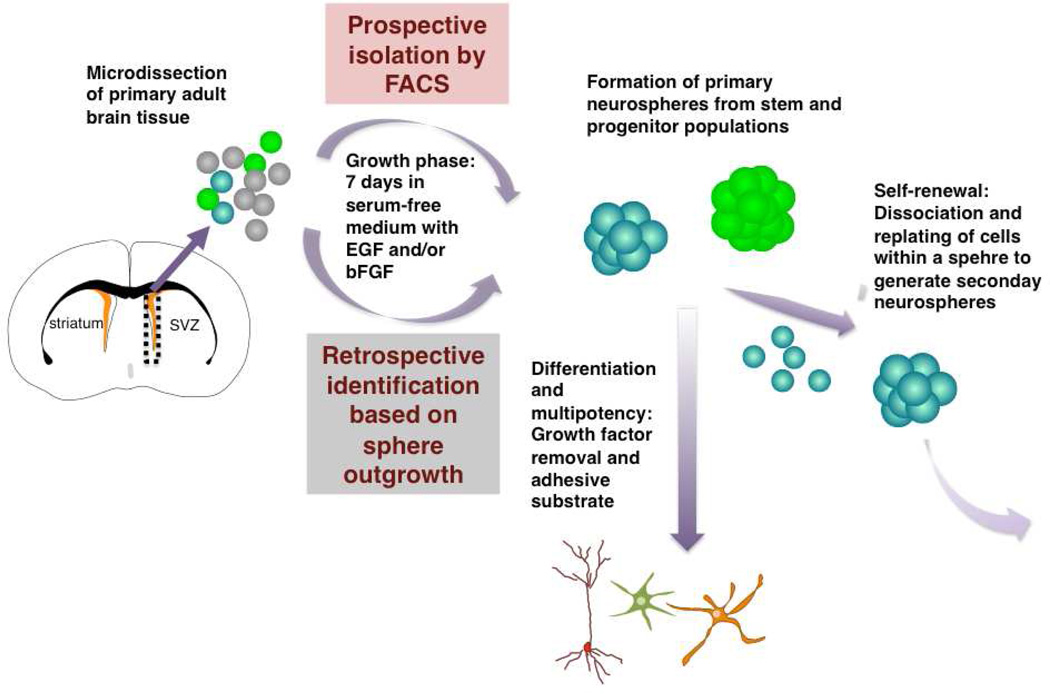
At the same time, Reynolds and Weiss first cultured cells that exhibit stem cell properties as free-floating spheres, called neurospheres, from the adult brain (Reynolds and Weiss, 1992). They dissected striatal tissue, which included the periventricular area encompassing the SVZ, enzymatically dissociated the tissue to single cells and plated them in non-adherent conditions in serum-free medium in the presence of epidermal growth factor (EGF) (Figure 1). A small population of cells began to divide, initially adhering to the plate, and after a few days detaching and forming spheres of proliferating cells. The majority of cells within these neurospheres expressed nestin, an intermediate filament present in neuroepithelial stem cells in the embryonic brain. To assess whether cells could be propagated as secondary cultures, to show self-renewal, the neurospheres were mechanically dissociated and cultured again in the presence of EGF, with a subset forming secondary neurospheres (Figure 1). When plated on an adherent substrate, they differentiated into both neurons and glial cells. This provided the first evidence that multipotent stem cells were present in the adult mammalian brain.
Figure 1. Schema of the neurosphere assay.
Microdissection experiments subsequently revealed that the SVZ (also called the subependymal zone) is the source of neurospheres in vivo (Morshead et al., 1994). Moreover, it was also proposed that neurospheres only arise from relatively quiescent cells in vivo, based on a series of [3H]-thymidine kill experiments, in which neurospheres were cultured after the in vivo elimination of rapidly dividing cells (Morshead et al., 1994). However, as the cell types in the SVZ had not yet been defined, it was not clear which cells were present when neurospheres were cultured at different timepoints after the [3H]-thymidine kill. Further experiments revealed that neurospheres can be cultured from the entire ventricular axis of the central nervous system, including the spinal cord (Weiss et al., 1996; Vescovi et al., 1993). However, both bFGF and EGF are required for neurospheres to grow from these non-neurogenic brain regions. EGF neurospheres can only be cultured from the SVZ.
Since these early experiments, the neurosphere assay has evolved. It is now accepted that the assay needs to be performed at clonal density. To demonstrate multipotency, individual neurospheres must give rise to neurons, astrocytes and oligodendrocytes, the three main cell types in the brain, upon differentiation after withdrawal of growth factors. In addition, although at first neurospheres were considered to be a homogeneous population of nestin+ stem cells, it is now clear that individual neurospheres contain stem cells, progenitors and differentiated cells.
The neurosphere assay therefore appeared to provide a simple retrospective assay to identify cells exhibiting both functional properties of stem cells, self-renewal and differentiation, as well as a quantitative readout of the number of stem cells in vivo. Importantly, the identity of the in vivo stem cells had not yet been discovered and it was not feasible to prospectively isolate different cell populations from the SVZ to directly test which cells have the capacity to give rise to neurospheres.
Anatomy of the SVZ and identity of stem cells
A key step in identifying the stem cells responsible for adult neurogenesis, determining their in vivo lineage, and elucidating which cells give rise to neurospheres, was defining the cell types and architecture of the SVZ niche. The SVZ has several striking organizational features, best visualized in whole mount preparations that reveal the entire three-dimensional surface of the ventricular wall (Doetsch and Alvarez-Buylla, 1996). It has recently been shown that stem cells in the adult SVZ are regionally specified and that precursors for different interneuron subtypes reside in different regions (Hack et al., 2005; Merkle et al., 2007; Young et al., 2007; Kelsch et al., 2007; Ventura and Goldman, 2007). Newly generated neurons migrate from their sites of birth and collect in a network of migrating chains that extends throughout the SVZ to join the rostral migratory stream that leads to the olfactory bulb (Doetsch and Alvarez-Buylla, 1996). A small number of oligodendrocytes are also generated in the adult SVZ (Nait-Oumesmar et al., 1999; Menn et al., 2006). However it is still unknown if tripotent stem cells exist in vivo, or whether separate stem cells give rise to oligodendrocytes and neurons.
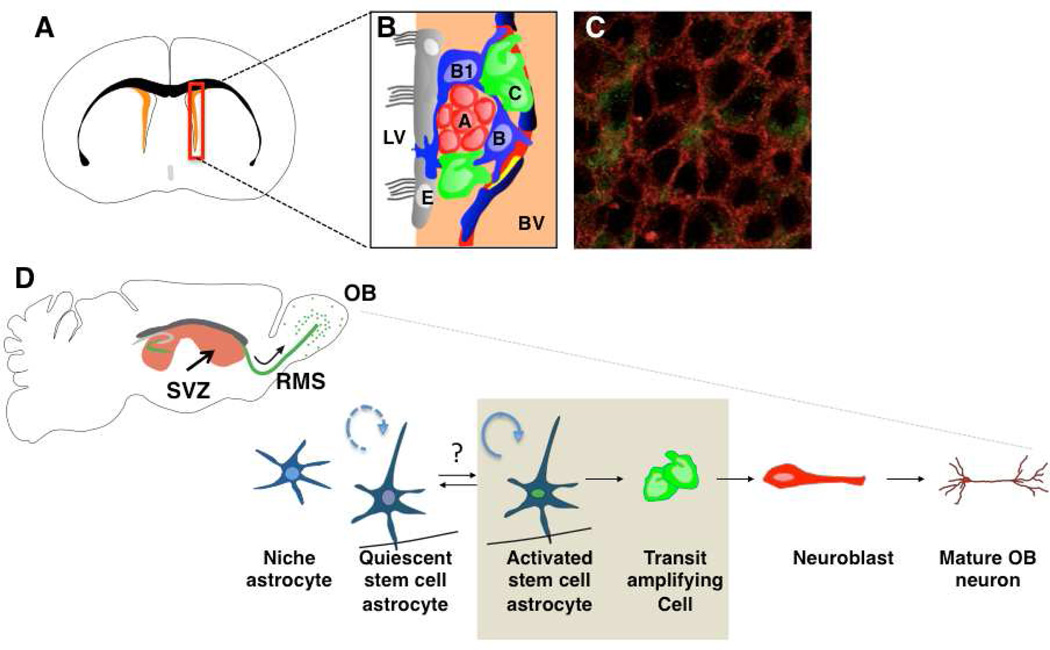
Initially, ultrastructural analysis was used to identify the cellular populations in the SVZ, as there were no markers available to distinguish the different cell types (Doetsch et al., 1997). Indeed, two markers commonly used to identify neural stem cells, nestin and Sox2, are expressed by all cell types in this region, and therefore cannot be used as unique markers of stem cells (Doetsch et al., 1997; Tavazoie et al., 2008). Four main classes of cells are present in the SVZ (Figure 2). Multi-ciliated ependymal cells (Type E cells) line the ventricles. The chains of neuroblasts (Type A cells) travel through tunnels formed by the processes of glial fibrillary acidic protein (GFAP)+ cells with many ultrastructural features of astrocytes (Type B cells). Rapidly dividing transit amplifying cells (Type C cells) are clustered adjacent to the chains of neuroblasts.
Figure 2. SVZ anatomy and lineage.
A, Frontal schema of adult mouse brain showing SVZ in orange adjacent to the lateral ventricle.
B, Summary schema of the organization of SVZ cells. GFAP+ stem cells (B, blue) transit amplifying cells (C, green), neuroblasts (A cells, red), are adjacent to ependymal cells (E, grey), which line the lateral ventricle (LV). A subset of GFAP+ cells (B1) extend a process between ependymal cells to contact the LV. Stem cells and transit amplifying cells directly contact the vasculature at specialized regions on blood vessels lacking astrocyte endfeet (dark blue) and pericyte coverage (yellow).
C, Confocal image of ependymal cell pinwheel with GFAP::GFP+ Type B1 cell contacting the ventricle at its center (Image from Angel Maldonado-Soto, generated according to the methods reported in Mirzadeh et al., 2008).
D, Stem cell lineage and sagittal schema of adult mouse brain showing whole mount perspective of SVZ adjacent to lateral ventricle (red). Newly generated neurons migrate along the rostral migratory stream (RMS) to the olfactory bulb (OB). Small numbers of oligodendrocytes are also generated in the SVZ but are not shown here. Beige boc indicates neurosphere forming cells in the lineage.
Cell ablation and lineage tracing studies established the SVZ stem cell lineage. Intriguingly, cells with several hallmarks of glial cells, long thought to be support cells in the brain and derived from a completely different lineage than neurons, are stem cells in both adult neurogenic regions (reviewed in Kriegstein and Alvarez-Buylla, 2009). Within the SVZ, GFAP positive (Type B) cells are stem cells both during regeneration and under homeostasis (Doetsch et al., 1999a). They divide to generate transit amplifying cells, which in turn give rise to neuroblasts (Figure 2). Furthermore, a subset of GFAP+ cells form neuropheres. At the time that GFAP+ cells were identified as stem cells, ependymal cells were also proposed to be stem cells in the SVZ (Johansson et al., 1999). This debate was largely resolved as more sophisticated genetic labeling and purification strategies were developed. This finding was not replicated by others (reviewed in Kokovay et al., 2008), and the original group proposing that ependymal cells form neurospheres has recently published that they do not give rise to neurons under homeostasis (Carlen et al., 2009). However, several recent papers have resurrected the idea that multi-ciliated ependymal cells are stem cells, based on the claim that CD133 and FoxJ1 are exclusively expressed by ependymal cells and can be used to purify them (Coskun et al., 2008; Meletis et al., 2008). However, both markers are also expressed by a subpopulation of GFAP+ cells (see below); the specificity of these markers in non-neurogenic brain regions needs to be better defined using high resolution ultrastructural and molecular analysis. Later experiments in which dividing GFAP+ cells were killed in GFAP-TK mice confirmed that GFAP+ cells are the source of adult generated neurons (Garcia et al., 2004; Imura et al., 2003; Morshead et al., 2003). However, even now, a critical issue is the lack of markers available to distinguish between GFAP+ stem cells and other brain astrocytes, as well as how they differ in their functional roles and potential.
Another important, and ongoing issue, is the identity and dynamics of putative quiescent stem cells in the adult SVZ. Based on regeneration studies, a pool of slowly dividing B cells escapes being killed by an anti-mitotic drug and rapidly regenerates the SVZ (Doetsch et al., 1999a). Markers have not yet been defined that allow the purification of these cells. Moreover it is still unknown whether these cells are only activated during injury or whether a deeply quiescent pool participates in neurogenesis under homeostasis as well.
Relationship of prospectively purified cells to neurosphere-forming cells
A crucial advance in defining which cells form neurospheres was the identification of markers that allow populations at different stages of the lineage to be isolated or killed using genetic approaches.
Heterogeneity of GFAP+ Type B cells
A very active effort in the neural stem cell field is to define the heterogeneity of GFAP+ cells within the SVZ and elsewhere in the brain and assess their stem cell potential. Important insights have arisen from anatomical, ultrastructural and functional analysis. Early studies using electron microscopy identified two populations of GFAP+ Type B cells in the SVZ. Type B1 cells extend a process between ependymal cells to contact the lateral ventricle, and have a primary cilium (Doetsch et al., 1999b). Type B2 cells are located closer to the vasculature and divide more frequently (Doetsch et al, 1997) (Figure 2). Recent work has uncovered important new features of the SVZ niche (Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008), which shed light on the functional organization of GFAP+ Type B cells. Ependymal cells are organized as a series of pinwheels along the ventricular wall with Type B1 astrocytes contacting the ventricle at the center of these pinwheels (Mirzadeh et al., 2008) (Figure 2). In addition, a planar vascular plexus extends throughout the length of the SVZ (Shen et al., 2008; Tavazoie et al., 2008). Both stem cells and transit amplifying cells are tightly associated with this vascular plexus and contact it at specialized sites that lack astrocyte endfeet (Shen et al., 2008; Tavazoie et al., 2008).
Several markers have now been identified that distinguish different subpopulations of Type B cells in the SVZ and have allowed their prospective purification (Pastrana et al., 2009; Beckervordersandforth et al., 2010). Importantly, some of these markers are co-expressed by multiple cell types, such as CD133 (prominin), which is expressed by both ependymal cells and a subset of B1 cells contacting the ventricle (Coskun et al., 2008; Mirzadeh et al., 2008, Beckervordersandforth et al., 2010). At least four populations of GFAP expressing B cells can be discerned within the SVZ: Type B1 cells contacting the ventricle (Doetsch et al., 1999b; Mirzadeh et al., 2008; Beckervordersandforth et al., 2010), some of which express CD133 (prominin); actively dividing (activated) EGFR+ astrocytes, some of which contact the lateral ventricle (Doetsch et al., 2002; Pastrana et al., 2009), and non-dividing multipolar niche astrocytes found closest to the striatum (García et al., 2004; Mirzadeh et al., 2008; Shen et al., 2008). The overlap of marker expression, proliferation state and morphology of these different subpopulations still needs to be elucidated.
Multiple populations give rise to neurospheres
Until recently, adult neural stem cells and their progeny have been difficult to purify using fluorescence activated cell sorting (FACS) due to the lack of markers that allow separation of cells at different stages in the lineage and direct comparison of their neurosphere-forming capacity. Diverse approaches have been used to attempt to isolate adult neural stem cells and their progeny, based on markers for cells at different stages in the lineage, cell cycle status, and putative general stem cell markers, including metabolic substrates, dye efflux, surface markers and fluorescently-complexed molecules and transgenic reporter mice (Table 1 and references therein). Table 1 summarizes the neurosphere formation efficiency of adult cells purified using different strategies. Interestingly, the side population purification method, in which Hoechst exclusion is used to prospectively isolate stem cells (reviewed in Golebiewska et al., 2011), is not selective for neurosphere-forming cells (Kim and Morshead, 2003). Similarly, aldehyde dehydrogenase activity does not significantly enrich for neurosphere-forming cells (Corti et al., 2006; Obermair et al., 2010).
Table 1.
Markers used to purify neurospheres from the adult brain and their reported efficiencies
| Marker | Isolation Strategy | Reported % NS formation from adult |
Reference |
|---|---|---|---|
| Bulk frequency | Bulk dissociated cells | 0.03%--1% | |
| Aldehyde dehydrogenase | ALDH activity with aldefluor substrate | 2.82% | Corti et al 2006 |
| Cell size/PNA/CD24 | Cells > 12µm/ anti-PNAlow cells/ anti-CD24low | 80% | Rietze et al 2001 |
| CD15 (Lex, SSEA-1) or CD24 | Anti-CD15 or Anti-CD24 | CD15+ 25% CD24+ 0.15% | Capela and Temple 2002 |
| CD133 (Prominin) | Anti-CD133 | 4% | Corti et al 2007, |
| CD133 or CD24 | Anti CD133 or Anti-CD24 | CD133+ 6% CD24+ 0.9% | Coskun et al 2008 |
| CD133/CD24 | Anti CD133/Anti-CD24 | CD133+/CD24+ 0% | Pfenninger et al.2011 |
| CD133/CD15/aldehye dehydrogenase | anti-CD133/anti-CD15/aldehyde dehydrogenase activity with aldefluor substrate | CD15+ 0.45% Aldh high 0.41% Aldh high/CD15+ 0.24% did not detect CD133+/CD15+ cells | Obermair et al 2010 |
| Dcx | Dcx::GFP | Dcx Low 0.8% Dcx- 1.2% | Walker et al 2007 |
| Dlx2/CD24 | Dlx2::LacZ/Anti-CD24 | Dlx2+CD24− 14% | Doetsch et al 2002 |
| Dil labeling (Intraventricular injection) | Intraventricular injection of DiI | Dil+ 6.2% | Johansson et al.1999 |
| Erythroagglutinin lectin (E-PHA) | FITC conjugated E-PHA | Low E-PHA 0.02% High E-PHA 0.26% | Hamanoue et al 2009 |
| FGF1B | F1B::GFP | 1% | Hsu et al 2009 |
| GFAP | GFAP-GFP adenoviral labelling | 11.65% | Doetsch,et al.,1999b. |
| GFAP/EGFR/CD24 | hGFAP::GFP/Fluorescent EGF ligand/anti-CD24 | GFAP+EGFR+CD24− 30% EGFR+ 7.5% CD24low 0% GFP+ 0% | Pastrana et al 2009 |
| GFAP/CD133 | hGFAP::GFP/antiCD133 | 78% | Beckervordersandforth et al 2010 |
| Id1 reporter activity | Id1::GFP | Id1GFP high 1% Id1GFP low 0.5% | Nam and Benezra 2009 |
| Mcm2 | Mcm2::GFP | 3% | Maslov et al 2007 |
| Nestin | Nestin::GFP | 0.30% | Kawahuchi et al 2001 |
| Notch 1 | Anti-Notch1 | Notch1+ 2.8% | Johansson et al.1999 |
| Notch reporter activity | TNR::EGFP | EGFP high70% EGFP low10% | Andreu-Agulló et al 2009 |
| Side population (SP) | exclusion of Hoechst 33342 | SP 2.1% Non-SP 0.28% | Kim and Morshead 2003 |
| Sox1 | Sox1::GFP | 1.70% | Barraud et al 2005 |
| Sox2/beta1 integrin | sox2::GFP/ beta1 integrin | Sox2GFP+beta1+ 0.05% Sox2GFP+beta1– 47% | Kazanis et al 2010 |
| Selective killing experiments | |||
| DLX2 | Dlx2-TK | 70% of ns are killed | Doetsch et al 2002 |
| GFAP | GFAP-TK | ~100 % of ns are killed | Imura et al., 2003; Morshead et al., 2003; Garcia et al., 2004 |
Some combinations of markers have allowed significant enrichment of neurosphere forming cells from the adult SVZ (Table 1). However, most of the markers employed in these studies are common to several stages in the lineage and yield mixed populations of neural progenitor cells. When combinations of markers are used that allow the simultaneous isolation of different stages of the lineage, and the neurosphere forming capacity of each population assessed, it has become clear that neurospheres arise from cells within the lineage that express EGFR and are in a proliferative state (activated GFAP+ stem cells and transit amplifying cells) (Table1; Doetsch et al., 2002; Pastrana et al., 2009). Consistent with these findings, killing of transit amplifying cells and dividing GFAP+ cells greatly reduces neurosphere formation (Doetsch et al., 2002; Garcia et al., 2004; Imura et al., 2003; Morshead et al., 2003). Both populations can be serially passaged and are multipotent. As such, the neurosphere assay does not provide an accurate readout of the number of stem cells in vivo. Moreover, the neurosphere assay likely does not detect quiescent stem cells, as the purified population containing putative quiescent stem cells does not give rise to neurospheres (Pastrana et al., 2009). The identification of markers that allow the isolation of quiescent stem cells will allow their sphere-forming capacity to be directly tested. Defining the populations of cells that form neurospheres after injury in various models will also be important to pinpoint potential latent stem cells elsewhere in the brain (Robel et al., 2011). It will be important to assess the long-term self-renewal capacity of different purified populations that can give rise to neurospheres both in vitro and after transplantation in vivo (Neumeister et al., 2009).
As revealed by this historical overview, the neurosphere assay cannot be used alone to define the in vivo stem cells. However, if performed carefully it can provide a useful tool to assay stem cell potential in vitro, in a relatively simple manner. Sphere-forming assays are increasingly used, both retrospectively and prospectively, to investigate stem cells and progenitors in many tissues during development and in the adult (Table 2), as well as in cancers and the cancer stem cell field (Hirschhaeuser et al., 2010; Clevers, 2011). Similarly, they are frequently employed to dissect the molecular regulation of self-renewal and differentiation, and to investigate how the intrinsic properties of stem cells/progenitors cells change with aging and pathology. For the appropriate interpretations of such experiments, it is essential to understand the strengths and limitations of this assay. Below we highlight critical considerations for sphere-forming assays that are relevant for all systems.
Table 2.
Tissues in which sphere-forming assays have been used
| Tissue | Isolation markers | References |
|---|---|---|
| Breast |
|
Dontu et al., 2003 Shackleton et al., 2006 |
| Cornea | Bulk microdissected human corneal epithelium | Yokoo et al., 2005 |
| Dermis | Bulk skin tissue | Toma et al., 2001 |
| Heart | Side Population | Tomita et al., 2005 |
| Pancreas |
|
Seaberg et al., 2004 Rovira, et al., 2010 |
| Pituitary gland | Side Population | Chen et al., 2005 |
| Prostate | Lin (CD45/CD31/Ter119)/Sca1/CD49f | Lawson et al., 2007 |
| Retina | Bulk microdissected ciliary margin of outer retinal pigmented epithelium | Tropepe et al., 2000 |
| Trachea |
|
Rock et al., 2009 |
Critical Considerations for Sphere-Forming Assays
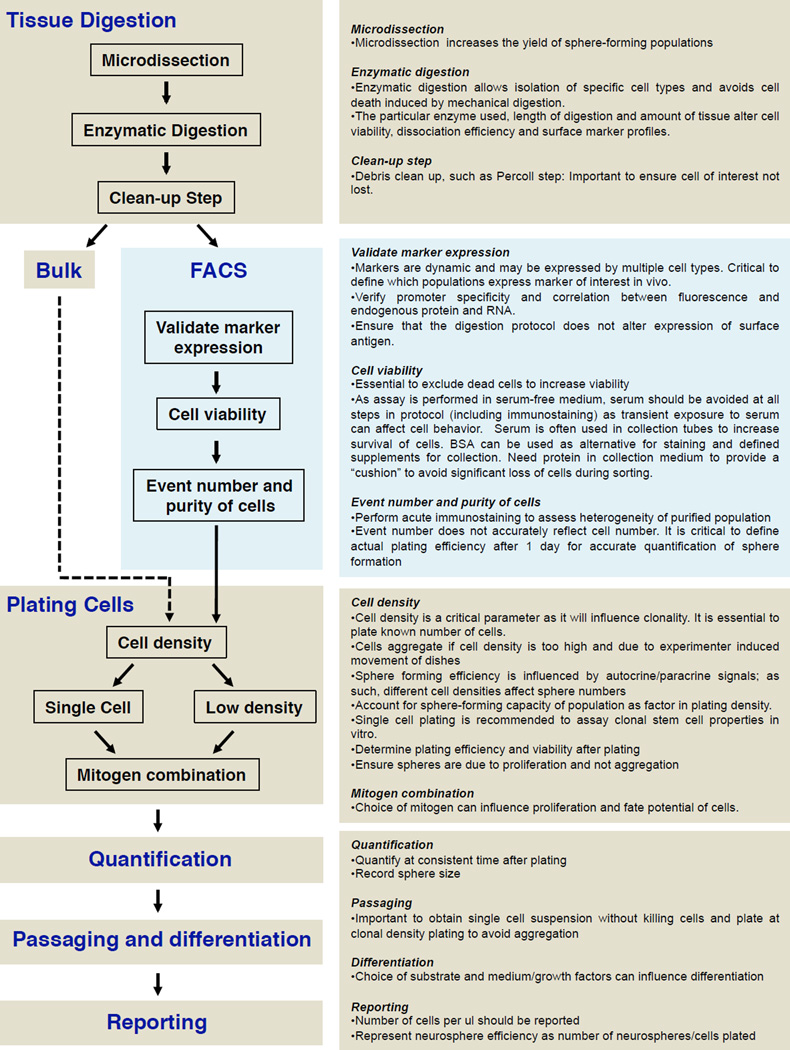
Over the years, experimental variability has been introduced into sphere-forming assays including medium composition and volume, cell density, surface area of the culture dish and duration in culture before quantification (reviewed by Chaichana, et al. 2006). This diversity in protocols has favored differing and sometimes conflicting results to arise from different groups. In Figure 3, we outline the steps and critical experimental parameters that are crucial in the design and execution of sphere assays. Below, we highlight general issues that are essential for the interpretation of sphere-forming assays (Table 3).
Figure 3.
Flowchart outlining design and critical experimental steps in sphere-forming assays
Table 3.
Overview of critical considerations for sphere-forming assays
| Cell density and clonality of spheres | Cell density is critical parameter as it influences clonality. Spheres are prone to aggregation due to both intrinsic locomotion and to experimenter-induced movement. Clonality is only guaranteed by single cell plating. Important to ensure that spheres are due to proliferation of cells and not to aggregation. |
| Sphere-forming assays may not detect quiescent stem cells | Sphere forming assays predominantly detect cells that are poised for, or are actively undergoing, proliferation. Quiescent cells may not be capable of forming spheres, either due to intrinsic cell properties or due to lack of additional extrinsic signals needed for their activation in this assay. |
| Sphere-forming assays are not a readout of in vivo stem cell frequency | Multiple populations in stem cell lineages, including both stem cells and transit amplifying cells, are able to form spheres that can be serially passaged and are multipotent. The long-term in vitro and in vivo potential of purified populations needs to be assessed. |
| Sphere size is not a read-out of in vivo stem cells | Large spheres are often assumed to arise from stem cells. However, independent of aggregation issues, sphere size simply reflects proliferation/ differentiation status and responsiveness to growth factors of the parental clone-forming cell. |
| Towards the prospective purification of sphere-forming cells | As FACS becomes an integral part of assaying the potential of different populations to form spheres, it is essential to ensure that enzymatic digestion does not alter surface marker profiles (different enzymes result in markedly different surface profiles). In addition, the cell type specificity of individual markers needs to be validated in vivo. |
| Markers are dynamic | Purified populations reflect the state of a population at a given moment in time. Within a purified population, cells may be in different states or stages of the cell cycle. Markers commonly used to purify stem cells change their expression with the cell cycle. Cells can shuttle between quiescent and activated states, or from more committed to more primitive states. |
| Differentiation potential bias due to culture with exogenous growth factors | Cells within a population may respond differently to distinct mitogens. Mitogens can also bias the differentiation capacity of cells. |
Cell density and clonality of spheres
Cell density is the most important and controversial parameter of sphere-forming assays as it has a critical impact on clonality. The final readout of sphere-forming assays is the size and number of spheres, whether primary or passaged. A central tenet of sphere-forming assays is that each sphere is derived from a single cell and is therefore clonal.
A wide variety of seeding cell densities, from presumably clonal to much higher cell concentrations, are used in different laboratories. Different groups consider a wide range of ratios of cells per volume of tissue culture medium to be consistent with a clonal density. Indeed, anywhere between 0.2 to 20 cells per µl is considered -or at least named- appropriate for clonal conditions of growth (Coles-Tabake et al., 2008, Ferrón et al.; 2007, Chojnacki and Weiss, 2008). Results obtained from high density seeded cultures are impossible to interpret due to fusion of spheres. Even low-density cultures can be problematic. Mixing experiments in which wild-type and fluorescently labeled cells are co-cultured suggest that a neurosphere can reliably be of clonal origin only when cells are plated at 10 cells/µl or 1 cell/µl when using primary cells and passaged spheres, respectively (Coles-Tabake et al., 2008). However, using imaging approaches, neurospheres were observed to frequently aggregate and fuse, even at low densities. Indeed, time-lapse imaging experiments show that free-floating neurospheres are highly dynamic structures, which undergo intrinsic, spontaneous locomotion (Singec et al., 2006; Mori et al., 2006), even if left untouched in the incubator. A second major cause of non-clonality is experimenter-induced aggregation (Coles-Tabake et al., 2008). Movement of plates to examine cultures under the microscope rapidly leads to the aggregation of spheres at the center of the plate. As such, true clonality can only be guaranteed by plating single cells per well.
Importantly, the choice of an appropriate cell density should be determined by the intended purpose of the individual sphere assay. If the experiment is designed to characterize and define the stem cell potential (self-renewal and differentiation) of a newly identified population in vitro, cells should be plated as single cells per well to ensure clonality. However, it is important to note that cell density directly impacts cell growth. Sphere-forming efficiency decreases significantly when cells are plated as single cells as compared to low-density conditions, due to autocrine/paracrine signals released by cells into the medium. If the purpose of the assay is to study other parameters, such as survival or proliferation, it may be possible to use low density cultures, as long as it is recognized that spheres may not be clonal. When spheres are passaged to assess self-renewal they should be re-plated at extremely low densities to avoid cell fusion and aggregation.
It is also important to perform clonal density cultures even at the primary sphere stage. Increasingly, primary spheres are cultured at very high density and then upon passaging plated at lower density. However, this practice can greatly impact the interpretation of results. To reiterate, both primary and passaged spheres should be cultured at clonal density. Finally, irregular clumps of cells resulting from cell aggregation can also appear in sphere cultures (Chen et al., 2005). For any stem cell system, it should be validated that spheres actually arise from proliferation (such as by a short pulse of a nucleoside analogue or by time-lapse imaging) and are not simply the result of the aggregation of cells.
To circumvent some of the above technical hurdles, variations of the classical sphere assay have recently been proposed. Encapsulating spheres or culturing them in semi-adherent conditions (i.e. methylcellulose or collagen), similar to colony forming assays in the hematopoietic field (Purton and Scadden, 2007), or microengineered hydrogel matrices may avoid problems associated with experimenter-induced aggregation and intrinsic mobility of spheres (Ignatova, et al., 2002; Cordey et al., 2008, Louis et al., 2008).
Sphere-forming assays may not detect quiescent stem cells
An important caveat of sphere forming assays is that they may not detect quiescent stem cells. Quiescent stem cells reside in a G0 state, which likely prevents their depletion in vivo and the possibility of the introduction of mutations during replication. In contrast, sphere-forming assays predominantly allow the expansion of cells that are either poised for proliferation in vivo or are already actively dividing, and can therefore be rapidly expanded in vitro with mitogens. As such, it may never be feasible to detect quiescent stem cells by sphere-forming assays, as the protocols used do not provide as yet unknown key components of the in vivo niche required for the activation of dormant stem cells, either during homeostasis or after injury. In addition, the intrinsic properties of quiescent stem cells may limit their rapid expansion in the presence of growth factors, such as inherently slower cell cycle kinetics, and an intrinsic limitation on the number of times they can divide before being exhausted. Once markers are identified that allow quiescent stem cells to be purified directly from their niche, insight will be gained into their physiology and molecular regulation.
Sphere-forming assays are not a read-out of in vivo stem cell frequency
Stem cell frequency is often calculated based on the number of spheres generated from a given tissue sample. This premise is based on the false assumption that all spheres are derived from a stem cell. Indeed, as described above, FACS purification of neural stem cells and their progeny have revealed that both stem cells and their transit amplifying progeny can give rise to neurospheres. Furthermore, this pattern has also been observed with spheres that arise from isolated mammary populations, termed mammospheres (Stingl, 2009). As such, sphere-forming assays are not a read-out of in vivo stem cell activity, but instead may reflect the potential of cells to exhibit stem cell traits.
Based on modeling of predicted and actual serial sphere forming capacity, it has been proposed that the neurosphere assay overestimates stem cell frequency by an order of magnitude (Reynolds and Rietze, 2005). Less than 6% of cells in neurospheres can be passaged more than seven times, suggesting that only a small fraction of cells exhibit extensive self-renewal (Louis et al., 2008). These findings were all based on retrospective analysis of neurosphere formation. Indeed, it has become common lore that serial passaging will eliminate more committed progenitors and select for self-renewing stem cells. However, both purified transit amplifying cells from the adult brain, which are short-lived cells in vivo, and multipotent progenitors in breast can be serially passaged and retain multipotency in vitro. Using prospectively purified populations, it will now be important to determine whether there is a difference in the number of times cells that were isolated at different stages can be serially passaged in vitro. While sphere culture conditions may allow long-term passaging of both cell types, this capacity might not be the case when cells are exposed to the in vivo niche. More challenging experimental conditions such as transplantation paradigms or in vivo lineage tracing are necessary complements of in vitro assays that will reveal differences in stem cell behaviour and potential.
Sphere size is not a read-out of in vivo stem cells
Significant heterogeneity exists in the size of individual spheres, independent of the problem of merging, and it has been posited that size indicates the nature of the founder clone. This premise is confounded by non-uniform criteria regarding what sized spheres to quantify, which typically ranges from 40–150 µm in diameter. Stem cells are believed to give rise to large spheres, and progenitors to smaller spheres. It has been postulated that only large spheres can be serially passaged, as opposed to smaller spheres, which cannot. However, this hypothesis has not been rigourously tested with prospectively purified cells. Indeed, the size of a sphere might also reflect responsiveness to growth factors as well as the proliferation/differentiation status of the parental clone-forming cell. This observation has important implications for interpreting sphere size in loss-of-function studies: smaller spheres could be a result of decreased self-renewal, or altered responsiveness to growth factors. Furthermore, the mode of division within a sphere can impact the size of a sphere. For example, smaller neurospheres (<100µm) grown in leukemia inhibitory factor (LIF) give rise to secondary neurospheres to the same extent as much larger neurospheres grown with EGF alone (Bauer, 2009). This fact is likely due to increased self-renewing divisions in the smaller spheres, and the presence of more differentiated cells in the large spheres, which are incapable of being passaged. Thus large clones may actually contain fewer stem cells than smaller clones.
Towards the prospective purification of sphere-forming cells
The ability to prospectively purify different populations of cells and assess their in vitro and in vivo behavior is a crucial advance in the stem cell field. Isolating stem and progenitor cells from solid tissues presents unique challenges to obtaining a viable single cell suspension. Stem cells from solid tissues are often relatively rare populations enmeshed in a complex extracellular-rich microenvironment. As such it is crucial to optimize each isolation step to maximize yields (Figure 3). The use of FACS to isolate cells from solid tissue has recently been reviewed elsewhere (Alexander et al., 2009). Particular care has to be taken when FACS is used to isolate different populations, as both the enzyme used and duration of digestion can profoundly affect surface antigen survival, thereby influencing marker expression. Indeed, the expression patterns of CD133, CD15 and CD24 on embryonic neural progenitors are dramatically different depending on whether trypsin, papain, collagenase/dispase or liberase 1 is utilized (Panchision et al., 2007), with distinct populations of cells appearing and disappearing depending on the enzyme. As more and more complex combinations of markers are used to FACS purify more refined populations of cells, this issue becomes increasingly important and might explain conflicting results observed by various groups that use different dissociation protocols. For example, it was recently reported that CD133+GFAP::GFP+ cells contain all neurosphere forming cells in the adult SVZ (Beckervordersandforth et al., 2010). However, when papain is used, instead of trypsin, both EGFR+GFAP::GFP+ and EGFR+ only cells give rise to neurospheres (Pastrana et al., 2009). As such it is crucial to ensure that the profile observed by FACS matches the in vivo expression pattern as well as to account for strain and species differences.
Another key issue is to validate the specificity of markers in vivo and to define the populations that express them. Sometimes it may be difficult to detect expression of a marker by rare cells if it is highly expressed by more abundant cells. An ongoing debate in the neural stem cell field is whether ependymal cells are stem cells in the SVZ (Johansson et al., 1999, Coskun et al., 2008). These claims are based on the putative selective expression of markers on ependymal cells. Both CD133 and FoxJ1 (Coskun et al., 2008; Meletis et al., 2008) have been suggested to be exclusively expressed by ependymal cells and been used to purify them, followed by neurosphere cultures. However, both markers are also expressed by non-ependymal, GFAP+ (Type B1) cells, which are highly enriched for neurosphere forming potential (Mirzadeh et al., 2008, Beckervordersandforth et al., 2010; Jacquet et al., 2009). This overlap highlights how important it is to establish the specificity of markers used to purify cells and assess their functional properties in vivo and in vitro.
A similar issue exists when labeling strategies are employed to prospectively identify cells based on their anatomical localization, for example the injection of lipophilic dyes, such as DiI, or of viruses encoding reporters, to label cells contacting a lumen. It is essential to ensure that the tracer is not transferred between cells, and that all of the populations that contact the lumen are known. In the brain, injection of tracers into the ventricles will lead to the labeling of both ependymal cells and GFAP+ stem cells that contact the ventricle (Figure 2C), which initially led to some confusion about the identify of neurosphere forming cells.
Markers are dynamic
Purified populations remain heterogeneous, and the iterative identification of additional markers will allow their further enrichment. However, an important point to consider is that two apparently distinct populations may actually be the same population of cells that are in different states or stages of the cell cycle. For example, the expression of Hes1, neurogenin2 and Delta1, proteins that are classically thought to distinguish various populations of cells in the developing brain, oscillates within the same cell in a cell cycle-dependent manner (Kageyama et al., 2010). CD133, a marker frequently used to isolate putative stem cells, is another excellent example of a protein that is influenced by the cell cycle state (Sun et al., 2009). In vivo, CD133 is expressed on primary cilia, yet in order to divide, cells must disassemble the primary cilium, as the centriole in the basal body is required for the centrosome. As such, these cells will lose CD133 expression. Finally, two other recent findings have important implications for the purification of different cells. An emerging theme in stem cell biology is that cells can shuttle between quiescent and activated states (Li and Clevers, 2010) and that even more committed progenitors can revert back to a more primitive state (Davies and Fuller, 2008), underscored by the neutral drift that occurs in populations over times in diverse stem cell systems (López-García et al., 2010; Snippert et al., 2010).
Differentiation potential bias due to culture with exogenous growth factors
Traditionally, spheres are cultured in high levels of growth factors, in the presence of EGF (20 ng/ml), bFGF (10ng/ml) or both in many systems. Such high concentrations may bias the differentiation potential of the cultured cells. For example, standard culture conditions for neurospheres use high concentrations of EGF, which heavily biases the cells towards glial differentiation, both in vitro and after transplantation in vivo. Lowering the concentration of EGF promotes more neuronal differentiation (Burrows et al., 1997), but the neural stem cell field continues to use high levels of EGF in the medium. A second key point is whether the same or different cells grow in the presence of different growth factors. With the advent of the ability to purify different populations of cells by FACS, this issue can now be addressed directly. An important question is whether the multipotency observed in vitro also translates to the same set of fates being adopted in vivo, or whether this multipotent capacity is only unmasked in the specific conditions present in culture.
Alternatives to sphere-forming assays
Modifications to sphere-forming assays have been developed which circumvent some of the limitations described above. These include bioengineering approaches using patterned substrates to mimic the in vivo extracellular matrix and substrate elasticity, adherent two dimensional and three dimensional cultures, such as CFU assays and co-culture configurations with different niche components (reviewed in Vunjak-Novakovic and Scadden, 2011). With any of these newer variants, it is important to assess the behaviour of purified populations in each assay.
An increasingly widely used assay in the neural stem cell field is the neuronal colony-forming cell assay (NCFCA) (Louis et al., 2008), in which cells are cultured in a collagen-containing semi-solid matrix with EGF and/or bFGF. This assay retrospectively defines stem cells based on the size of the colony formed, with large colonies over 1–2mm in size postulated to be derived from stem cells, and all other smaller colonies from progenitors. This assay is based on the premise that progenitor cells exhibit limited proliferative capacity in relation to stem cells, and that the diameter of a colony can be used to distinguish its founder cell type. It will be crucial to directly test this assumption using purified populations of cells. While this assay circumvents the issue of aggregation, it still suffers from several of the same limitations of neurosphere assays, namely, dormant stem cells may not divide to form large colonies, colony size may simply reflect growth factor responsiveness, and the same biases of culturing in high levels of growth factors also remain.
Within a sphere, significant differentiation occurs due to complex cell-cell interactions. Two-dimensional adherent culture, in which stem cells and their progeny are expanded as monolayers, significantly reduces the number of differentiated cells during stem cell expansion. For example, culture of neural stem cells as cell lines as a monolayer of adherent cells in the presence of EGF and bFGF allows the propagation of a reasonably uniform population of cells with much less differentiation than observed in non-adherent assays (Conti et al., 2005; Pollard et al., 2009). However, this approach makes it more difficult to monitor single clones. Another promising approach is the co-culture of purified stem cells with different “niche” cells, in the absence of additional growth factors, which provide more physiological signals. Both endothelial cells and astrocyte co-cultures support the growth of neural stem cells (Lim and Alvarez-Buylla, 1999; Song et al., 2002; Shen et al., 2004; Cheng et al., 2009). An attractive feature of 2D cultures is that morphological analysis and molecular characterization can easily be assessed. Moreover, time-lapse imaging of single colonies can reveal lineage dynamics of individual cells (Qian et al., 1998; Scheffler et al., 2005; Cohen et al., 2010; Costa et al., 2011).
Finally, three-dimensional Matrigel cultures have been very powerful in providing a proper microenvironment for clonal mini-organs to grow from single cells combined with other niche cells (Sato et al., 2009). Such three-dimensional cultures may constitute the ideal system to start assessing and manipulating quiescent stem cells in vitro that have not yet been able to grow in culture.
Towards the future
As our understanding of the in vivo biology of adult stem cells and their niche deepens, it is crucial to develop new in vitro assays that overcome the limitations and practical pitfalls of sphere-forming assays and their modifications highlighted in this review. These new assays will need to assess self-renewal and multipotency at a clonal level without biasing cells by introducing saturating levels of exogenous growth factors. Further development of technologies such as engineered culture matrices that allow single cell assays in both adherent and floating conditions will be an important step towards high throughput assays that can assess the role of different molecules on stem cell physiology. In combination with increasingly sophisticated purification methods these studies will enable the rigorous comparison of the biological differences between stem cells and their progeny. It will be important for the community to extend and standardize the use of these purification methods so that different assays can be cross-compared and their effect on the different populations better assessed. Importantly, stem cells cannot only be studied in isolation. In addition to the development of 3D culture models that recapitulate the in vivo niche it will remain essential to explore the biology of stem cell populations in vivo using transplant paradigms and in vivo lineage tracing of endogenous populations. Transplantation of purified stem cell populations or cultured cells back into their endogenous niches can complement in vitro assays in evaluating the in vivo potential of these populations. Moreover, serial transplantation studies could help determine the self-renewal capacities of these cells. Great leaps forward will continue to be made by the synergy between in vivo and in vitro approaches, which mutually inform each other.
Acknowledgements
We thank Angel Maldonado-Soto for the image of the pinwheel. We apologize to the many studies we could not cite due to space limitations. VSV is supported by a Human Frontiers Scientific Program Long-Term Fellowship, EP was supported by Grant T32 MH15174–29 and by a fellowship from the Spanish Ministerio de Educación y Ciencia, FD is a Packard Foundation Fellow and Irma T. Hirschl Fellow. We receive support from NIH NINDS, NYSTEM, and the Jerry and Emily Spiegel Laboratory for Cell Replacement Therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander CM, Puchalski J, Klos KS, Badders N, Ailles L, Kim CF, Dirks P, Smalley MJ. Separating stem cells by flow cytometry: reducing variability for solid tissues. Cell Stem Cell. 2009;5:579–583. doi: 10.1016/j.stem.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC, Beckers J, Yoder B, Irmler M, Götz M. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Barraud P, Thompson L, Kirik D, Björklund A, Parmar M. Isolation and characterization of neural precursor cells from the Sox1-GFP reporter mouse. Eur. J. Neurosci. 2002;22:1555–1569. doi: 10.1111/j.1460-9568.2005.04352.x. [DOI] [PubMed] [Google Scholar]
- Bauer S. Cytokine control of adult neural stem cells. Ann. NY. Acad. Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisén J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Chaichana K, Zamora-Berridi G, Camara-Quintana J, Quiñones-Hinojosa A. Neurosphere assays: growth factors and hormone differences in tumor and nontumor studies. Stem Cells. 2006;24:2851–2857. doi: 10.1634/stemcells.2006-0399. [DOI] [PubMed] [Google Scholar]
- Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146:3985–3998. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki A, Weiss S. Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat. Protoc. 2008;3:935–940. doi: 10.1038/nprot.2008.55. [DOI] [PubMed] [Google Scholar]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat. Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Gomes FL, Roysam B, Cayouette M. Computational prediction of neural progenitor cell fates. Nat Methods. 2010;7:213–218. doi: 10.1038/nmeth.1424. [DOI] [PubMed] [Google Scholar]
- Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, van der Kooy D. Don't look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26:2938–2944. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells. 2008;26:2586–2594. doi: 10.1634/stemcells.2008-0498. [DOI] [PubMed] [Google Scholar]
- Corti S, Nizzardo M, Nardini M, Donadoni C, Locatelli F, Papadimitriou D, Salani S, Del Bo R, Ghezzi S, Strazzer S, Bresolin N, Comi GP. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol. 2007;205:547–562. doi: 10.1016/j.expneurol.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, de Vellis J, Sun YE. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc. Natl. Acad. Sci. U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Ortega F, Brill MS, Beckervordersandforth R, Petrone C, Schroeder T, Götz M, Berninger B. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. doi: 10.1242/dev.061663. [DOI] [PubMed] [Google Scholar]
- Davies EL, Fuller MT. Regulation of self-renewal and differentiation in adult stem cell lineages: lessons from the Drosophila male germ line. Cold Spring Harb Symp Quant Biol. 2008;73:137–145. doi: 10.1101/sqb.2008.73.063. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc. Natl. Acad. Sci. U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrón SR, Andreu-Agullo C, Mira H, Sanchez P, Marques-Torrejon MA, Fariñas I. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat. Protoc. 2007;2:849–859. doi: 10.1038/nprot.2007.104. [DOI] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- García AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frölichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Götz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hermann A, Suess C, Fauser M, Kanzler S, Witt M, Fabel K, Schwarz J, Höglinger GU, Storch A. Rostro-caudal gradual loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells. 2009;27:928–941. doi: 10.1002/stem.21. Erratum in: Stem Cells. 2010.28, 843. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Matsuzaki Y, Sato K, Okano HJ, Shibata S, Sato I, Suzuki S, Ogawara M, Takamatsu K, Okano H. Cell surface N-glycans mediated isolation of mouse neural stem cells. J Neurochem. 2009;110:1575–1584. doi: 10.1111/j.1471-4159.2009.06256.x. [DOI] [PubMed] [Google Scholar]
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J. Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Lee DC, Chen SL, Liao WC, Lin JW, Chiu WT, Chiu IM. Brain-specific 1B promoter of FGF1 gene facilitates the isolation of neural stem/progenitor cells with self-renewal and multipotent capacities. Dev Dyn. 2009;238:302–314. doi: 10.1002/dvdy.21753. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J. Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet BV, Salinas-Mondragon R, Liang H, Therit B, Buie JD, Dykstra M, Campbell K, Ostrowski LE, Brody SL, Ghashghaei HT. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman SA, Okano H. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Niwa Y, Shimojo H, Kobayashi T, Ohtsuka T. Ultradian oscillations in Notch signaling regulate dynamic biological events. Curr Top Dev Biol. 2010;92:311–331. doi: 10.1016/S0070-2153(10)92010-3. [DOI] [PubMed] [Google Scholar]
- Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME, ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J. Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. U S A. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J. Neurosci. 2003;23:10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Shen Q, Temple S. The incredible elastic brain: how neural stem cells expand our minds. Neuron. 2008;60:420–429. doi: 10.1016/j.neuron.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- López-García C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26:988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Bailey KJ, Mielnicki LM, Freeland AL, Sun X, Burhans WC, Pruitt SC. Stem/progenitor cell-specific enhanced green fluorescent protein expression driven by the endogenous Mcm2 promoter. Stem Cells. 2007;25:132–138. doi: 10.1634/stemcells.2006-0032. [DOI] [PubMed] [Google Scholar]
- Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Ninomiya K, Kino-oka M, Shofuda T, Islam MO, Yamasaki M, Okano H, Taya M, Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J. Neurosci. Res. 2006;84:1682–1691. doi: 10.1002/jnr.21082. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur.J. Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister B, Grabosch A, Basak O, Kemler R, Taylor V. Neural progenitors of the postnatal and adult mouse forebrain retain the ability to self-replicate, form neurospheres, and undergo multipotent differentiation in vivo. Stem Cells. 2009;27:714–723. doi: 10.1634/stemcells.2008-0985. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Obermair FJ, Fiorelli R, Schroeter A, Beyeler S, Blatti C, Zoerner B, Thallmair M. A novel classification of quiescent and transit amplifying adult neural stem cells by surface and metabolic markers permits a defined simultaneous isolation. Stem Cell Res. 2010;5:131–143. doi: 10.1016/j.scr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J. Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl. Acad. Sci. U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger CV, Steinhoff C, Hertwig F, Nuber UA. Prospectively isolated CD133/CD24-positive ependymal cells from the adult spinal cord and lateral ventricle wall differ in their long-term in vitro self-renewal and in vivo gene expression. Glia. 2011;59:68–81. doi: 10.1002/glia.21077. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, Squire JA, Smith A, Dirks P. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Qian X, Goderie SK, Shen Q, Stern JH, Temple S. Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development. 1998;125:3143–3152. doi: 10.1242/dev.125.16.3143. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl. Acad. Sci. U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder E. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat. Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stingl J. Detection and analysis of mammary gland stem cells. J. Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sun Y, Kong W, Falk A, Hu J, Zhou L, Pollard S, Smith A. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One. 2009;4:e5498. doi: 10.1371/journal.pone.0005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;13:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJL, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of neural precursors from skin. Nature Cell Biology. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J. Cell Biol. 2005;170:1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Coles BKL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, Van der Kooy D. Retinal Stem Cells in the Adult Mammalian Eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J. Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGFgenerated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Scadden D Biomimetic Platforms for Human Stem Cell Research. Cell Stem Cell. 2011;8:252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, Yasuda T, Adams DJ, Bartlett PF. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J. Neurosci. 2007;27:3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo S, Yamagami S, Yanagi Y, Uchida S, Mimura T, Usui T, Amano S. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest. Ophthalmol. Vis. Sci. 2005;46:1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J. Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]