Abstract
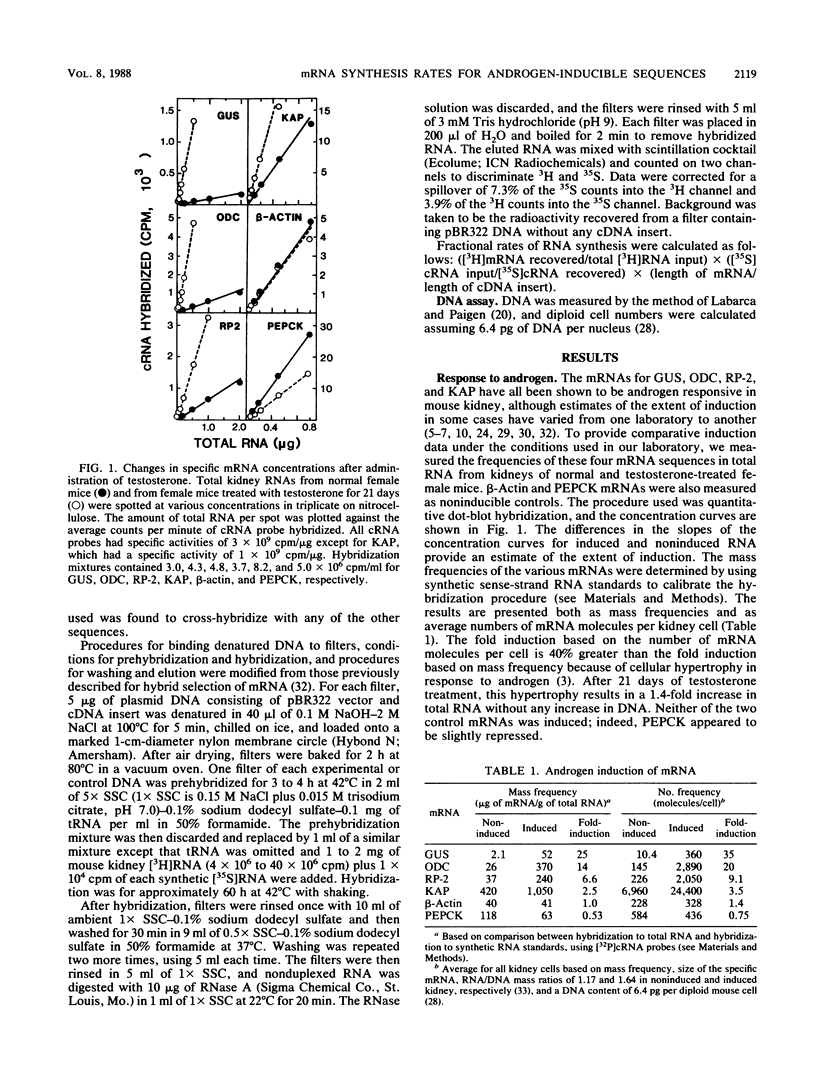
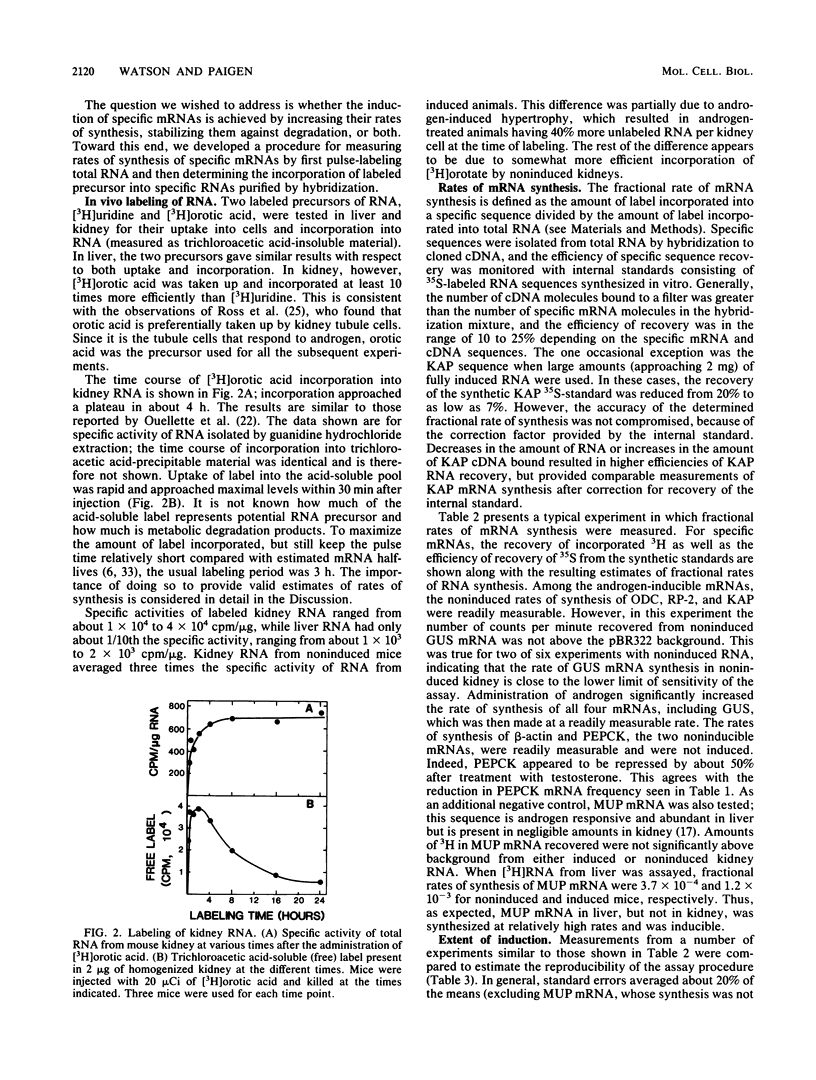
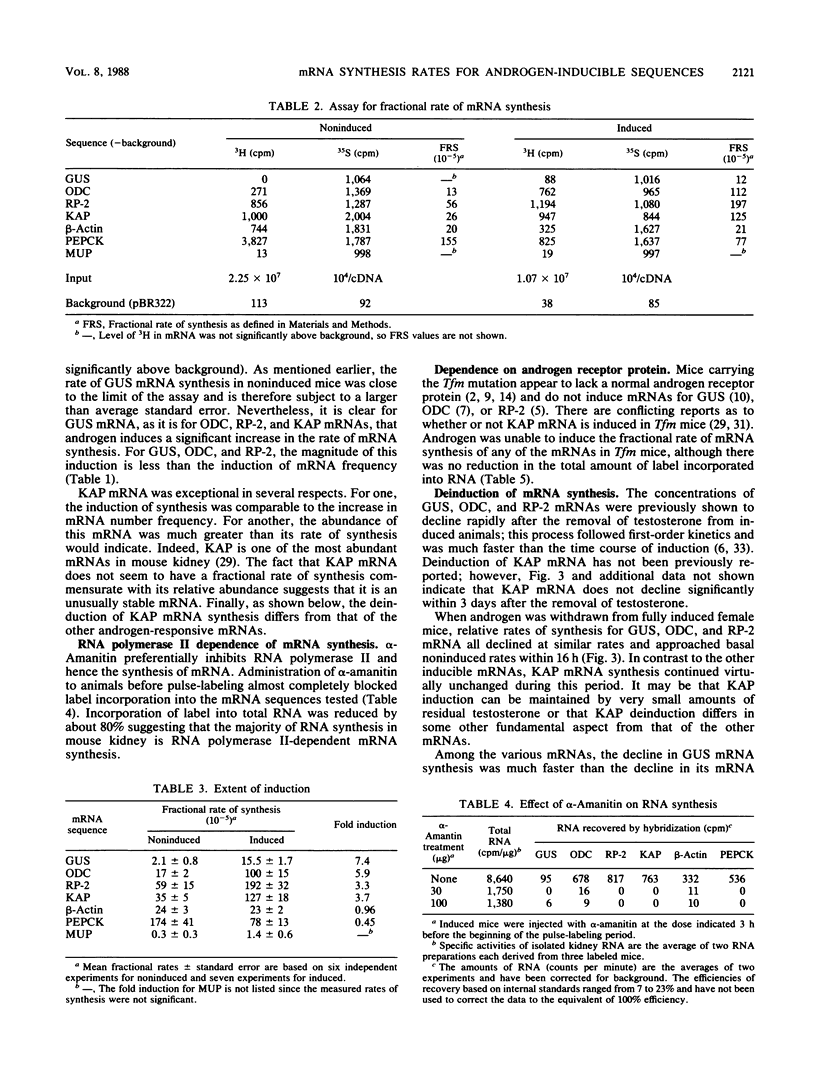
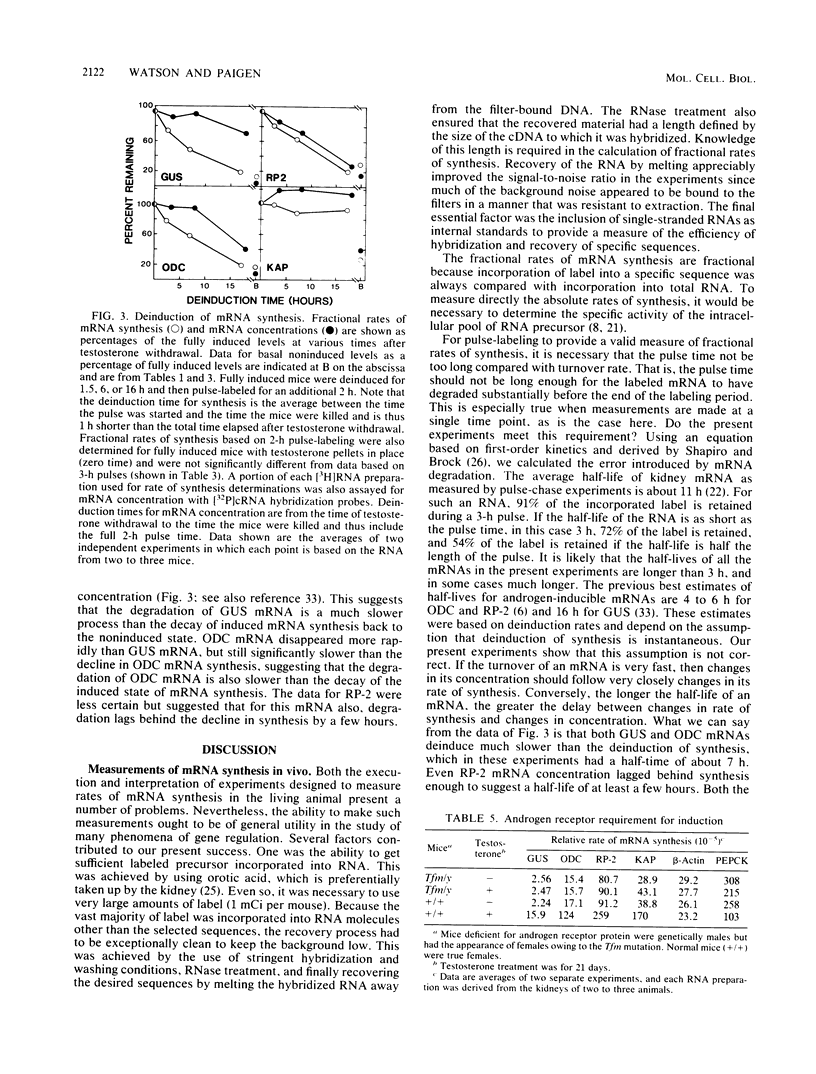
A method was developed for measuring in vivo rates of mRNA synthesis in mice by pulse-labeling with the RNA precursor [3H]orotate and then using hybridization to recover specific mRNAs. The efficiency of recovery is determined with synthetic RNAs as internal hybridization standards. The method is particularly applicable to the kidney since this organ shows a strong preferential uptake of the label. Rates of synthesis, expressed as a fraction of total RNA synthesis, were measured for the androgen-inducible mRNAs coding for beta-glucuronidase (GUS), ornithine decarboxylase (ODC), the protein coded by the RP-2 gene, and the so-called kidney androgen-regulated protein (KAP). Control mRNAs coded for beta-actin, phosphoenolpyruvate carboxykinase, and major urinary protein. Testosterone markedly increased the synthesis of the androgen-inducible mRNAs, but not the control mRNAs. Induction was not seen in mutant mice lacking functional androgen receptor protein. For GUS, ODC, and RP-2 mRNAs, the fold induction of synthesis was less than the fold induction of concentration, suggesting that mRNA stabilization also plays a part in the response to androgen. For GUS, ODC, and RP-2 mRNAs, but not KAP mRNA, induction of synthesis was rapidly reversed after testosterone removal. KAP mRNA was also exceptional in that its concentration was disproportionately high compared with its rate of synthesis, implying that it is a particularly stable mRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Ono S. Cytosol androgen receptor from kidney of normal and testicular feminized (Tfm) mice. Cell. 1974 Aug;2(4):205–212. doi: 10.1016/0092-8674(74)90012-9. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Catterall J. F. Testosterone: a major determinant of extragenital sexual dimorphism. Science. 1981 Mar 20;211(4488):1285–1294. doi: 10.1126/science.7010603. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Berger F. G., Gross K. W., Watson G. Isolation and characterization of a DNA sequence complementary to an androgen-inducible messenger RNA from mouse kidney. J Biol Chem. 1981 Jul 10;256(13):7006–7013. [PubMed] [Google Scholar]
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Bullock L. P., Bardin W. C. Androgen receptors in testicular feminization. J Clin Endocrinol Metab. 1972 Dec;35(6):935–937. doi: 10.1210/jcem-35-6-935. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Leary S. L. Detection of early changes in androgen-induced mouse renal beta-glucuronidase messenger ribonucleic acid using cloned complementary deoxyribonucleic acid. Biochemistry. 1983 Dec 20;22(26):6049–6053. doi: 10.1021/bi00295a001. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fraser N., Ziff E., Weber J., Wilson M., Darnell J. E. The initiation sites for RNA transcription in Ad2 DNA. Cell. 1977 Nov;12(3):733–739. doi: 10.1016/0092-8674(77)90273-2. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. Characterization of a hormone receptor defect in the androgen-insensitivity mutant. Cell. 1974 Sep;3(1):59–64. doi: 10.1016/0092-8674(74)90040-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A., Toole J. J. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979 Jun;17(2):449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Woodworth-Gutai M., Gross K. W., Held W. A. Subfamilies of the mouse major urinary protein (MUP) multi-gene family: sequence analysis of cDNA clones and differential regulation in the liver. Nucleic Acids Res. 1984 Aug 10;12(15):6073–6090. doi: 10.1093/nar/12.15.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. MRNA-directed synthesis of catalytically active mouse beta-glucuronidase in Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4462–4465. doi: 10.1073/pnas.74.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Ouellette A. J., Ordahl C. P., Van Ness J., Malt R. A. Mouse kidney nonpolysomal messenger ribonucleic acid: metabolism, coding function, and translational activity. Biochemistry. 1982 Mar 16;21(6):1169–1177. doi: 10.1021/bi00535a010. [DOI] [PubMed] [Google Scholar]
- Page M. J., Parker M. G. Effect of androgen on the transcription of rat prostatic binding protein genes. Mol Cell Endocrinol. 1982 Aug;27(3):343–355. doi: 10.1016/0303-7207(82)90099-5. [DOI] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. S., Malamud D., Caulfield J. A., Malt R. A. Differential labeling with orotic acid and uridine in compensatroy renal hypertrophy. Am J Physiol. 1975 Oct;229(4):952–954. doi: 10.1152/ajplegacy.1975.229.4.952. [DOI] [PubMed] [Google Scholar]
- Snider L. D., King D., Lingrel J. B. Androgen regulation of MAK mRNAs in mouse kidney. J Biol Chem. 1985 Aug 15;260(17):9884–9893. [PubMed] [Google Scholar]
- Toole J. J., Hastie N. D., Held W. A. An abundant androgen-regulated mRNA in the mouse kidney. Cell. 1979 Jun;17(2):441–448. doi: 10.1016/0092-8674(79)90170-3. [DOI] [PubMed] [Google Scholar]
- Watson C. S., Catterall J. F. Genetic regulation of androgen-induced accumulation of mouse renal beta-glucuronidase messenger ribonucleic acid. Endocrinology. 1986 Mar;118(3):1081–1086. doi: 10.1210/endo-118-3-1081. [DOI] [PubMed] [Google Scholar]
- Watson C. S., Salomon D., Catterall J. F. Structure and expression of androgen-regulated genes in mouse kidney. Ann N Y Acad Sci. 1984;438:101–114. doi: 10.1111/j.1749-6632.1984.tb38279.x. [DOI] [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Watson G., Paigen K. Genetic variations in kinetic constants that describe beta-glucuronidase mRNA induction in androgen-treated mice. Mol Cell Biol. 1987 Mar;7(3):1085–1090. doi: 10.1128/mcb.7.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. L., Parker M. G. Regulation of prostatic steroid binding protein mRNAs by testosterone. Mol Cell Endocrinol. 1985 Dec;43(2-3):151–154. doi: 10.1016/0303-7207(85)90078-4. [DOI] [PubMed] [Google Scholar]


