Abstract
Protonophorous uncouplers causing a partial decrease in mitochondrial membrane potential are promising candidates for therapeutic applications. Here we showed that hydrophobic penetrating cations specifically targeted to mitochondria in a membrane potential-driven fashion increased proton-translocating activity of the anionic uncouplers 2,4-dinitrophenol (DNP) and carbonylcyanide-p-trifluorophenylhydrazone (FCCP). In planar bilayer lipid membranes (BLM) separating two compartments with different pH values, DNP-mediated diffusion potential of H+ ions was enhanced in the presence of dodecyltriphenylphosphonium cation (C12TPP). The mitochondria-targeted penetrating cations strongly increased DNP- and carbonylcyanide m-chlorophenylhydrazone (CCCP)-mediated steady-state current through BLM when a transmembrane electrical potential difference was applied. Carboxyfluorescein efflux from liposomes initiated by the plastoquinone-containing penetrating cation SkQ1 was inhibited by both DNP and FCCP. Formation of complexes between the cation and CCCP was observed spectophotometrically. In contrast to the less hydrophobic tetraphenylphosphonium cation (TPP), SkQ1 and C12TPP promoted the uncoupling action of DNP and FCCP on isolated mitochondria. C12TPP and FCCP exhibited a synergistic effect decreasing the membrane potential of mitochondria in yeast cells. The stimulating action of penetrating cations on the protonophore-mediated uncoupling is assumed to be useful for medical applications of low (non-toxic) concentrations of protonophores.
Introduction
The concept of “mild uncoupling” implies favorable therapeutic action of low concentrations of uncouplers which slightly decrease membrane potential thereby preventing fast production of reactive oxygen species (ROS) in mitochondria [1]–[3]. In fact, low concentrations of the protonophore 2,4-dinitrophenol (DNP) exhibit favorable effects in some pathological laboratory models related to oxidative stress and increase lifespan of yeast [4], Drosophila [5] and mice [6].
DNP was used in the early 1900’s as a weight loss-causing drug but was prohibited because of some toxicity and complications resulting in a few deaths (reviewed in [7]). Some efforts have been carried out to the search for uncouplers with a therapeutic window broader than DNP [8]. It has been assumed that toxic action of DNP and its analogues is related to an excessive decrease in mitochondrial membrane potential. However, uncouplers are known to have additional intracellular targets, e.g., plasma membrane potential and ΔpH [9], [10]. It has also been shown that DNP inhibits chloride channel of erythrocytes [11] and blocks glutathione-S-conjugate forming ATP-ase [12]. Comparison of toxic doses for mice with uncoupling concentrations for isolated mitochondria has revealed poor correlation of these parameters for a large set of DNP analogues as well as for some other uncouplers [13]. These findings suggest that the toxic effect of at least some uncouplers in vivo could be unrelated to their action on mitochondria. It seems possible that the therapeutic window for uncouplers could be enlarged by agents promoting the uncoupling action of their small concentrations.
It has been shown that protonophorous action of CCCP (carbonyl cyanide m-chlorophenylhydrazone) and FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone) on lipid vesicles can be enhanced upon the addition of the potassium transporter valinomycin and the penetrating cation tetraphenylphosphonium (TPP) [14]. These effects were explained by formation of complexes between anionic forms of the protonophores and valinomycin-bound K+ or TPP cations in lipid membranes [14]–[16]. Addition of the cationic anesthetic procaine to isolated mitochondria led to substantial acceleration of respiration mediated by a CCCP derivative due to the formation of complexes between these compounds [17]. Recently, a new generation of penetrating cations capable of targeted delivery of antioxidants and some other compounds into mitochondria was synthesized and characterized. It was shown that conjugates of penetrating cations and quinones, such as MitoQ and SkQ, protected brain and kidney from ischemia injuries accompanied by generation of ROS [18]–[20]. In the present paper, we demonstrate the enhancing effects of SkQ and related compounds on the protonophorous action of DNP and FCCP in different experimental systems, including model lipid membranes, isolated mitochondria and yeast cells. According to our recent findings [21], SkQ1 (10-(6-plastoquinonyl)decyl triphenylphosphonium) is able to transport organic anions of various structures through lipid bilayer membranes (BLM) and liposomes. In particular, interaction of SkQ with fatty acid anions strongly stimulated the uncoupling effect of the latter on mitochondria [22]. Importantly, fatty acids per se do not exhibit protonophorous action and induce uncoupling via ATP/ADP-antiporter [23]. Below, we show that SkQ and its analogues promote the uncoupling activity of FCCP and DNP much more efficiently than TPP, being operative already at micromolar and even submicromolar concentrations. This suggests that a combined use of uncouplers and mitochondrially-targeted cationic antioxidants may be promising in an attempt to lower effective concentrations of protonophores as therapeutic tools.
Materials and Methods
Chemical Substances
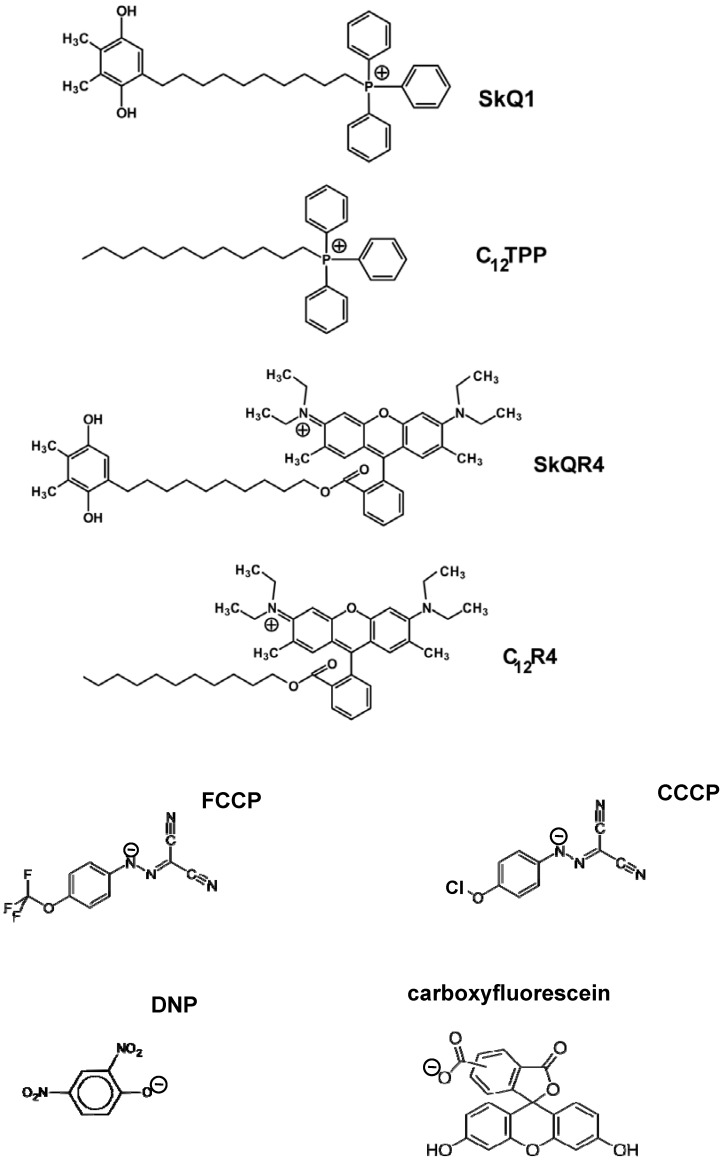
Synthesis of a series of mitochondria-targeted antioxidants composed of substituted 1,4-benzoquinone rings conjugated to hydrophobic triphenylphosphonium (SkQ1) or rhodamine cations (SkQR1) with a decyl linker was described in our group by G.Korshunova and N.Sumbatyan [21], [24]. Structures of SkQ1 and some other compounds used in this work are shown in Fig. 1. FCCP, CCCP, DNP, MOPS, oligomycin, L-malic acid disodium salt, L-glutamic acid potassium salt monohydrate and fatty acid-free BSA were from Sigma-Aldrich; EGTA, safranin O, and potassium phosphate were from Serva; sucrose was purified via twice precipitation from a concentrated solution in ethanol.
Figure 1. Chemical structures of C12TPP, SkQ1, SkQR4, C12R4, FCCP, CCCP, DNP, and carboxyfluorescein.
Planar Bilayers
Bilayer lipid membrane (BLM) was formed from 2% decane solution of diphytanoylphosphatidylcholine (DPhPC) on a 0.6-mm aperture in a Teflon septum separating the experimental chamber into two compartments of equal size (volumes, 3 ml). The current measured by a patch-clamp amplifier (OES-2, OPUS, Moscow) was digitized using an NI-DAQmx device (National Instruments, Austin, TX) and analyzed with a personal computer using WinWCP Strathclyde Electrophysiology Software designed by J. Dempster. Electrical potential was measured with two AgCl electrodes placed into the solutions on both sides of the BLM, using a Keithley 6517 amplifier (Cleveland, Ohio, USA). The incubation mixture contained 50 mM Tris-HCl, and 50 mM KCl, pH 7.0.
Liposome Leakage Assay
Liposomes loaded with 5,6-carboxyfluorescein (CF, Sigma) were prepared by extrusion through a 100-nm filter (Avanti Mini-Extruder) from diphytanoylphosphatidylcholine (Avanti Polar Lipids) in solution containing 100 mM CF titrated with TRIS-base. The unloaded CF was then removed by passage through a Sephadex G-50 coarse column using 100 mM KCl, 10 mM Tris, pH 7.4 as the eluting buffer. To measure the rate of CF efflux, the liposomes were diluted with a buffer containing 100 mM KCl, 10 mM Tris, pH 7.4. The fluorescence at 520 nm (excitation, 490 nm) was monitored with a Panorama Fluorat 02 spectrofluorimeter (Lumex, Russia). At the end of each recording, 0.1% Triton-X100 was added to complete the efflux process.
Isolation of Rat Liver Mitochondria
Rat liver mitochondria were isolated by differential centrifugation [25] in a medium containing 250 mM sucrose, 10 mM MOPS, 1 mM EGTA, and bovine serum albumin (0.1 mg/ml), pH 7.4. The final washing was performed in the medium of the same composition. Protein concentration was determined using biuret method. Handling of animals and experimental procedures were conducted in accordance with the international guidelines for animal care and use and were approved by the Ethics Committee of A.N. Belozersky Institute of Physico-Chemical Biology at Moscow State University.
Membrane Potential (ΔΨ) Measurement in Isolated Mitochondria
ΔΨ was estimated using the safranin O dye [26]. The difference in the absorbance between at 555 and 523 nm (ΔA) was recorded with an Aminco DW-2000 spectrophotometer in the dual wavelength mode. The following incubation medium was used: 250 mM sucrose, BSA (0.1 mg/ml), a respiratory substrate, 1 mM EGTA, 100 µM potassium phosphate, 10 mM MOPS-KOH (pH 7.4), 10 µM safranin O. The mitochondrial protein content was 0.6–0.9 mg protein/ml, The temperature was 26°C.
Yeast Strains and Cell Growth Conditions
In our experiments, we used standard laboratory yeast strain W303 (Mat a genetic background) and its respiratory incompetent derivative (petite strain). The petite strain was generated by growing cells on plates supplemented with ethidium bromide [27]. Rich liquid medium contained yeast extract (1%) supplemented with bactopeptone (2%) and 2% glycerol (YPGly medium), or 2% glucose, or 2% glycerol and 0.1% glucose (YP medium) [28]. The cells were typically grown in liquid media to the density of 2×106 cells/ml. For testing the biomass accumulation, the respiratory-incompetent cells were grown for 72 hours to reach the stationary phase in liquid medium supplemented with 0.1% glucose.
Cell Respiration
Respiration of yeast cells was measured using a standard polarographic technique with a Clark-type oxygen electrode [29]. The incubation medium for yeast cells contained 50 mM KH2PO4, pH 5.5, and 0.005% glucose.
Mitochondrial Membrane Potential in Yeast Cells
For estimation of mitochondrial membrane potential, intact yeast cells grown on glycerol-containing rich medium were stained with 2 µM JC-1 [30]. Under our experimental conditions, we did not observe any significant red shifts in fluorescence maxima and, therefore, we assumed that JC-1 did not form J-aggregates. After incubation with JC-1, cells were photographed with an Olympus U-MNIABA3 filter set (excitation, 470–495 nm; split, 505 nm; emission, 510–550 nm) and analyzed with ImageJ software. For each cell, the local intensity maximum was determined. The average intensities of the square areas (0.6 µm2) with the center of determined maximum were measured. As a result, an average of JC-1 fluorescence intensity was assigned for individual cells. For each treatment conditions, about 60 cells from a least 2 different experiments were analyzed. The average intensities of local maximum areas of the control (solvent-treated) cells were taken as 100%.
In all figures, the typical results of 4–6 experiments were shown.
Results
Diffusion Potential and Electrical Current on Planar Bilayer Lipid Membranes
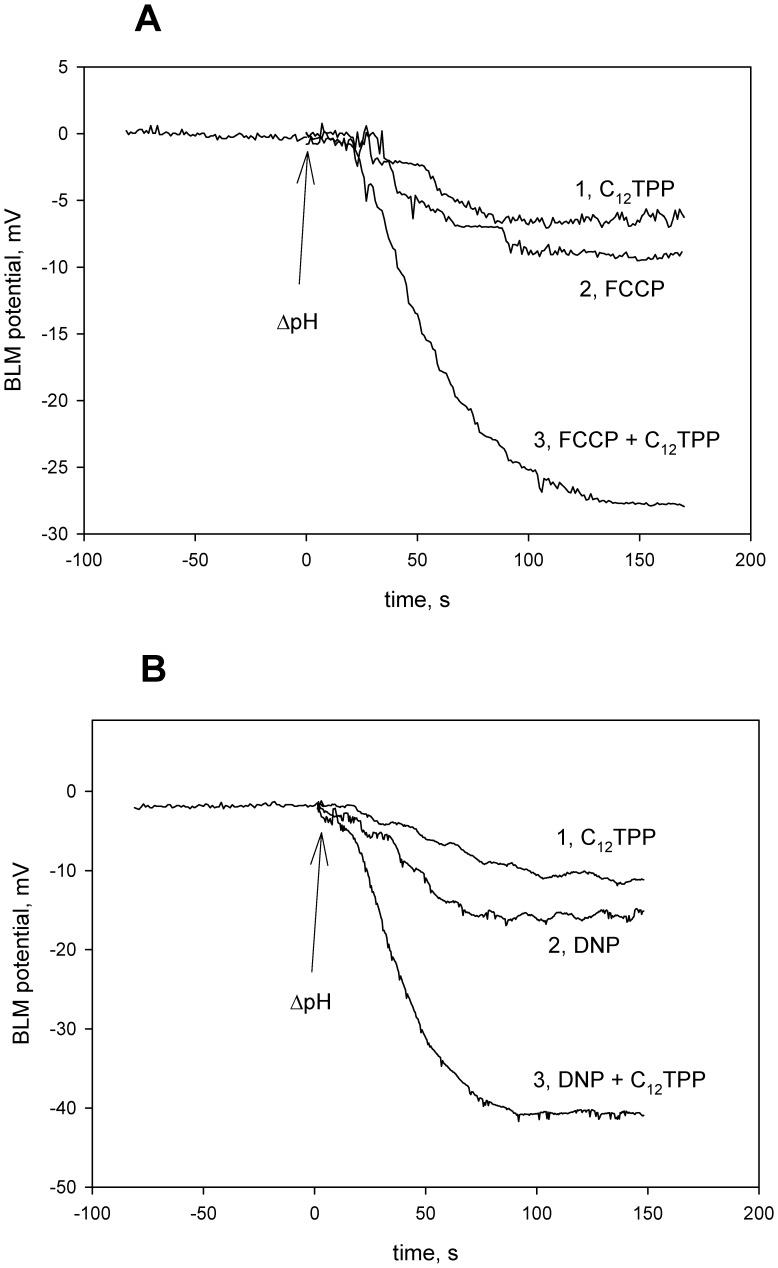
To study the effect of the mitochondria-targeted penetrating cations on the uncoupler-mediated proton flux through the planar bilayer lipid membrane (BLM), we measured proton diffusion potential, i.e. an electrical potential difference (ΔΨ) across the BLM, generated as a result of transmembrane H+ flux facilitated by a protonophore under the conditions of pH difference between two BLM-separated compartments [31]–[33]. The penetrating cation C12TPP substantially increased the value of H+ diffusion potential if added in combination with FCCP (Fig. 2A) or DNP (Fig. 2B). In these experiments, protonophores (FCCP or DNP) were added at low concentrations which were far below the level sufficient to generate the theoretical (Nernstian) potential, i.e. 59 mV at ΔpH = 1. However, in the presence of 0.1 µM C12TPP, the magnitude of ΔΨ increased and reached approximately 28 mV in the case of FCCP and 42 mV in the case of DNP. The growth of ΔΨ proved that BLM preserved proton selectivity under these conditions. C12TPP alone generated only a small diffusion H+ potential (Fig. 3A). Importantly, TPP was unable to substitute for C12TPP in these experiments (data not shown). Higher concentrations of FCCP or DNP allowed us to observe theoretical (about 60 mV) values of ΔΨ even without hydrophobic cations (not shown).
Figure 2. C12TPP enhances protonophorous effect of 2 nM FCCP (panel A) and 10 µM DNP (panel B) in bilayer lipid membrane (BLM).
Diffusion potential was recorded upon the addition of KOH in one compartment to create ΔpH = 1. Incubation mixture, 10 mM Tris, 10 mM MES, 10 mM KCl, pH 7; C12TPP, 0.1 µM. Control, a record without C12TPP and uncouplers. Plus sign of the potential in the compartment of high pH.
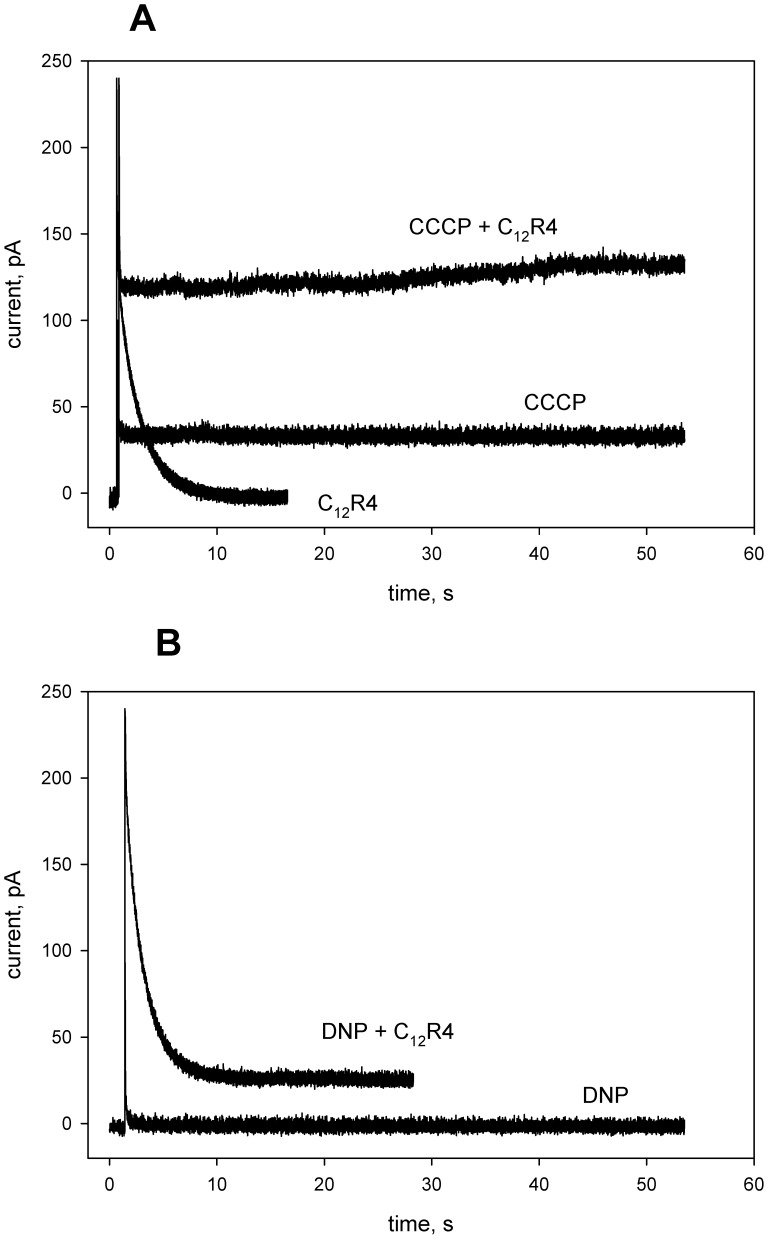
Figure 3. C12R4 (rhodamine B dodecyl ester) strongly increases CCCP- (panel A) or DNP-mediated (panel B) electric current through bilayer lipid membrane (BLM).
Where indicated, 0.1 µM CCCP or 1 µM DNP were added to both compartments prior to the measurements; incubation mixture, 0.1 M KCl, 10 mM Tris, 10 mM MES, pH 7.0; at t = 1.5 s, 100 mV voltage was applied to the BLM. Concentration of C12R4 was 0.1 µM.
In further experiments, we applied the current relaxation technique to measure the protonophore-mediated electric current across planar BLM under voltage-clamp conditions. As was shown previously in our group, voltage application to a planar BLM in the presence of SkQ1 or related penetrating cations results in a jump of electric current followed by its relaxation [21], [34]. With rhodamine B dodecyl ester (C12R4, Fig. 1), current across the membrane, which was maximal immediately after application of the voltage, also decreased spontaneously with time from an initial to a steady-state level (the current relaxation process). Figure 3 shows current relaxation recordings obtained upon a voltage jump of 100 mV with CCCP (panel A) or DNP (panel B) added symmetrically to the bathing solution in the absence and in the presence of C12R4. Note that the steady-state level of electric current with C12R4 was negligible if no protonophore (CCCP) was added. The current was higher in the samples with 0.1 µM CCCP. The addition of 0.1 µM C12R4 to the CCCP-containing sample led to an increase in the steady-state current from about 40 pA up to 120 pA. An experiment with another protonophore, DNP, showed similar results: the addition of C12R4 led to an increase in the steady-state current, and the magnitude of the effect was even higher than with FCCP, namely about 30-fold.
Spectrophotometrical Evidence for Interactions of a Protonophorous Uncoupler and Penetrating Ions
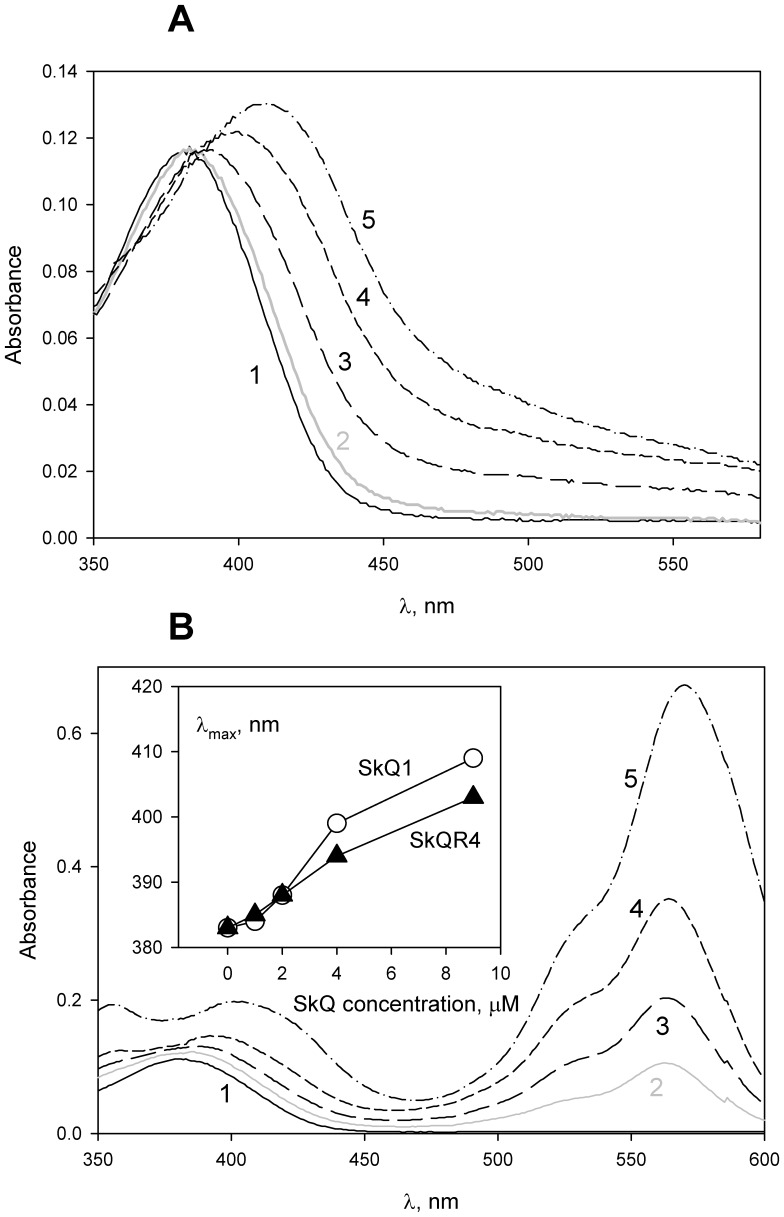
Data on planar lipid membranes favored the idea of hydrophobic cations being carriers of anionic forms of protonophores. The carrier-type mechanism of transport implies formation of complexes between a substrate (protonophore anion) and a hydrophobic cation. The interaction between CCCP and penetrating cations was studied by measuring CCCP absorption spectra in the presence of SkQ1 (Fig. 4A) and SkQR4 (Fig. 4B) in a suspension of liposomes. The addition of the hydrophobic cations led to a red shift in the absorption maximum (λmax) of CCCP, indicating formation of complexes.
Figure 4. SkQ1 (10-(6-plastoquinonyl)decyl triphenylphosphonium) affects absorption spectra of CCCP in the presence of liposomes.
Incubation mixture: 4 µM CCCP, 1 mM Tris, 1 mM MES, pH = 7.4, containing DPhPC (diphytanoylphosphatidylcholine) liposomes (20 µg/ml) in the absence (curve 1) and in the presence of 1 µM, 2 µM, 4 µM and 9 µM SkQ1 (panel A, curves 2–5, respectively) or in the presence of 1 µM, 2 µM, 4 µM and 9 µM SkQR4 (panel B, curves 2–5, respectively). Insert to panel B shows the dependence of λmax on the concentration of the SkQ derivatives.
Effect of CCCP and DNP on the SkQ1-induced Carboxyfluorescein Efflux from Liposomes
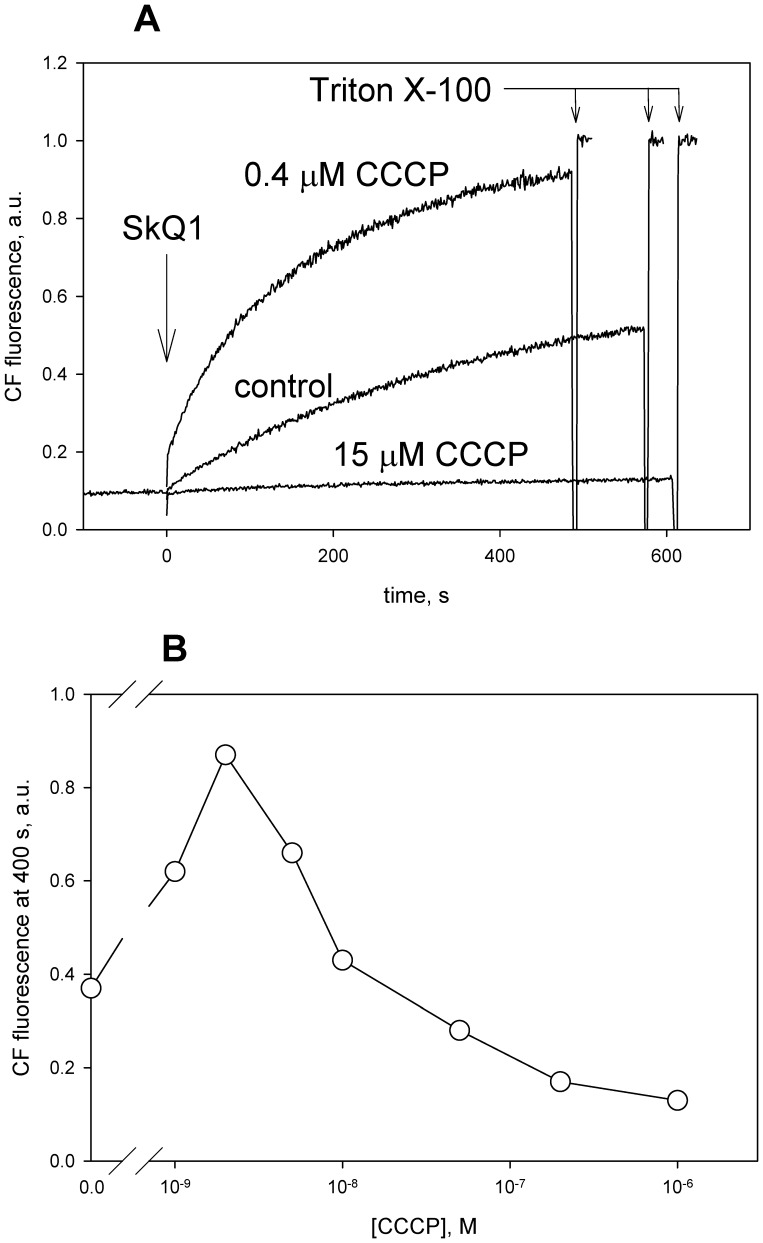
It was previously shown in our group that SkQ1 and other penetrating cations initiated efflux of carboxyfluorescein (CF) anions from liposomes via the carrier-type mechanism [21]. Importantly, the ability to induce the efflux of CF strongly depended on the number of carbon atoms in the alkyl chain (n) of alkyltriphenylphosphonium being maximal at n about 10–12 [21]. The process was inhibited by fatty acids which competed with CF for the formation of a complex with SkQ1. It could be expected that the anionic protonophores would inhibit the CF efflux as well. Fig. 5 shows the effect of CCCP on SkQ1-induced CF efflux from phosphatidylcholine liposomes loaded with CF at a self-quenching concentration. Efflux of CF from liposomes entailed dilution of CF which increased CF fluorescence. As expected, high concentrations of CCCP blocked the SkQ1-induced CF efflux. However, low CCCP concentrations accelerated the process (top curve in Fig. 5A). Two phases in the concentration dependence were also observed for another penetrating cation, SkQR1 (Fig. 5B) and were also found with DNP (data not shown). The accelerating effect of low concentrations of CCCP or DNP might be associated with the electrogenic nature of CF efflux which should result in the formation of a diffusion potential on membranes of liposomes. Apparently, addition of protonophores decreases this ΔΨ, thus stimulating the CF efflux. As to the inhibitory effect of high concentrations of the protonophores, it could result from formation of their complexes with the penetrating cations.
Figure 5. CCCP increases the SkQ-induced efflux of carboxyfluorescein from liposomes.
Carboxyfluorescein (CF) efflux from DPhPC (diphytanoylphosphatidylcholine) liposomes (50 µg/ml), induced by 2.5 µM SkQ1 (panel A) or by 0.5 µM SkQR1 (panel B) was measured with or without CCCP. The efflux was accompanied by an increase in CF fluorescence due to dilution and a relief of CF self-quenching. In panel B, the CF efflux was measured 400 s after the addition of SkQR1. Incubation mixture, 10 mM Tris, 10 mM MES, 100 mM KCl, pH 7.
Effect of Penetrating Cations on DNP- and FCCP-mediated Uncoupling of Isolated Rat Liver Mitochondria
Transport of electrons along the mitochondrial respiratory chain is accompanied by the formation of electrochemical gradient of hydrogen ions (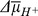 )at the inner mitochondrial membrane. The electrical part of
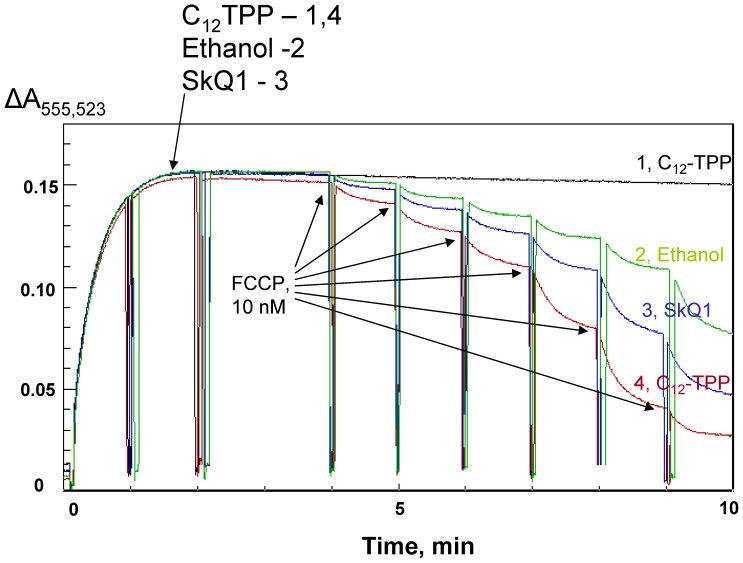
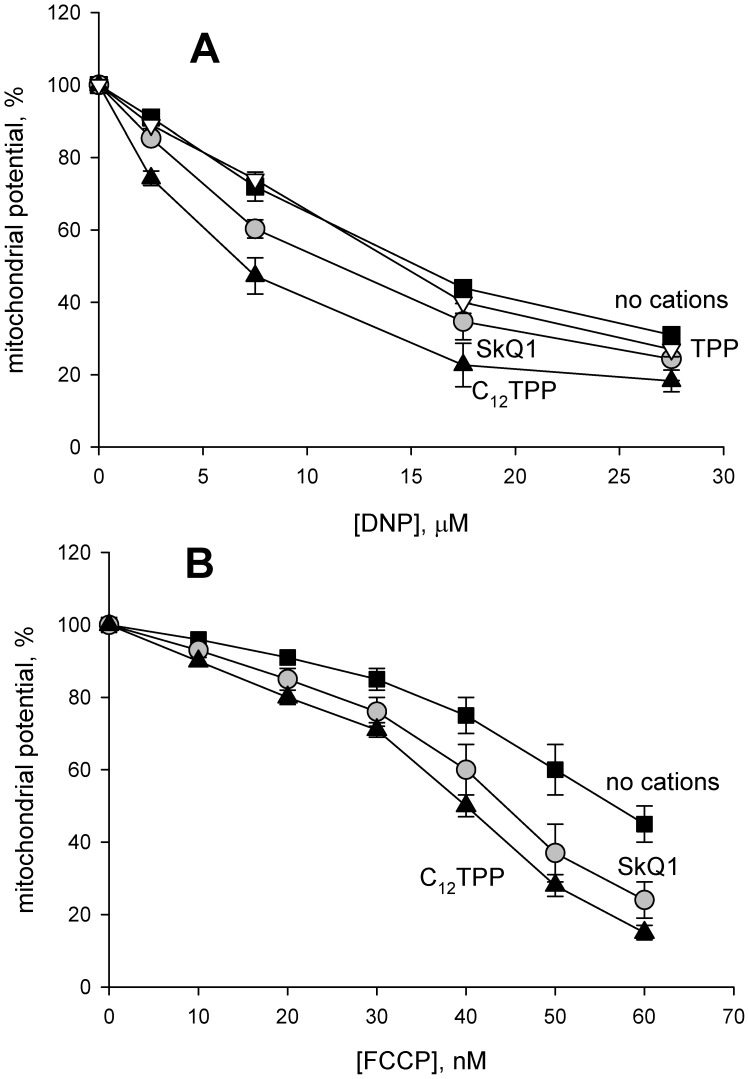
)at the inner mitochondrial membrane. The electrical part of 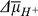 , i.e. ΔΨ, can be measured in isolated mitochondria by changes in the absorbance of the cationic dye safranin O [26]. Fig. 6 shows the results of ΔΨ measurements on rat liver mitochondria upon the addition of dodecyl tetraphenylphosphonium cation (C12-TPP, curve 1), FCCP (curve 2) or FCCP together with SkQ1 (curve 3) or C12-TPP (curve 4). One can see that C12-TPP did not decrease ΔΨ under the experimental conditions but substantially increased the FCCP-induced ΔΨ lowering. Fig. 7A and 7B show the dose dependence of the ΔΨ decrease by DNP and FCCP. In contrast to C12-TPP, the less hydrophobic cation TPP had no effect even at a concentration of 200 µM (Fig. 7A).
, i.e. ΔΨ, can be measured in isolated mitochondria by changes in the absorbance of the cationic dye safranin O [26]. Fig. 6 shows the results of ΔΨ measurements on rat liver mitochondria upon the addition of dodecyl tetraphenylphosphonium cation (C12-TPP, curve 1), FCCP (curve 2) or FCCP together with SkQ1 (curve 3) or C12-TPP (curve 4). One can see that C12-TPP did not decrease ΔΨ under the experimental conditions but substantially increased the FCCP-induced ΔΨ lowering. Fig. 7A and 7B show the dose dependence of the ΔΨ decrease by DNP and FCCP. In contrast to C12-TPP, the less hydrophobic cation TPP had no effect even at a concentration of 200 µM (Fig. 7A).
Figure 6. SkQ1 and C12TPP stimulate the uncoupling action of FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone) in rat liver mitochondria.
Mitochondrial potential was assayed by measuring the absorbance of safranin O. 10 nM FCCP was added at 4, 5, 6, 7, 8, and 9 min (curves 2–4). 0.2% ethanol or ethanol solutions leading to 2 µM C12TPP (curves 1 and 4) or 2 µM SkQ1 (curve 3) were added at 2 min. Oligomycin (1 µg/ml) was added at 1 min. For other conditions, see Materials and methods.
Figure 7. Comparison of SkQ1 and C12TPP effects on the uncoupling activity of DNP and FCCP.
Effects of 2 µM SkQ1, 2 µM C12TPP or 200 µM TPP on the dependence of mitochondrial membrane potential on the concentration of DNP (panel A) or FCCP (panel B). The experiments were conducted in a way shown in Fig. 6. Shown are Mean±S.E. of 4–6 experiments.
Effect of Penetrating Cations on FCCP- and DNP-mediated Uncoupling of Mitochondria in Intact Yeast Cells
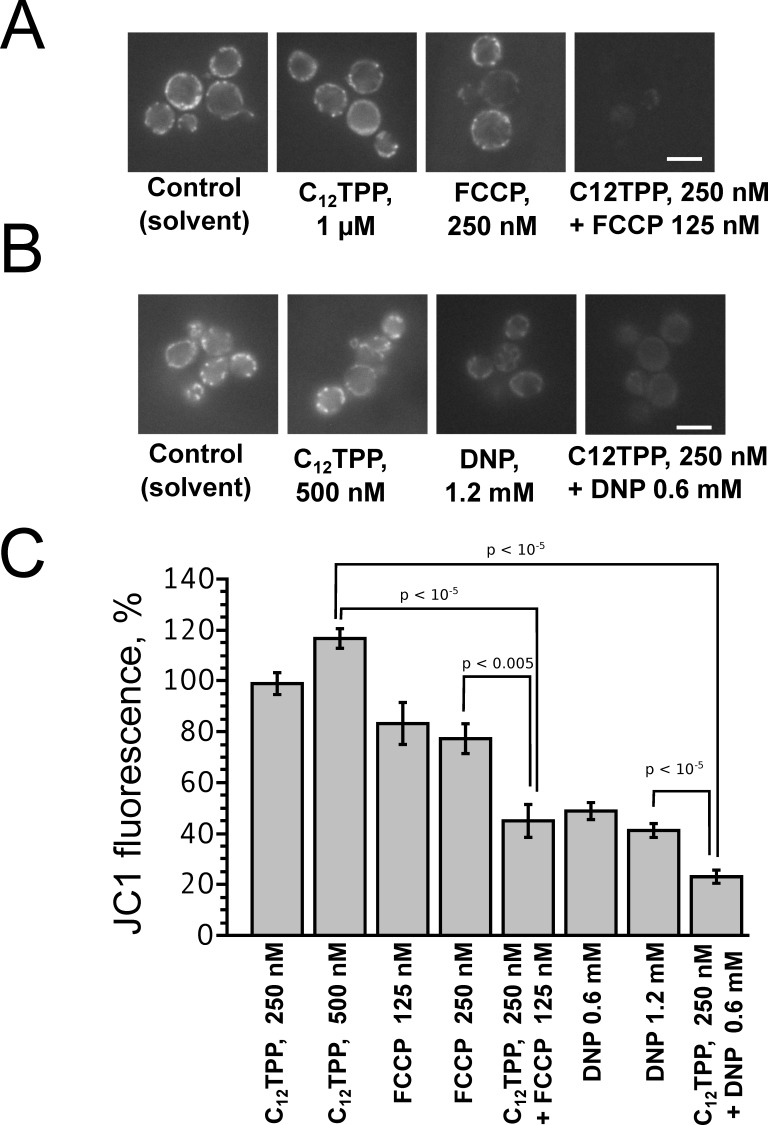
To test whether the penetrating cations enhanced the action of uncouplers at a cellular level, we studied the effects of C12TPP, FCCP and DNP on intact yeast cells. Unlike isolated mitochondria and artificial planar bilayer membranes or liposomes, intact eukaryotic cells have several membraneous compartments differing in values of transmembrane proton gradient. Therefore, the effects of uncouplers on intact cells could be rather complicated. To address this question, we first studied the combined action of the uncouplers and penetrating cations on the ability of Saccharomyces cerevisiae cells to accumulate the hydrophobic cationic fluorescent dye JC-1, that is widely used to estimate ΔΨ on the mitochondrial inner membrane in living cells [30].
C12TPP added together with the anionic uncouplers FCCP (Fig. 8 A,C) or DNP (Fig. 8 B,C) significantly enhanced the uncoupling effect of these protonophores in yeast mitochondria, as judged by the accumulation of JC-1. Importantly, C12TPP added alone in submicromolar concentrations did not affect the ability of yeast cells to accumulate JC-1 (Fig. 8).
Figure 8. C12TPP augments the FCCP- and DNP-induced decrease of mitochondrial membrane potential (ΔΨ) in intact yeast cells.
Mitochondrial membrane potential in yeast was estimated by staining cells with the ΔΨ probe JC-1. S.cerevisiae cells were incubated with C12TPP and/or FCCP (A), C12TPP and/or DNP (B) and then loaded with 2 µM JC-1. (C) Levels of JC-1 fluorescence in yeast cells (see Materials and methods). Data are presented as averages with standard errors. Samples were compared by Wilcoxon signed ranked unpaired test. Results of at least three experiments performed on separate days (number of cells, from 30 to 170). Bar, 5 µm.
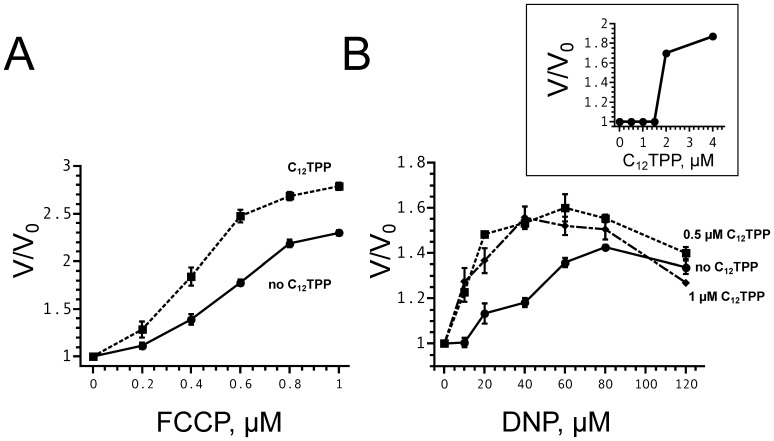
Figure 9A shows the dependence of the yeast cell respiration rate on the concentration of FCCP in the absence (solid line) and in the presence (dotted line) of 1 µM C12TPP. It is seen that the combination of C12TPP and FCCP stimulated respiration stronger than FCCP alone. Under these conditions, C12TPP per se did not produce any effect up to concentrations of 2 µM (insert), in agreement with earlier data [8]. C12TPP activated the DNP-mediated respiration too (Figure 9B).
Figure 9. C12TPP enlarges the FCCP- and DNP-induced increase in respiration rates of intact yeast cells.
The ordinate represents the ratio of the oxygen consumption rates after (V) and before (V0) the addition of an uncoupler. (A) FCCP-mediated stimulation of yeast respiration in the absence (solid line) and in the presence (dotted line) of 1 µM C12TPP. (B) DNP stimulation of yeast respiration in the absence (solid line) and in the presence of 0.5 µM (dotted line) or 1 µM (dot and dash line) C12TPP. In the absence of the anionic uncoupler, C12TPP did not increase the rate of oxygen consumption at concentrations below 2 µM (insert). Shown are Mean±S.E. of 4 experiments.
Discussion
Summarizing the above presented data, one can conclude that C12-TPP, SkQ1 and other hydrophobic penetrating cations are able to promote the protonophorous action of anionic uncouplers on model lipid membranes (Figs.2, 3) and certain natural (mitochondrial) membranes (Figs.6, 7, 8, and 9). The planar bilayer experiments revealed a stimulating effect of the penetrating cations both on the uncoupler-assisted transmembrane proton flux manifesting itself in augmenting the H+ diffusion potentials on BLM (Fig. 2) and on the steady-state current in voltage-jump relaxation experiments (Fig. 3) reflecting an increase in the transmembrane flux of charged forms of an uncoupler [33]. Spectral measurements (Fig. 4) together with liposome leakage experiments (Fig. 5) allowed us to shed light on the mechanism of the uncoupler activation, most likely proceeding via formation of complexes between the penetrating cations and anionic forms of the uncouplers. High hydrophobicity of cation and anion seems to be required for formation of such complexes because electrostatic interactions are much stronger in hydrophobic membrane core than in water solutions. In line with this assumption, the cation of tetraphenylphosphonium (TPP), being rather hydrophilic, was of no effect in our systems in contrast to C12TPP (Fig. 7A). As the penetrating cations are known to accumulate electrophoretically in mitochondria [35]–[38], their promoting effect on the action of DNP or FCCP should be more pronounced at low uncoupler concentrations which decrease ΔΨ to a small extent. Importantly, our planar bilayer data showed that the influence of penetrating cations was not associated with their possible detergent effects because they (i) did not induce fluctuations of the electric current (Fig. 3), and (ii) did not reduce proton selectivity of the membrane (Fig. 2).
It was previously found that penetrating cations stimulated uncoupling action of endogeneous free fatty acids present in mitochondria [22]. In this paper, we used comparatively low concentrations of SkQ1, C12TPP and other cations which did not affect the mitochondrial ΔΨ (Figs.6) or decreased it only slightly (Figs.7). Importantly, the combined action of SkQ1 (or C12TPP) and DNP (or FCCP) was stronger, as compared to the sum of their separate effects (Figs.6, 8, 9). This observation supported our conclusion that direct interaction of the studied cations and artificial uncouplers in the membrane caused their synergistic action.
Although C12TPP increased the uncoupling action of FCCP and DNP on S. cerevisiae cells (Fig. 8, 9), it did not enhance inhibiting effects of anionic uncouplers on biomass accumulation (see Supplemental information, Fig. S1 and S2). Apparently, the stationary biomass level was controlled primarily by the efficiency of ATP production. Therefore, it can be concluded that the combination of C12TPP and anionic uncouplers, while decreasing ΔΨ in tightly coupled fraction of mitochondria, still did not significantly inhibit the rate of ATP synthesis. In other words, DNP and FCCP, at small concentrations, being supplemented with C12TPP, become more efficient but still remain rather mild uncouplers. Hence, the promoting action of penetrating cations on the uncoupling effect of anionic protonophores seems promising for the development of drugs against obesity. Known uncouplers display relatively narrow windows between favorable and toxic concentrations which makes their medical use problematic [8]. It is generally accepted that the major cause of such toxicity is inhibition of oxidative phosphorylation. On the other hand, DNP and some other uncouplers are known to decrease the electrical potential on plasma membranes of erythrocytes [9], [10]. These and other data suggest that toxicity of uncouplers, apart from their action on oxidative phosphorylation, is at least partially unrelated to mitochondria. Our data on the inhibition of the yeast cell growth (Figs. S1 and S2) support the idea of non-mitochondrial toxic action of DNP and FCCP, which is in line with results of Ilivichy and Casida [13] showing poor correlation between the effect of DNP and its derivatives on the respiration of isolated mitochondria and the toxic action on mice. In particular, LD50 for toxicity of DNP and 6-cyclohexyl-DNP differed 1.5-fold, while uncoupling concentrations differed more than 30-fold [13]. It is essential that penetrating cations, being mitochondria-targeted compounds, should specifically stimulate mitochondria-linked activity of uncouplers rather than any other effects. In any case, there is a chance that the use of DNP in combination with hydrophobic penetrating cations as an obesity-lowering drug could be effective at substantially smaller (non-toxic) DNP doses. This idea seems to be even more attractive if one takes into account the data on several beneficial effects of SkQ or MitoQ used separately in a number of animal models of various diseases [18], [19].
Supporting Information
FCCP and C12TPP do not display synergistic inhibition of the increase in biomass of S. cerevisiae . Yeast cells were incubated in YPGly medium (see “Materials and methods”) supplemented with C12TPP and FCCP separately or with equimolar mixture of these compounds. For “C12TPP+FCCP” curve, the sum of the concentrations is shown, i.e. the data point “2 µM” corresponds to the growth medium supplemented with 1 µM of each compound. Incubation time was 5 hours. After incubation, the optical density (OD) was measured (λ = 550 nm). The OD of mock treated probe was set as 100%. Figure S1 shows that C12TPP did not enhance the inhibitory effect of FCCP on yeast growth, if the cells were grown on nonfermentable carbon source (glycerol). This result suggests that FCCP inhibited the growth not via arrest of oxidative phosphorylation but rather due to some other activity, for instance due to changing the proton potential on the plasma or vacuolar membranes.
(TIFF)
C12TPP did not affect the ability of neither FCCP (A) nor DNP (B) to decrease optical density of yeast respiratory-incompetent cells grown up to the stationary phase. The cells were incubated in YP medium for 72 hours. Respiratory incompetent cells were taken to ensure that ethanol (added as solvent of uncouplers and C12TPP) was not used by cells as a carbon source. Curves in the left plots represent the separate effects of the tested compounds, the right bar plots indicate the combined effects of each uncoupler supplemented with Cl2TPP in concentrations shown by the tables below the graphs. The OD of the mock-treated probe was set as 100%. In the experiments shown in Figure S2, we used petite (respiratory incompetent) yeast strain cells which were not able to utilize 0.1% ethanol added to the medium as a mock or as a solvent of uncouplers. Moreover, it is shown that in this system C12TPP did not increase the efficiency of the inhibition of cell growth by the anionic uncouplers. One may speculate that in this case inhibition of cell growth by uncouplers was also a result of their action on the plasma or vacuolar membrane rather than on mitochondria. Indeed, partial inactivation of Pma1p (plasma membrane H+-ATPase) was reported to increase the level of ATP in yeast cells (Holyoak et al., 1996 Appl Environ Microbiol 62: 3158-3164.). This indicates that maintenance of proton gradient on a plasma membrane is highly energy-consuming process and dissipation of this gradient could result in a decrease of biomass production.
(TIFF)
Funding Statement
This work was supported in part by the Russian Foundation for Basic Research grant 12-04-00199 and the Institute of Mitoengineering, Moscow State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Skulachev VP (1996) Role of uncoupled and non coupled oxidations in maintenance of safely low levels of oxygen and its one electron reductants. Quarterly Reviews Of Biophysics 29: 169–202. [DOI] [PubMed] [Google Scholar]
- 2. Harper JA, Dickinson K, Brand MD (2001) Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2: 255–265. [DOI] [PubMed] [Google Scholar]
- 3. Cunha FM, Caldeira da Silva CC, Cerqueira FM, Kowaltowski AJ (2011) Mild Mitochondrial Uncoupling as a Therapeutic Strategy. Curr Drug Targets 12: 783–789. [DOI] [PubMed] [Google Scholar]
- 4. Barros MH, Bandy B, Tahara EB, Kowaltowski AJ (2004) Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem 279: 49883–49888. [DOI] [PubMed] [Google Scholar]
- 5. Padalko VI (2005) Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry (Mosc ) 70: 986–989. [DOI] [PubMed] [Google Scholar]
- 6. Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ (2008) Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7: 552–560. [DOI] [PubMed] [Google Scholar]
- 7. Grundlingh J, Dargan PI, El Zanfaly M, Wood DM (2011) 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol 7: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lou PH, Hansen BS, Olsen PH, Tullin S, Murphy MP, et al. (2007) Mitochondrial uncouplers with an extraordinary dynamic range. Biochem J 407: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jennings ML, Adame MF (2001) Direct estimate of 1:1 stoichiometry of K(+)-Cl(-) cotransport in rabbit erythrocytes. Am J Physiol Cell Physiol 281: C825–C832. [DOI] [PubMed] [Google Scholar]
- 10. Nicholls DG (2006) Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem 281: 14864–14874. [DOI] [PubMed] [Google Scholar]
- 11. Omachi A, Scott CB, Glader BE (1968) 2,4-dinitrophenol inhibition of P32 release from human red cells. Experientia 24: 244–245. [DOI] [PubMed] [Google Scholar]
- 12. Winter CG, DeLuca DC, Szumilo H (1994) 2,4-Dinitrophenol and carbonylcyanide p-trifluoromethoxyphenylhydrazone activate the glutathione S-conjugate transport ATPase of human erythrocyte membranes. Arch Biochem Biophys 314: 17–22. [DOI] [PubMed] [Google Scholar]
- 13. Ilivicky J, Casida JE (1969) Uncoupling action of 2,4-dinitrophenols, 2-trifluoromethylbenzimidazoles and certain other pesticide chemicals upon mitochondria from different sources and its relation to toxicity. Biochem Pharmacol 18: 1389–1401. [DOI] [PubMed] [Google Scholar]
- 14. Ahmed I, Krishnamoorthy G (1990) Enhancement of transmembrane proton conductivity of protonophores by membrane-permeant cations. Biochim Biophys Acta 1024: 298–306. [DOI] [PubMed] [Google Scholar]
- 15. Orlov VN, Antonenko YN, Bulychev AA, Yaguzhinsky LS (1994) Combination of the electrogenic ionophores, valinomycin and cccp, can lead to non-electrogenic k+/h+ exchange on bilayer lipid membranes. FEBS Lett 345: 104–106. [DOI] [PubMed] [Google Scholar]
- 16. O’Brien TA, Nieva-Gomez D, Gennis RB (1978) Complex formation between the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and valinomycin in the presence of potassium. J Biol Chem 253: 1749–1751. [PubMed] [Google Scholar]
- 17. Kolajova M, Antalik M, Sturdik E (1993) Study of the complex formation between amine local anesthetics and uncouplers of oxidative phosphorylation carbonyl cyanide phenylhydrazones. Gen Physiol Biophys 12: 213–229. [PubMed] [Google Scholar]
- 18. Murphy MP, Smith RA (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656. [DOI] [PubMed] [Google Scholar]
- 19. Skulachev MV, Antonenko YN, Anisimov VN, Chernyak BV, Cherepanov DA, et al. (2011) Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Current Drug Targets 12: 800–826. [DOI] [PubMed] [Google Scholar]
- 20. Hoye ATDJ, Wipf P, Fink MP, Kagan VE (2008) Targeting mitochondria. Acc Chem Res 41: 87–97. [DOI] [PubMed] [Google Scholar]
- 21. Rokitskaya TI, Sumbatyan NV, Tashlitsky VN, Korshunova GA, Antonenko YN, et al. (2010) Mitochondria-targeted penetrating cations as carriers of hydrophobic anions through lipid membranes. Biochim Biophys Acta 1798: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 22. Severin FF, Severina II, Antonenko YN, Rokitskaya TI, Cherepanov DA, et al. (2010) Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc Natl Acad Sci U S A 107: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andreyev AY, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, et al. (1989) The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem 182: 585–592. [DOI] [PubMed] [Google Scholar]
- 24. Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, et al. (2008) Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc ) 73: 1273–1287. [DOI] [PubMed] [Google Scholar]
- 25. Johnson D, Lardy H (1967) Isolation of liver or kidney mitochondria. Methods Enzymol 10: 94–96. [Google Scholar]
- 26. Akerman KE, Wikstrom MK (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68: 191–197. [DOI] [PubMed] [Google Scholar]
- 27. Slonimski PP, Perrodin G, Croft JH (1968) Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal “petites”. Biochem Biophys Res Commun 30: 232–239. [DOI] [PubMed] [Google Scholar]
- 28. Sherman F (2002) Getting started with yeast. Methods Enzymol 350: 3–41. [DOI] [PubMed] [Google Scholar]
- 29. Pozniakovsky AI, Knorre DA, Markova OV, Hyman AA, Skulachev VP, et al. (2005) Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J Cell Biol 168: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, et al. (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A 88: 3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skulachev VP, Sharaf AA, Liberman EA (1967) Proton conductors in the respiratory chain and artificial membranes. Nature 216: 718–719. [DOI] [PubMed] [Google Scholar]
- 32. Skulachev VP, Sharaf AA, Yagujzinsky LS, Jasaitis AA, Liberman EA, et al. (1968) The effect of uncouplers on mitochondria, respiratory enzyme complexes and artificial phospholipid membranes. Curr Mod Biol 2: 98–105. [DOI] [PubMed] [Google Scholar]
- 33. McLaughlin SG, Dilger JP (1980) Transport of protons across membranes by weak acids. Physiol Rev 60: 825–863. [DOI] [PubMed] [Google Scholar]
- 34. Rokitskaya TI, Klishin SS, Severina II, Skulachev VP, Antonenko YN (2008) Kinetic analysis of permeation of mitochondria-targeted antioxidants across bilayer lipid membranes. J Membr Biol 224: 9–19. [DOI] [PubMed] [Google Scholar]
- 35. Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP (1969) Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 222: 1076–1078. [DOI] [PubMed] [Google Scholar]
- 36. Asin-Cayuela J, Manas AR, James AM, Smith RA, Murphy MP (2004) Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett 571: 9–16. [DOI] [PubMed] [Google Scholar]
- 37. Fetisova EK, Avetisyan AV, Izyumov DS, Korotetskaya MV, Chernyak BV, et al. (2010) Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress. FEBS Lett 584: 562–566. [DOI] [PubMed] [Google Scholar]
- 38. Antonenko YN, Avetisyan AV, Cherepanov DA, Knorre DA, Korshunova GA, et al. (2011) Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J Biol Chem 286: 17831–17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FCCP and C12TPP do not display synergistic inhibition of the increase in biomass of S. cerevisiae . Yeast cells were incubated in YPGly medium (see “Materials and methods”) supplemented with C12TPP and FCCP separately or with equimolar mixture of these compounds. For “C12TPP+FCCP” curve, the sum of the concentrations is shown, i.e. the data point “2 µM” corresponds to the growth medium supplemented with 1 µM of each compound. Incubation time was 5 hours. After incubation, the optical density (OD) was measured (λ = 550 nm). The OD of mock treated probe was set as 100%. Figure S1 shows that C12TPP did not enhance the inhibitory effect of FCCP on yeast growth, if the cells were grown on nonfermentable carbon source (glycerol). This result suggests that FCCP inhibited the growth not via arrest of oxidative phosphorylation but rather due to some other activity, for instance due to changing the proton potential on the plasma or vacuolar membranes.
(TIFF)
C12TPP did not affect the ability of neither FCCP (A) nor DNP (B) to decrease optical density of yeast respiratory-incompetent cells grown up to the stationary phase. The cells were incubated in YP medium for 72 hours. Respiratory incompetent cells were taken to ensure that ethanol (added as solvent of uncouplers and C12TPP) was not used by cells as a carbon source. Curves in the left plots represent the separate effects of the tested compounds, the right bar plots indicate the combined effects of each uncoupler supplemented with Cl2TPP in concentrations shown by the tables below the graphs. The OD of the mock-treated probe was set as 100%. In the experiments shown in Figure S2, we used petite (respiratory incompetent) yeast strain cells which were not able to utilize 0.1% ethanol added to the medium as a mock or as a solvent of uncouplers. Moreover, it is shown that in this system C12TPP did not increase the efficiency of the inhibition of cell growth by the anionic uncouplers. One may speculate that in this case inhibition of cell growth by uncouplers was also a result of their action on the plasma or vacuolar membrane rather than on mitochondria. Indeed, partial inactivation of Pma1p (plasma membrane H+-ATPase) was reported to increase the level of ATP in yeast cells (Holyoak et al., 1996 Appl Environ Microbiol 62: 3158-3164.). This indicates that maintenance of proton gradient on a plasma membrane is highly energy-consuming process and dissipation of this gradient could result in a decrease of biomass production.
(TIFF)