Abstract
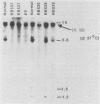
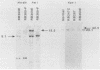
Retinoblastoma (RB) tumors develop when both alleles of a gene (RB1) are mutated and unable to function normally. Recently, Friend et al. [S. H. Friend, R. Bernards, S. Rogelj, R. A. Weinberg, J. M. Rapaport, D. M. Albert, and T. P. Dryja, Nature (London) 32:643-646, 1986] reported the cloning of a gene, 4.7R, with some properties expected for the RB1 gene, namely, a high frequency (30%) of genomic rearrangements in tumors and absence of message in all RB tumors examined. To extend the characterization of this gene, we used 4.7R probes to search for genomic rearrangements of DNA and to study the expression of the 4.7R gene in RB tumors, osteosarcoma (OS) tumors arising in RB patients, and other normal and malignant tissues. In 34 previously unreported RB and OS tumors arising in RB patients, we observed only four (12%) with genomic abnormalities. Transcripts of 4.7R were present in 12 of 17 RB tumors, 2 of 2 OS tumors, and all non-RB tumors and normal tissues tested. We were unable to confirm the high frequency of truncated messages of 4.7R in RB tumors reported by Lee et al. (W. H. Lee, R. Bookstein, F. Hong, L. J. Young, J. Y. Shaw, and E. Y. Lee, Science 235:1394-1399, 1987) and Fung et al. (Y. K. Fung, A. L. Murphree, A. Tang, J. Qian, S. H. Hinrichs, and W. F. Benedict, Science 236:1657-1661, 1987) but did confirm the presence of a truncated transcript in the RB cell line Y79. Of the RB and RB-related OS tumors which appeared normal on Southern blots, 2 of 26 or 12% had abnormal transcripts, giving a combined frequency of 22% abnormalities in the 4.7R gene detectable by Southern and Northern (RNA) blot analyses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Byrd P., Brown K. W., Gallimore P. H. Malignant transformation of human embryo retinoblasts by cloned adenovirus 12 DNA. Nature. 1982 Jul 1;298(5869):69–71. doi: 10.1038/298069a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper G. J., Sanders B. M., Kingston J. E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 May;53(5):661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A. M., Morgan C., Gallie B. l., Phillips R. A., Squire J. Re-evaluation of the sublocalization of esterase D and its relation to the retinoblastoma locus by in situ hybridization. Cytogenet Cell Genet. 1987;44(2-3):153–157. doi: 10.1159/000132361. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gallie B. L., Albert D. M., Wong J. J., Buyukmihci N., Pullafito C. A. Heterotransplantation of retinoblastoma into the athymic "nude" mouse. Invest Ophthalmol Vis Sci. 1977 Mar;16(3):256–259. [PubMed] [Google Scholar]
- Gallie B. L., Holmes W., Phillips R. A. Reproducible growth in tissue culture of retinoblastoma tumor specimens. Cancer Res. 1982 Jan;42(1):301–305. [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H. Molecular cloning of the human esterase D gene, a genetic marker of retinoblastoma. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6337–6341. doi: 10.1073/pnas.83.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Wheatley W., Benedict W. F., Lee E. Y. A null allele of esterase D is a marker for genetic events in retinoblastoma formation. Hum Genet. 1987 May;76(1):33–36. doi: 10.1007/BF00283046. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- McFall R. C., Sery T. W., Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977 Apr;37(4):1003–1010. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T. W., Albert D. M., Rabson A. S., Russell P., Craft J., Chu E. W., Tralka T. S., Wilcox J. L. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974 Aug;53(2):347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- Sakai K., Kanda N., Shiloh Y., Donlon T., Schreck R., Shipley J., Dryja T., Chaum E., Chaganti R. S., Latt S. Molecular and cytologic analysis of DNA amplification in retinoblastoma. Cancer Genet Cytogenet. 1985 Jun;17(2):95–112. doi: 10.1016/0165-4608(85)90020-2. [DOI] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M., Grzeschik K. H., Naylor S. L., Sakaguchi A. Y., Brodeur G., Trent J. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984 Mar 15;308(5956):288–291. doi: 10.1038/308288a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Squire J., Dryja T. P., Dunn J., Goddard A., Hofmann T., Musarella M., Willard H. F., Becker A. J., Gallie B. L., Phillips R. A. Cloning of the esterase D gene: a polymorphic gene probe closely linked to the retinoblastoma locus on chromosome 13. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6573–6577. doi: 10.1073/pnas.83.17.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J., Gallie B. L., Phillips R. A. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70(4):291–301. doi: 10.1007/BF00295364. [DOI] [PubMed] [Google Scholar]
- Squire J., Goddard A. D., Canton M., Becker A., Phillips R. A., Gallie B. L. Tumour induction by the retinoblastoma mutation is independent of N-myc expression. Nature. 1986 Aug 7;322(6079):555–557. doi: 10.1038/322555a0. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A., Meo P., Rovera G. Regulation of acid phosphatase activity in human promyelocytic leukemic cells induced to differentiate in culture. J Cell Biol. 1979 Nov;83(2 Pt 1):300–307. doi: 10.1083/jcb.83.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger L., Winger C., Shastry P., Russell A., Longenecker M. Efficient generation in vitro, from human peripheral blood cells, of monoclonal Epstein-Barr virus transformants producing specific antibody to a variety of antigens without prior deliberate immunization. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4484–4488. doi: 10.1073/pnas.80.14.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]