Abstract
Studying Pneumocystis has proven to be a challenge from the perspective of propagating a significant amount of the pathogen in a facile manner. The study of several fungal pathogens has been aided by the use of invertebrate model hosts. Our efforts to infect the invertebrate larvae Galleria mellonella with Pneumocystis proved futile since P. murina neither caused disease nor was able to proliferate within G. mellonella. It did, however, show that the pathogen could be rapidly cleared from the host.
Keywords: Galleria mellonella, infection model, Pneumocystis murina
Introduction
Pneumocystis is a fungal pathogen that causes morbidity and mortality in patients that are immunodeficient or immunosuppressed. Studying Pneumocystis has proven difficult due to the particular challenges of growing Pneumocystis for research purposes. The lack of an effective means to grow the microbe in cell culture prohibits sufficient and facile propagation for research on the biology of the microbe. Attempts at in vitro propagation through cell culture have shown only a modest rate of replication [1]. Mammalian models have been identified but non-mammalian models have yet to be established to propagate or study Pneumocystis.
The study of many pathogens has been greatly aided by the use of invertebrate models such as Caenorhabditis elegans, Drosophila melanogaster, Galleria mellonella, Dictyostelium discoideum and Acanthamoeba castellanii [2]. The study of Pneumocystis has thus far relied on more complicated mammalian models. Models that have been used to isolate Pneumocystis include rats, mice and ferrets [3, 4]. The mouse and rat models require immunosuppression in order to study Pneumocystis. Thus development of an invertebrate model to study Pneumocystis would be an asset to the study of this fungal pathogen.
In this study, we explore G. mellonella as a potential model host for P. murina. G. mellonella has been used as a model host to study fungal pathogens such as Aspergillus spp. [5–7], Candida albicans [8–10] and Cryptococcus neoformans [11]. As an infection model it offers the attractive attributes of being able to be maintained at 37°C post infection in order to study pathogenicity at mammalian temperature conditions and it can be injected with a specific amount of pathogen [2].
Our investigation found that G. mellonella, although susceptible to a number of medically relevant microbes, is not susceptible by P. murina. P. murina do not replicate within G. mellonella and are cleared from the G. mellonella larvae. We hope that the information contained in this report will allow others to benefit from our experience in attempting to establish a non-vertebrate model for P. murina studies utilizing G. mellonella.
Materials and Methods
Strains and media
P. murina were partially purified from mouse lung homogenate with Ficoll-Hypaque gradient centrifugation, frozen using Recovery Cell Culture Freezing Medium (Gibco) and stored at −80°C. The stored cells were thawed in a water bath at 35°C and then an equal volume of RPMI was added containing 20% heat-inactivated fetal bovine serum (FBS). P. murina were collected with centrifugation at 1000 × g for five min then suspended in 1 ml of phpsphate buffered saline (PBS). Pneumocystis organisms were quantified prior to infection using a real-time quantitative PCR (qPCR) assay based on the P. murina dhfr gene as previously described [12].
As a positive control in monitoring disease development, C. neoformans were included in our G. mellonella infection assays. C. neoformans (wild type strain KN99α) inocula were prepared by growing a culture overnight at 30°C in YPD (1% yeast extract, 2% peptone and 2% dextrose). The cells were collected with centrifugation and washed twice with PBS. C. neoformans were then counted with a hemocytometer and used immediately.
G. mellonella survival assay
G. mellonella larvae (Vanderhorst Wholesale, St. Marys, Ohio) were injected at the last left pro-leg using a Hamilton syringe as previously described [11]. G. mellonella infected with C. neoformans received 1.5 × 105 cells per larvae. P. murina was delivered at an inoculum of 4.85 × 105 or 4.85 × 106 cells per larvae. All G. mellonella received a 10 μl inoculum volume of fungal cells suspended in PBS. Each infection group contained sixteen larvae of the appropriate weight (330 ± 25 mg). Larvae were kept at 37°C in the dark and scored daily for survival. Killing curves were plotted and statistical analysis (log rank test) was performed by the Kaplan-Meier method using STATA 6 statistical software (Stata). Killing curves were performed in three independent replicates and a representative graph was reported.
Hemocyte density
Hemolymph was collected from larvae 72 h post-infection into pre-chilled collection tubes. The hemolymph from three larvae were pooled into a single sample and three samples were examined per infection group. Hemolymph was diluted in PBS then hemocytes were counted with a hemocytometer. We did not differentiate between the various types of hemocytes within the hemolymph.
Tissue staining
G. mellonella were infected as described above. The internal structures of three larvae per infection group were collected at 96 h post infection and placed in formalin. The tissue was fixed overnight at 4°C, serially dehydrated with ethanol then transferred to xylene for three h and embedded in paraffin. Sections were cut and stained with a 4D7 monoclonal antibody specific for P. murina and an Alexa Fluor 488 conjugated anti-mouse IgG secondary antibody [13, 14]. DAPI was included when mounting the slides. The tissue sections were examined with light and fluorescent microscopy. A Nikon eclipse TE2000-U microscope was used with 100x magnification.
P. murina quantification from G. mellonella
Hemolymph and internal body structures were collected from G. mellonella at the indicated time points, weighed and stored at −80°C for DNA isolation. To the frozen material, 100 μl of glass beads (425–600 μm) were added along with 1 ml DNA isolation buffer (100mM Tris HCl pH 7.5, 700mM sodium chloride, 10 mM ethyldiaminetetraacentic acid, and 0.05% sodium dodecyl sulfate). The mixture was agitated vigorously for 5 min to disrupt the tissue then β-mercaptoethanol was added to 1% followed by 300 μg/ml proteinase K. The slurry was then incubated at 65°C for 30 min then allowed to cool to room temperature. DNA was isolated with phenol:chloroform then isopropanol precipitated. The isolated DNA was used as a template in a qPCR reaction to quantify the Pneumocystis organisms within G. mellonella.
Results and Discussion
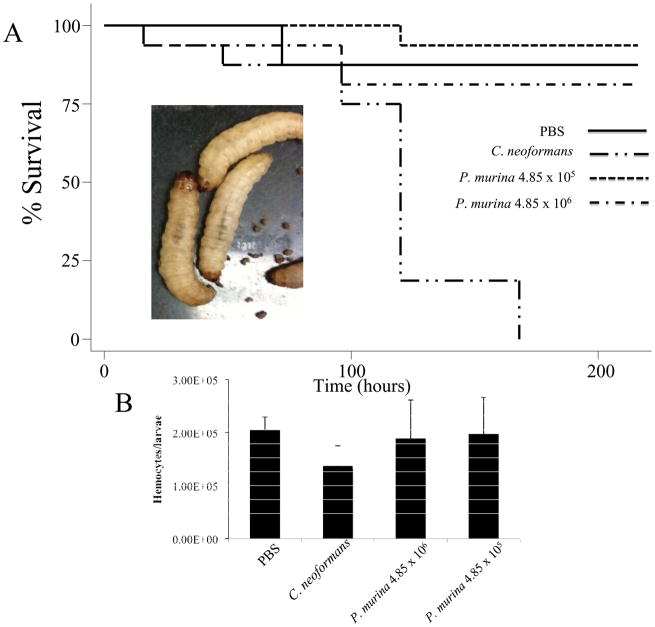
Our goal for this study was to determine if G. mellonella was a useful infection model to study P. murina. 81–93% of G. mellonella inoculated with P. murina survived for 216 h without exhibiting signs of a lethal infection (Fig. 1). We included a C. neoformans infected group of G. mellonella larvae as a control. None of the larvae infected with C. neoformans were alive at 216 h post infection. Survival curves were plotted using the Kaplan-Meier analysis, and the differences in survival were evaluated for significance using the log rank test. There was no significant difference between P. murina infected larvae and larvae injected with PBS. Symptoms that can accompany fungal infections include lethargy and melanization of the larvae and eventually death. In the case of P. murina infected larvae, they remained active and did not melanize (Fig. 1A).
Figure 1.
A. A Kaplan-Meier plot of G. mellonella survival after injection with 1.5 × 105 cfu/larvae of C. neoformans showed that C. neoformans caused a lethal infection whereby 50% mortality was reached by 120 h post-infection. G. mellonella infected with 4.85 × 105 or 4.85 × 106 P. murina did not shows signs of infection. At 216 h post infection, 15 out of 16 P. murina infected G. mellonella remained alive from the group infected with 4.85 × 105 cells per larvae and 13 out of 16 were alive in the group infected with 4.85 × 106 cells per larvae. The image to the left shows that G. mellonella did not melanize after injection with P. murina. B. Hemocyte density was reduced when larvae were infected with C. neoformans. However, larvae infected with P. murina had a hemocytes density similar to that of uninfected larvae.
Another infection symptom that can be followed with G. mellonella larvae is a change in hemocytes density. Fungi that are pathogenic to larvae lead to a reduction in G. mellonella hemocytes density while inoculations with fungi that are non-pathogenic result in G. mellonella maintaining a hemocytes density similar to uninfected larvae [9]. At 72 h post-infection with C. neoformans we observed a reduction in G. mellonella hemocyte density (Fig. 1B). This was congruent with observations associated with other fungi that cause lethal infections [9]. Larvae inoculated with P. murina, however, retained a hemocytes density comparable to larvae injected with PBS (Fig. 1B).
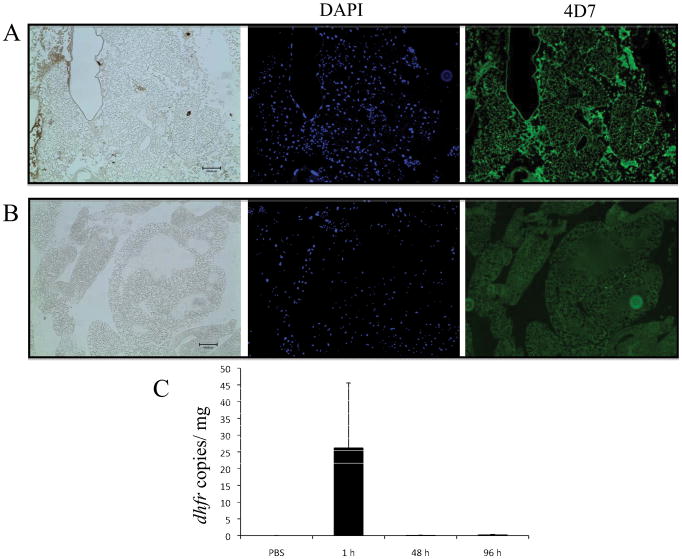
Although P. murina did not cause a lethal infection, we were still interested in determining of the fungi were retained with the larvae. Further, we examined the internal structures of G. mellonella to see if P. murina could be identified within the larvae or replicate within G. mellonella. Tissue collected from G. mellonella 96 h post infection were stained and then observed for the presence of P. murina. We did not find any P. murina in sections stained with 4D7 monoclonal antibody observation with fluorescent microscopy (Fig. 2).
Figure 2. G. mellonella tissue was fixed 96 h post infection and then embedded in paraffin. Paraffin sections were stained with 4D7 monoclonal antibody and a secondary IgG anti-mouse antibody conjugated to Alexa Fluor 488 was used to identify the presence of P. murina cells in the tissue. The tissue was also stained with DAPI.
A. No fungal cells were observed in C. neoformans infected tissue as expected since the antibody is specific to Pneumocystis. B. Pneumocystis was also not identified in tissue from larvae previously inoculated with P. murina. Scale bar, 100 μm (bottom right corner). C. The amount of P. murina within G. mellonella tissue was quantified at 1, 48 and 96 h post infection using qPCR to amplify dhfr.
The fungal burden within G. mellonella was assessed through qPCR of the dihydrofolate reductase gene (dhfr), which is a single copy gene that is amenable for P. murina quantification in mouse models [12]. The hemolymph and tissue was collected from G. mellonella at 1, 48 and 96 h post infection with P. murina. As a control, hemolymph and tissue was also collected from G. mellonella at 1 h post injection with PBS. DNA was isolated from the collected material and qPCR was used to measure the number of copies of DHFR, thus indicating the number of Pneumocystis nuclei within the tissue. The decrease in G. mellonella fungal burden indicates that P. murina is rapidly cleared from the host (Fig. 2C). It is not known at this time how the G. mellonella facilitate the clearing of P. murina from the system but one possibility is through an immune response either in which the G. mellonella presents adverse conditions to which P. murina cannot survive or they may be engulfed and subsequently destroyed by hemocytes which are G. mellonella phagocytic cells.
Thus it does not appear that G. mellonella sustains an infection by P. murina and is not a suitable model for the proliferation of the fungus for the study of pathogenesis. It has been a difficult challenge to identify alternative hosts to study Pneumocystis. One particular aspect to the challenge is that Pneumocystis appears, by present available evidence, to be species specific or to need species-specific requirements to establish an infection [15, 16]. What we did find from our endeavor of infecting G. mellonella with Pneumocystis was that Pneumocystis could be eliminated from the host system rapidly and without symptoms of disease. There is the potential that species-specific infection requirements were not fulfilled within G. mellonella and perhaps the fungal cells were not able to remain viable in that environment. Another possibility is that insects share a soil environment with a number of various fungal pathogens and this close proximity and constant bombardment by fungi have aided in the evolution of a means to effectively combat Pneumocystis.
The innate immune system of insects including G. mellonella uses multiple means to defend against fungal pathogenesis including phagocytosis of organisms by hemocytes. The hemocoel environment is further made hostile by host production of enzymes and reactive oxygen species. Also, within the G. mellonella defense arsenal is a gallerimycin peptide [17] and several moricin-like peptides that exhibit antifungal activity [18].
Further research is needed to identify what host biological facets are essential for the propagation of Pneumocystis within a viable host or conversely, from the host perspective, identifying which host responses prevent propagation of Pneumocystis and the development of PCP.
Abbreviations
- FBS
fetal bovine serum
- PBS
phosphate buffered saline
References
- 1.Sloand E, Laughon B, Armstrong M, Bartlett MS, Blumenfeld W, Cushion M, Kalica A, Kovacs JA, Martin W, Pitt E, et al. The challenge of Pneumocystis carinii culture. J Eukaryot Microbiol. 1993;40:188–95. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs BB, Mylonakis E. Using non-mammalian hosts tostudy fungal virulence and host defense. Curr Opin Microbiol. 2006;9:346–51. doi: 10.1016/j.mib.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Oz HS, Hughes WT, Vargas SL. Search for extrapulmonary Pneumocystiscarinii in an animal model. J Parasitol. 1996;82:357–9. [PubMed] [Google Scholar]
- 4.Tamburrini E, Ortona E, Visconti E, Mencarini P, Margutti P, Zolfo M, Barca S, Peters SE, Wakefield AE, Siracusano A. Pneumocystis carinii infection in young non-immunosuppressed rabbits. Kinetics of infection and of the primary specific immune response. Med Microbiol Immunol. 1999;188:1–7. doi: 10.1007/s004300050098. [DOI] [PubMed] [Google Scholar]
- 5.Scully LR, Bidochka MJ. Serial passage of the opportunistic pathogen Aspergillus flavus through an insect host yields decreased saprobic capacity. Can J Microbiol. 2005;51:185–9. doi: 10.1139/w04-124. [DOI] [PubMed] [Google Scholar]
- 6.St Leger RJ, Screen SE, Shams-Pirzadeh B. Lack of host specialization in Aspergillus flavus. Appl Environ Microbiol. 2000;66:320–4. doi: 10.1128/aem.66.1.320-324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves EP, Messina CG, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia. 2004;158:73–9. doi: 10.1023/b:myco.0000038434.55764.16. [DOI] [PubMed] [Google Scholar]
- 8.Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol. 2000;27:163–9. doi: 10.1111/j.1574-695X.2000.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003;5:1389–95. doi: 10.1016/j.micinf.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002;34:153–7. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 11.Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–50. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. Quantitative real-time polymerase chain-reaction assay allows characterization of pneumocystis infection in immunocompetent mice. J Infect Dis. 2004;189:1540–4. doi: 10.1086/382486. [DOI] [PubMed] [Google Scholar]
- 13.Angus CW, Tu A, Vogel P, Qin M, Kovacs JA. Expression of variants of the major surface glycoprotein of Pneumocystiscarinii. J Exp Med. 1996;183:1229–34. doi: 10.1084/jem.183.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutty G, Hernandez-Novoa B, Czapiga M, Kovacs JA. Pneumocystis encodes a functional S-adenosylmethionine synthetase gene. Eukaryot Cell. 2008;7:258–67. doi: 10.1128/EC.00345-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand-Joly I, Aliouat el M, Recourt C, Guyot K, Francois N, Wauquier M, Camus D, Dei-Cas E. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol. 2002;40:1862–5. doi: 10.1128/JCM.40.5.1862-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gigliotti F, Harmsen AG, Haidaris CG, Haidaris PJ. Pneumocystis carinii is not universally transmissible between mammalian species. Infect Immun. 1993;61:2886–90. doi: 10.1128/iai.61.7.2886-2890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuhmann B, Seitz V, Vilcinskas A, Podsiadlowski L. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleriamellonella. Arch Insect Biochem Physiol. 2003;53:125–33. doi: 10.1002/arch.10091. [DOI] [PubMed] [Google Scholar]
- 18.Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleriamellonella. Insect Biochem Mol Biol. 2008;38:201–12. doi: 10.1016/j.ibmb.2007.10.009. [DOI] [PubMed] [Google Scholar]