Abstract
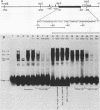
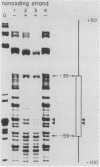
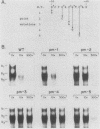
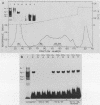
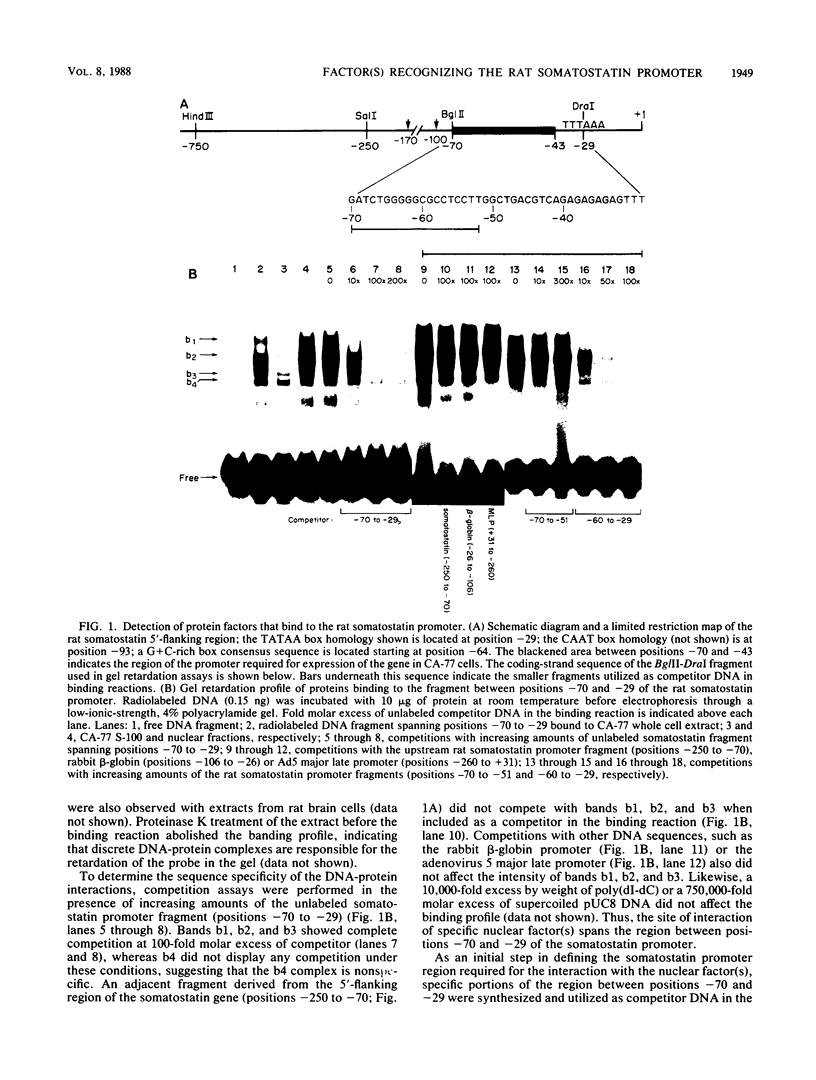
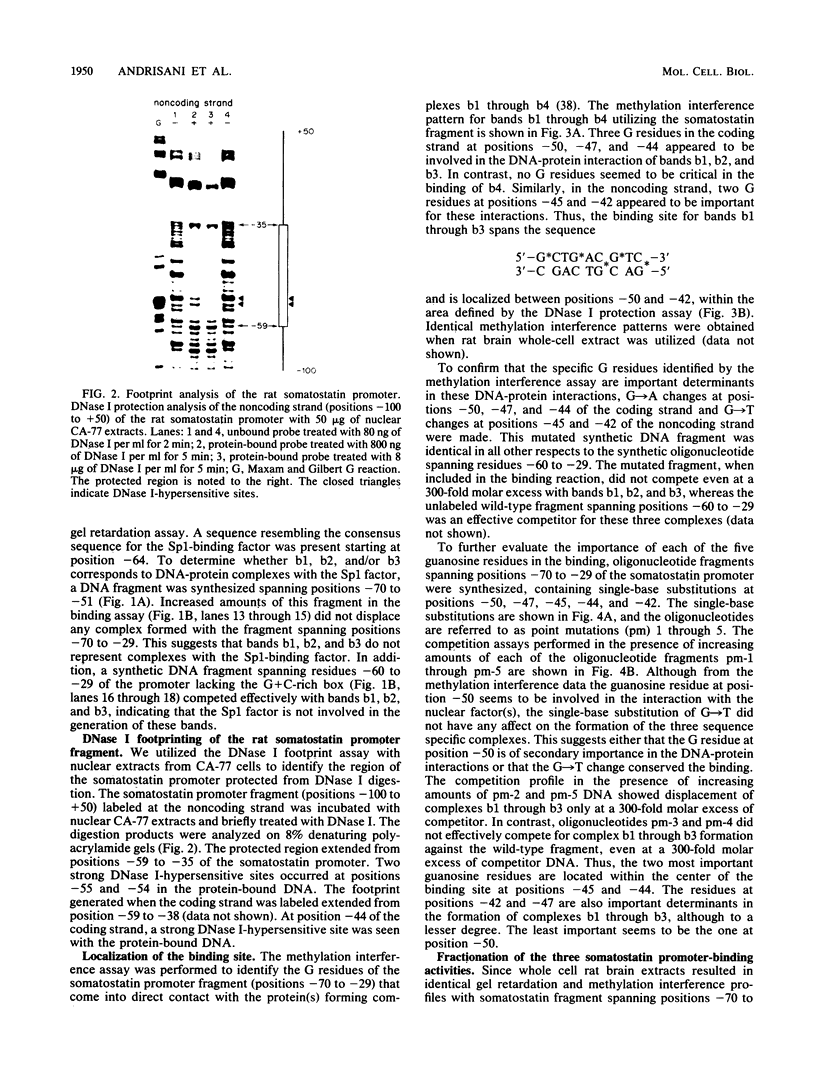
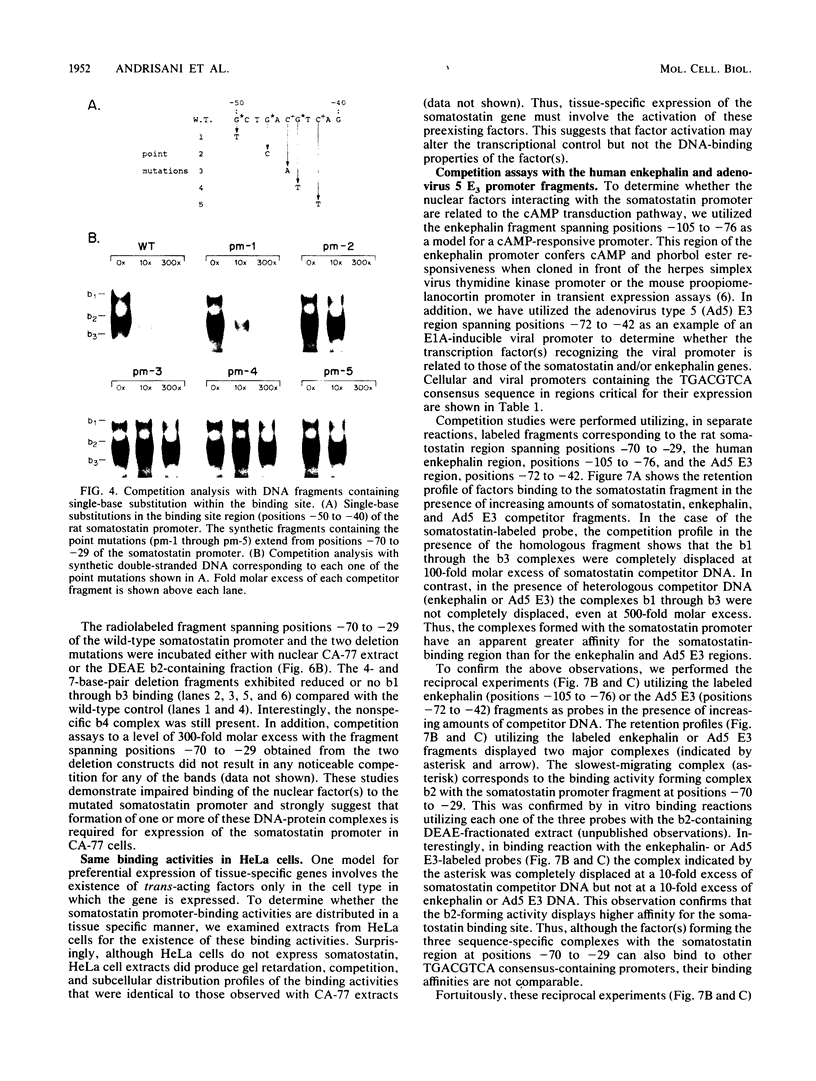
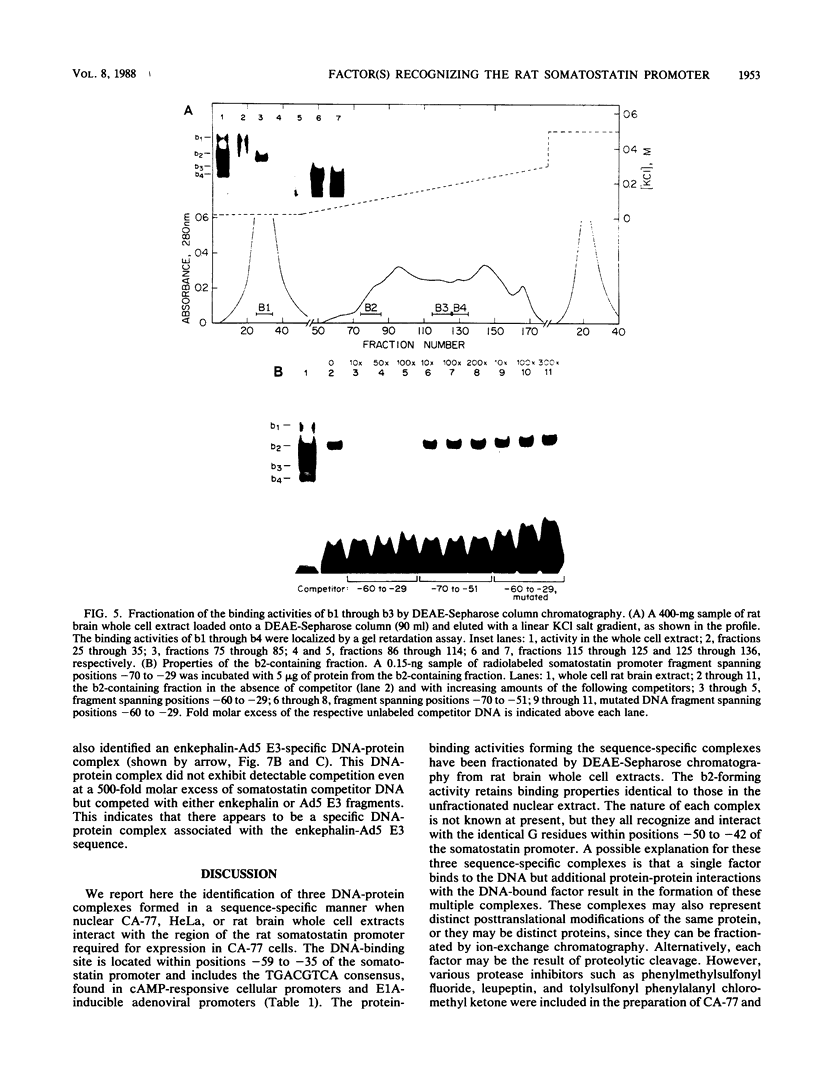
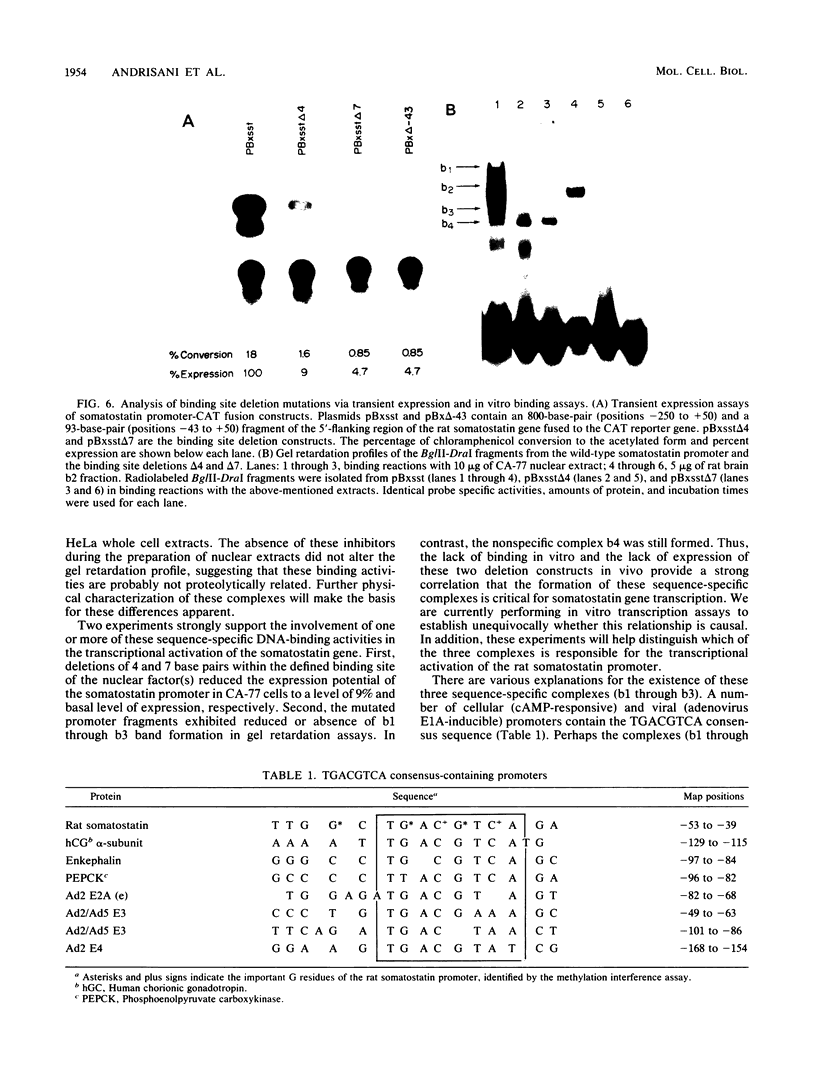
We identified three sequence-specific DNA-protein complexes which are formed after in vitro binding of nuclear extracts, derived from neuronal (CA-77, rat brain) or non-neuronal (HeLa) cells, to positions -70 to -29 of the rat somatostatin promoter. The protein(s) responsible for the formation of the three sequence-specific complexes was fractionated from rat brain whole cell extracts by DEAE-Sepharose chromatography. The critical contact residues of the factor(s) in each complex, as determined by methylation interference analyses, are located within positions -59 to -35, which is protected from DNase I digestion; these include the G residues of a TGACGTCA consensus also found in the cAMP-responsive human enkephalin (positions -105 to -76) and E1A-inducible adenovirus type 5 E3 (positions -72 to -42) promoters. Competition assays with these heterologous promoters reveal that the factor(s) of each complex displays approximately 50-fold greater affinity for the somatostatin promoter-binding site. Synthetic oligonucleotides spanning positions -70 to -29 of the somatostatin promoter and containing single-base substitutions of the G residues in the TGACGTCA consensus were utilized in competition assays. The G residues located in the center of the module are the most critical determinants in the formation of the three sequence-specific complexes. Deletions disrupting the TGACGTCA consensus abolish not only formation of the three complexes in vitro but also expression of the somatostatin promoter in vivo, suggesting that formation of one or more of these complexes is essential for transcription of the rat somatostatin gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrisani O. M., Hayes T. E., Roos B., Dixon J. E. Identification of the promoter sequences involved in the cell specific expression of the rat somatostatin gene. Nucleic Acids Res. 1987 Jul 24;15(14):5715–5728. doi: 10.1093/nar/15.14.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron D. C., Muszynski M., Birnbaum R. S., Sabo S. W., Roos B. A. Somatostatin elaboration by monolayer cell cultures derived from transplantable rat medullary thyroid carcinoma: synergistic stimulatory effects of glucagon and calcium. Endocrinology. 1981 Dec;109(6):1830–1834. doi: 10.1210/endo-109-6-1830. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Crabb D. W., Dixon J. E. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal Biochem. 1987 May 15;163(1):88–92. doi: 10.1016/0003-2697(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Haun R. S., Funckes C. L., Dixon J. E. A gene encoding rat cholecystokinin. Isolation, nucleotide sequence, and promoter activity. J Biol Chem. 1985 Jan 25;260(2):1280–1286. [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funckes C. L., Minth C. D., Deschenes R., Magazin M., Tavianini M. A., Sheets M., Collier K., Weith H. L., Aron D. C., Roos B. A. Cloning and characterization of a mRNA-encoding rat preprosomatostatin. J Biol Chem. 1983 Jul 25;258(14):8781–8787. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T. E., Dixon J. E. Z-DNA in the rat somatostatin gene. J Biol Chem. 1985 Jul 5;260(13):8145–8156. [PubMed] [Google Scholar]
- Hayes T. E., Kitchen A. M., Cochran B. H. Inducible binding of a factor to the c-fos regulatory region. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1272–1276. doi: 10.1073/pnas.84.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerker D. J., Ruch W., Chideckel E., Palmer J., Goodner C. J., Ensinck J., Gale C. C. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science. 1974 Apr 26;184(4135):482–484. doi: 10.1126/science.184.4135.482. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Green M. R. A cellular transcription factor E4F1 interacts with an E1a-inducible enhancer and mediates constitutive enhancer function in vitro. EMBO J. 1987 May;6(5):1345–1353. doi: 10.1002/j.1460-2075.1987.tb02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minth C. D., Andrews P. C., Dixon J. E. Characterization, sequence, and expression of the cloned human neuropeptide Y gene. J Biol Chem. 1986 Sep 15;261(26):11974–11979. [PubMed] [Google Scholar]
- Minth C. D., Bloom S. R., Polak J. M., Dixon J. E. Cloning, characterization, and DNA sequence of a human cDNA encoding neuropeptide tyrosine. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4577–4581. doi: 10.1073/pnas.81.14.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Reichlin S. Somatostatin in hypothalamus, extrahypothalamic brain, and peripheral tissues of the rat. Endocrinology. 1978 Feb;102(2):523–530. doi: 10.1210/endo-102-2-523. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Grimelius L., Bloom S. R. Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet. 1975 May 31;1(7918):1220–1222. doi: 10.1016/s0140-6736(75)92198-4. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Inducible binding of a factor to the c-fos enhancer. Cell. 1986 Dec 5;47(5):777–784. doi: 10.1016/0092-8674(86)90520-9. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Tavianini M. A., Hayes T. E., Magazin M. D., Minth C. D., Dixon J. E. Isolation, characterization, and DNA sequence of the rat somatostatin gene. J Biol Chem. 1984 Oct 10;259(19):11798–11803. [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. Adenovirus E3-early promoter: sequences required for activation by E1A. Nucleic Acids Res. 1985 Jul 25;13(14):5389–5402. doi: 10.1093/nar/13.14.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Lugo T. G., Short J. M., Fournier R. E., Hanson R. W. Identification of a cAMP regulatory region in the gene for rat cytosolic phosphoenolpyruvate carboxykinase (GTP). Use of chimeric genes transfected into hepatoma cells. J Biol Chem. 1984 Oct 10;259(19):12161–12169. [PubMed] [Google Scholar]