Abstract
Purpose
Polyamines are essential for normal growth; however, the requirement for, and the metabolism of, polyamines are frequently dysregulated in cancer. Polyamine analogues have demonstrated promising preclinical results in multiple model systems of cancer, but their clinical utility has been limited by apparent toxicity. A representative compound of a new generation of short chain, conformationally restricted polyamine analogues, CGC-11047 has been synthesized and ongoing phase I clinical trials indicate it to be well tolerated at weekly doses of 610 mg (dose escalation is still in progress). Therefore, studies were designed to gain a better understanding of its effects on cellular polyamine biochemistry and efficacy in the treatment of human lung cancer models in vitro and in vivo.
Methods
Human lung cancers cell lines representing non-small cell and small cell lung cancers were investigated for their growth and biochemical response to CGC-11047. Effects of in vitro treatment with CGC-11047 on cell growth, the activity of the polyamine biosynthetic enzyme ornithine decarboxylase (ODC), and the expression and activity of the polyamine catabolic enzymes spermidine/spermine N1-acetyltransferase (SSAT) and spermine oxides (SMO) were measured. Additionally, the overall effects on intracellular polyamine pools were monitored. Finally, the in vivo efficacy of CGC-11047 in the treatment of a nude mouse model of human non-small cell lung cancer was evaluated.
Results
CGC-11047 effectively inhibited the growth of both small cell and non-small cell lung cancer cells in vitro. The greatest biochemical effects were observed in the non-small cell lung cancer cells where in addition to a profound down regulation of ODC activity, there was a significant increase in polyamine catabolism leading to a greater degree of polyamine pool depletion and greater accumulation of CGC-11047 when compared with the changes observed for the small cell lines. Importantly, CGC-11047 was found to be highly significant (P < 0.0001) in delaying the progression of established tumors in an in vivo model of human non-small cell lung cancer.
Conclusion
CGC-11047 represents a promising new polyamine analogue that warrants further preclinical and, potentially, clinical evaluation in lung cancer.
Keywords: Polyamines, Analogues, Lung cancer, SSAT, SMO, ODC
Introduction
The natural polyamines, putrescine, spermidine, and spermine are small polycationic alkylamines that have been shown to be essential for cell growth. Polyamine concentrations are increased and polyamine metabolism dysregulated in multiple tumor cell types, thus making this pathway a rational target for antineoplastic therapies [10, 20, 28, 34]. Targeting the key enzymes involved in the polyamine biosynthetic pathway, ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC) has been demonstrated to be successful as a means of targeting polyamines and regulating cell growth, but has had limited clinical success [2, 33, 38]. In an attempt to overcome the shortcomings of the inhibitors of polyamine biosynthesis, several groups have synthesized and tested polyamine analogues that were designed to take advantage of the self-regulatory properties of the natural polyamines [4, 18, 26, 55].
In addition to having the expected results of down-regulating biosynthesis, several analogues of spermine were found to have the unusual property of super-inducing the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase (SSAT) which results in the depletion of intracellular polyamines, inhibits cell growth, and in a tumor type-specific manner, induces apoptotic cell death [11, 15, 23, 29, 35, 41]. N1.N12-Bisethylspermine (BESpm), a symmetrically substituted spermine analogue has been previously shown to induce SSAT to very high levels in numerous tumor cell types, leading to a rapid cytotoxic response of the cells.[16]. Despite impressive in vitro activity, BESpm was not effective in xenograft models of various tumor types [6]. A closely related analogue, N1, N11-diethylnorspermine (DENSpm), demonstrated very similar in vitro effects with respect to induction of SSAT and cytotoxicity, and also demonstrated impressive in vivo activity against multiple solid tumors [7, 21, 42]. As a result, phases I and II clinical trials have been completed with DENSpm [24, 30, 48, 56]; however, to date DENSpm has not been effective as a single agent. One of the potential problems limiting the effectiveness of DENSpm may have been the daily administration schedule in most of its clinical trials. Such a schedule may have contributed to the observed does-limiting toxicities, thus impeding its clinical utility [30]. Therefore, the search has continued for a polyamine analogue with similar antineoplastic characteristics as the symmetrically substituted analogues, BESpm and DENSpm, but whose dosing schedule could be adjusted to allow efficacy in the absence of serious dose-limiting toxicities.
CGC-11047 is a second-generation analogue of BESpm that is also symmetrically substituted, but that is conformationally restricted with a cis double bond between the central carbons (Fig. 1a). CGC-11047 has shown positive results in the treatment of non-Hodgkin’s lymphoma and is currently in several clinical trials. Treatment with CGC-11047 has demonstrated significant growth inhibition in numerous tumor cell lines with ID50s as low as 50 nM in DU145 cells [43]. The growth inhibition was concurrent with analogue accumulation and decreased natural polyamines. However, the basis for this decrease in polyamine pools was not determined [43]. Consequently, despite some very promising results with CGC-11047, further studies on its biochemistry and potential mechanism of action are needed. Here we report on the effects of CGC-11047 on four human lung cancer cell lines that represent the major forms of lung cancer, with an emphasis on biochemical mechanisms that may contribute to its antiproliferative effects. Importantly, we demonstrate impressive antitumor effects in vivo against a human xenograft model of lung adenocarcinoma that suggests that a clinical trial of CGC-11047 in lung cancer may be warranted.
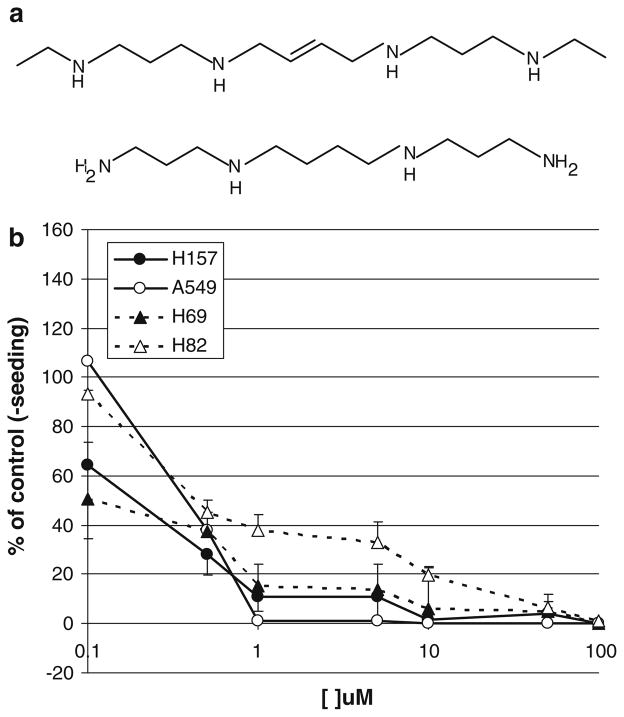
Fig. 1.
Effects of the conformationally restricted polyamine analogue, CGC-11047 of growth of human lung cancer cells in vitro. a Structures of CGC-1047 and the natural polyamine spermine. b CGC-11047 inhibits growth in human non-small cell and small cell lung cancer lines in vitro. Growth inhibition induced by increasing concentrations of CGC-11047 in the non-small cell (H157, A549) and small cell (H82, H69) lung cancer lines was determined by trypan blue exclusion cell after 96-hour treatment. Each point represents three independent experiments ±SE
Materials and methods
Chemicals and reagents
[(1)N,(12)N]Bis(Ethyl)-cis-6,7-dehydrospermine (CGC11047) was obtained from Progen Pharmaceuticals (Redwood City, CA). Other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO), Invitrogen/Life Technologies, Inc. (Rockville, MD), BioRad (Hercules, CA), Aldrich Chemical Co. (Milwaukee, WI), Hyclone (Logan, UT), and J.T. Baker Inc. (Phillipsburg, NJ).
Cell culture and analysis of growth inhibition of human lung cancer cell lines
The non-small cell cancer lines, NCI A549, NCI H157, and the small cell lung carcinoma lines, NCI H82 and NCI H69, were cultured as previously reported [15]. Cells were treated for the indicated times and concentrations. Cell growth was determined by standard trypan blue exclusion.
RNA purification and Northern blot analysis
Extraction of total cellular RNA was performed using Trizol Reagent (Invitrogen) according to the manufacturer-supplied protocol. For Northern blotting, total RNA (20 μg) was separated on a denaturing 1.5% agarose gel containing 6% formaldehyde and transferred to Zetaprobe membrane (Bio-Rad) and hybridized to random primer-labeled PAOh1/SMO or SSAT cDNAs to determine SMO and SSAT expression [14, 52]. Blots were stripped and reprobed with a labeled 18S ribosomal cDNA as a loading control. Hybridized membranes were analyzed and images generated on a PhosphoImager using Image Quant software (Molecular Dynamics).
Analysis of polyamine content, SSAT, SMO, and ODC activity
Intracellular polyamine concentrations were determined using the precolumn dansylation labeling, reverse phase high-pressure liquid chromatography method as reported by Kabra et al. [32] using 1,7-diaminoheptane as an internal standard. Polyamine concentrations were reported as nmol/mg protein for each sample, where lysate protein content was measured by the method of Bradford [9]. SSAT activity of cellular extracts was measured as previously published [11]. The SMO enzyme activity in the cell lysates was assayed as previously reported [53]. ODC activity was measured by the methods of Seely and Pegg [45].
Western blot analysis
Total cell extracts were obtained by lysing cells on ice in RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 30 μg/ml aprotinin, 100 μM sodium orthovanadate, 10 μg/ml PMSF) and insoluble material was pelleted at 12,000g for 20 min at 4°C. Thirty μg of total protein was loaded per lane and separated on a 10% SDS-PAGE gels transferred electrophoretically to Immunoblot PVDF membrane (BioRad) for immunobloting. Briefly, membranes were blocked for 1 hour in Odyssey blocking buffer, per manufacturer’s instructions. The primary antibodies, an affinity purified antisera to human SMO [3], SSAT [17] and a mouse anti-β-actin purchased from Santa Cruz (Santa Cruz, CA) were all used at dilutions of 1:1000 with 0.1% Tween 20 in blocking buffer for 1 h at room temperature. Following washes, blots were incubated with appropriate fluorescent dye-conjugated secondary antibodies (1:4000 each, 0.1% Tween 20, in blocking buffer protected from light, for 45 min). Western blot results were quantified using the LICOR immunofluorescence system (LI-COR Biosciences, Lincoln, NE).
In vivo studies
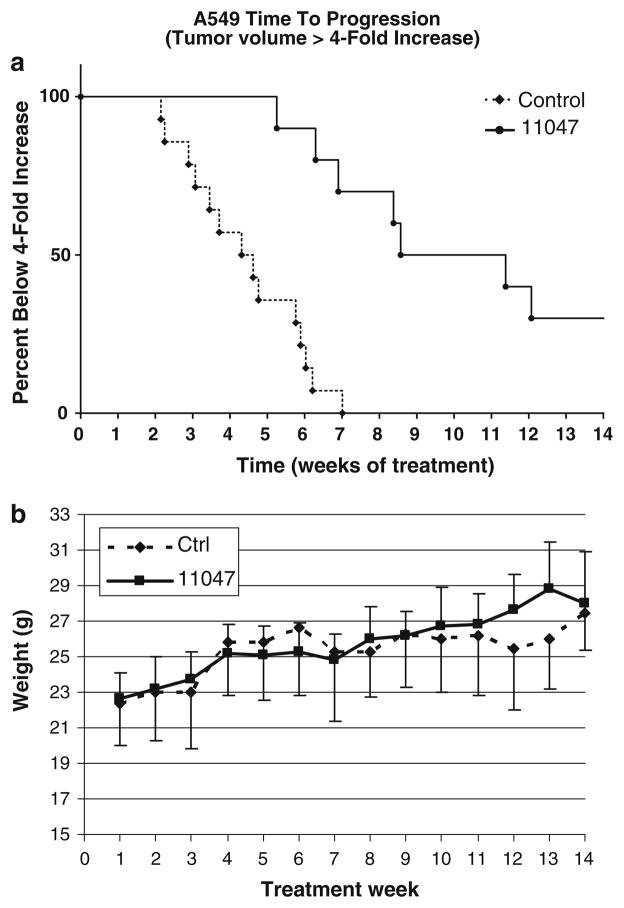
A549 non-small cell lung cancer cells (1.0 × 107) suspended in Hank’s Balance Salt Solution (HBSS) were injected subcutaneously into the right flank of 4–6-week-old female Athymic nude nu/nu mice (Harlan, Indianapolis, IN). Tumors were allowed to grow for 13 days resulting in an average volume of >0.3 cm3. Tumor-bearing animals were then randomly sorted into two groups, control (n = 15) and treatment (n = 10). The treatment group received 100 mg/kg of CGC-11047 in 200 μl HBSS by IP injection once a week for 3 weeks on and 1 week off for a total of 14 weeks by intraperitoneal injection (IP). Control animals were injected with 200 μl HBSS. Mice were weighed and tumor volumes were measured weekly. A Kaplan–Meier time to progression model was used to analyze the data with time to progression is defined as time for the individual tumor to increase fourfold after the initiation of treatment. The log-rank test was used to determine statistical significance.
Results
Inhibition of cell growth by CGC-11047
We have previously demonstrated differential sensitivity of human lung cancer phenotypes to specific polyamine analogues. Specifically, human non-small cell lung cancer cells were found to be more sensitive to non-conformationally restricted analogues than small cell lung cancer cells and this differential sensitivity was associated with differential induction of polyamine catabolism [12, 15, 19]. To determine if a similar differential sensitivity was observed with the conformationally restricted CGC-11047, we compared cellular responses of two representative lines of small cell (H69 and H82) to two representative lines of non-small cell lung cancers (A549 and NCI-H157). CGC-11047 effectively inhibited cell growth in both small cell and non-small cell lung cancers in a concentration-dependent manner after 96 h of treatment (Fig. 1b). The IC50 values for all cell lines ranged from 0.1 to 0.5 μM, with the order of sensitivity A549 > H69 = H157 > H82. Although there appears to be some differences in sensitivity between the different cell lines, these data indicate that CGC-11047 effectively inhibits in vitro cell growth of multiple human lung cancer lines regardless of their phenotype.
Effects of CGC-11047 on polyamine metabolism
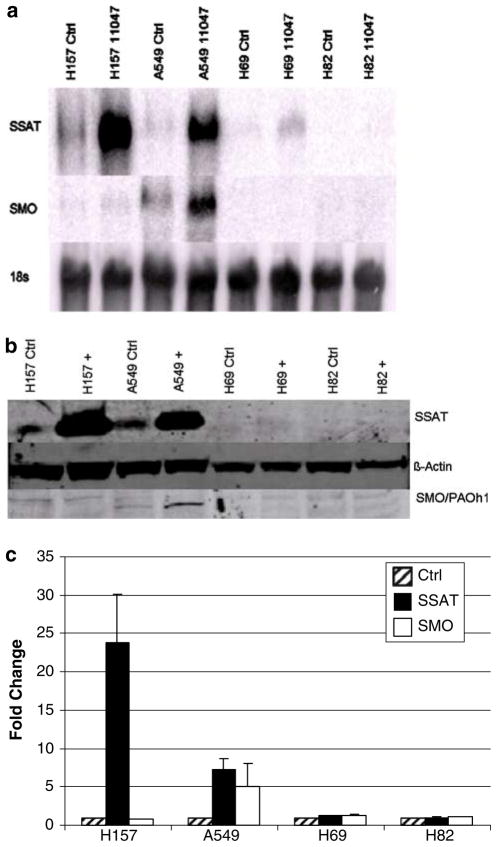
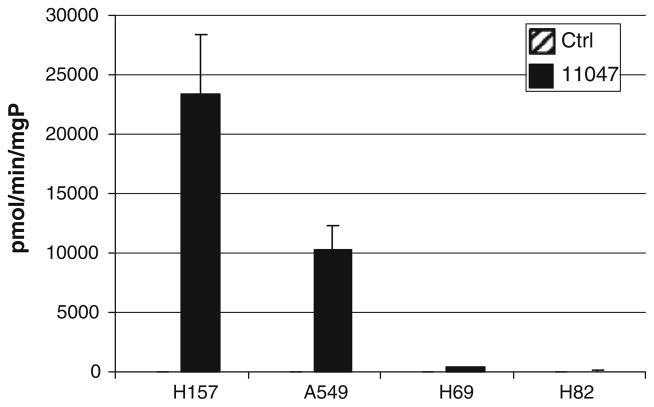
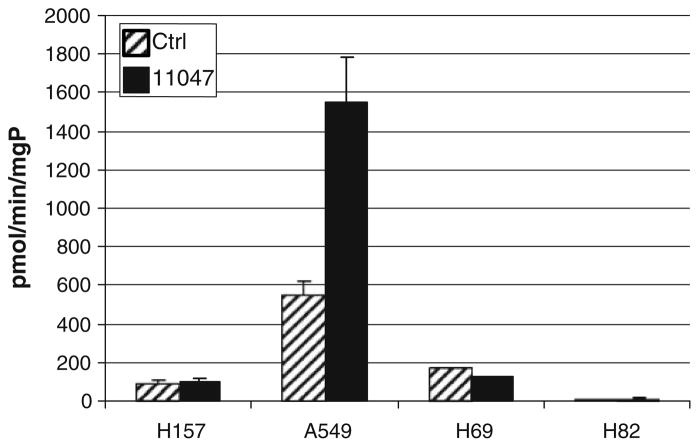
Several classes of polyamine analogues alter polyamine metabolism through multiple regulatory pathways [20]. The original basis for the synthesis of polyamine analogues for the treatment of cancer was based on the self-regulating nature of polyamine metabolism [39, 40]. Therefore, the effects of CGC-11047 on polyamine metabolism were examined in the multiple lung cancer cell lines. The response to treatment with respect to alterations in polyamine metabolism were greater in the non-small cell phenotype than observed in the small cell phenotype, consistent with previous findings with other analogues [19]. In H157 and A549 cells, 24-h treatment with 10 μM CGC-11047 up-regulated the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase (SSAT) and down-regulated the polyamine biosynthetic enzyme ornithine decarboxylase (ODC). The induction of SSAT occurred at the level of mRNA (Fig. 2a), protein (Fig. 2b, c), and activity (Fig. 3), with SSAT enzyme activity increasing >1,000-fold in both A549 cells and H157 cells (Fig. 2). By contrast, SSAT enzyme activity was not altered in H82 and H69 after 24-h treatment exposure to10 μM of CGC-11047. Spermine oxidase (SMO) was significantly induced only in the A549 cells (Figs. 2, 4), as has been previously observed with other polyamine analogues [25], demonstrating an approximate threefold increase in SMO activity after 24 h exposure to 10 μM CGC-11047 (Fig. 4).
Fig. 2.
Induction of polyamine catabolic enzymes at the level of mRNA and protein. a Northern blot analysis of CGC-11047 human lung cancer cell lines. Cells were exposed to 10 μM CGC-11047 for 24 h, where indicated. Each lane was loaded with 10 μg of total RNA and blots were sequentially probed with labeled SMO and SSAT cDNAs with 18s rRNA serving as a loading control. The illustrated blot is representative of three independent experiments. b Western analysis of CGC-11047-treated human lung cancer cells. Cells were exposed to10 μM CGC-11047 for 24 h, where indicated and 15 μg of total cellular protein was loaded per lane. After transfer, membranes were sequentially incubated with SSAT, SMO, or β-actin antbodies, as indicated. The illustrated blot is representative of three independent experiments. c Quantified values of protein expression normalized to β-actin. Values represent the mean of three independent experiments (±SD) and are shown as the fold change over the control
Fig. 3.
CGC-11047 induces SSAT enzyme activity in non-small cell lung cancer cell lines. Cells were treated with 10 μM CGC-11047 for 24 h and SSAT activity was determined. Striped bars represent control levels and solid bars represent treated cells. The activity values are shown in pmols/min/mg protein. Each bar represents three independent experiments performed in quadruplicate (±SE)
Fig. 4.
CGC-11047 induces SMO activity in the A459 non-small cell lung cancer cell line. Cells were treated with 10 μM CGC-11047 for 24 h and assayed for SMO activity. The striped bars represent controls and solid bars represent treated cells. The activity values are shown in pmols/min/mg protein. Each bar represents three independent experiments performed in triplicate (±SE)
The activity of one rate-limiting polyamine biosynthetic enzyme, ODC, demonstrated a reduction with CGC-11047 treatment to nearly undetectable levels in the H69, H157, and A549 cells, and to ~20% of control in H82 cells. The decreases observed were more prominent in H157 and A549 cells since they have a higher basal level of ODC activity as compared to H69 and H82 cells (Fig. 5).
Fig. 5.
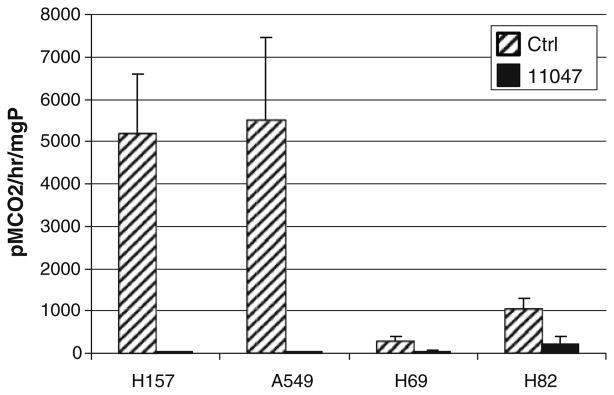
CGC-11047 treatment decreases ODC activity in human lung cancer cells. Cell lines were treated with 10 μM CGC-11047 for 24 h and ODC activity in treated a control cells was determined. The striped bars represent controls and solid bars represent treated cells. The activity values are shown in pmols CO2/hour/mg protein. Each bar represents a minimum of two independent experiments performed in quadruplicate (±SE)
The changes in polyamine concentrations resulting from analogue treatment are consistent with the observed changes in polyamine metabolic enzyme activities in the representative cell lines (Table 1). Specifically, the non-small cell lung cancer lines H157 and A549 demonstrated the greatest depletion of all three polyamines, consistent with their profile of decreased ODC activity and increased polyamine catabolism (Figs. 2, 3, 4), whereas the small cell line H69 was intermediate in its depletion of polyamine and H82 cells were the least affected, again consistent with their profile of polyamine metabolic enzyme activity in response to analogue treatment (Figs. 2, 3, 4). It is also important to note that analogue accumulation by the individual cell lines was inversely proportional to the level of natural polyamine depletion (Table 1).
Table 1.
Effects of CGC-11047 on intracellular polyamine pools in human lung cancer cells
| Cell type and treatment | Polyamines (nmol/mg protein)
|
|||
|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | CGC-11047 | |
| H157 Control | 1.5 ± 0.9a | 4.7 ± 1.7 | 5.1 ± 1.4 | ND |
| H157 Treated | ND | ND | 0.24 ± 0.12 | 24.2 ± 5.1 |
| A549 Control | 3.2 ± 1.7 | 11.6 ± 3.7 | 3.25 ± 1.5 | ND |
| A549 Treated | 0.1 ± 0.1 | ND | 0.24 ± 0.13 | 22.3 ± 2.8 |
| H69 Control | 10.4 ± 1.3 | 7.5 ± 3.5 | 7.7 ± 2.4 | ND |
| H69 Treated | 1.0 ± 0.7 | 1.9 ± 1.9 | 3.9 ± 2.1 | 15.4 ± 4.7 |
| H82 control | 6.2 ± 2.1 | 8.3 ± 2.2 | 5.7 ± 1.2 | ND |
| H82 Treated | 1.8 ± 0.7 | 4.6 ± 1.5 | 4.9 ± 1.3 | 11.6 ± 2.1 |
Indicated cells were exposed to 10 μM CGC-11047 for 24 h and intracellular polyamine pools and analogue accumulation were measured
ND indicates values below the 0.05 nmol/mg protein limit of detection
Polyamine pool values represent the mean of three independent experiments performed in duplicate ± SE
In vivo efficacy of CGC-11047
The in vivo therapeutic effect of CGC-11047 was assessed using A549 xenografts in athymic nude nu/nu mutant mice. The A549 cell line was chosen for use in nude mice since in the in vitro setting CGC-11047 induced the greatest increase in polyamine catabolic enzyme activity, the greatest depletion of intracellular polyamines, and the largest growth inhibitory effects of all the cell lines studied. Thirteen days after implantation of 1.0 × 106 A549 cells the average tumor volume was 0.3 cm3 (SD = 0.18) (n = 25) and average weight of the mice was 22.5 g (SD = 1.96). Mice were randomized into treated (n = 10) and control (n = 15) groups. Animals received intraperitoneal injections of 100 mg/kg of CGC-11047 in 200 μl of Hanks balanced salt solution or of 200 μl of Hanks alone. The treatment schedule was 100-mg/kg dose once weekly for 3 weeks followed a week of rest, for a total of 14 weeks. The once weekly dosing ×3 every 4 weeks was selected since previous xenograft studies with other polyamine analogues have demonstrated once or twice weekly schedules are equivalent to the 5× per week dosing, but with greater tolerability [31]. Treatment of established tumors with a weekly dose of CGC-11047 resulted in a significant delay in progression (P ≤ 0.0001, Fig. 6a). By week seven all control animals had progressed (>fourfold increase in tumor starting volume), whereas only 30% of the treated animals had progressed. Even after 14 weeks of treatment, 30% of the treatment group had still not progressed and one mouse was found to be completely tumor free. Importantly, there were no overt signs of drug-induced toxicities and all treated animals continued to feed and gain weight normally (Fig. 6b).
Fig. 6.
a Treatment of CGC-11047 on A549 cells in vivo increases the time to progression. Tumor cells were implanted in mice and allowed to grow until they reached a minimum of 200 mm3 prior to treatment. Animals with established tumors were then treated once a week as indicate in “Materials and Methods” with either 100 mg/kg CGC-11047 or HBSS. Tumors were measured weekly as described and the fold-increase over week one was calculated. Kaplan–Meier Analysis indicates that CGC11047-treatment significantly slows in vivo tumor growth compared to untreated controls (P ≤ 0.0001, using the Mantel–Cox log rank test). b CGC11047 does not effect normal weight gain of treated animals
Discussion
The frequent dysregulation of polyamine metabolism in tumor cells make polyamine metabolism and function a rational target for antineoplastic therapy [20, 46, 47, 50, 51]. Although inhibitors of virtually all of the biosynthetic enzymes in polyamine metabolism have been synthesized, none has demonstrated clinical efficacy as a single agent in clinical trails for cancer (see [20]). In an attempt to overcome the limitations encountered with specific inhibitors of enzyme function, we and others have exploited the self-regulatory properties of polyamine metabolism for therapeutic advantage through the use of structural analogues of the natural polyamines [4, 5, 8, 18, 39, 43, 44, 49]. The initial studies with polyamine analogues, particularly with BENSpm, were promising, but clinical trials with a number of analogues in lung, breast, and other tumors did not reveal efficacy as single agents. This might be due in part to less-than-optimal dosing schedules and resulting dose-limiting toxicities, but probably also due to the inherent properties of the analogues utilized. Here, we report on the effects of a conformationally restricted polyamine analogue, CGC-11047, that is effective in inhibiting the growth of four human lung cancer cell lines that are representative of the major forms of lung cancer. Further, we demonstrate that CGC-11047 is well tolerated and effective in delaying tumor progression in established tumors in vivo.
Our laboratory originally demonstrated a phenotype-specific response to polyamine analogues that was linked to increased polyamine catabolism, a finding that was subsequently corroborated by several other laboratories [11, 13–15, 22, 41]. Specifically, analogues that were most effective in inducing SSAT were found to be selective in their cytotoxic effects against non-small cell lung cancer cells. As these studies were performed with the first generation symmetrically substituted and second generation asymmetrically substituted polyamine analogues, we sought to determine if the symmetrically substituted, conformationally restricted polyamine analogue, CGC-11047 would demonstrate similar selectivity in vitro. Consistent with previous polyamine analogue studies, the small cell line H82 was the most resistant to CGC-11047 treatment [11, 12, 25]. However, at higher concentrations, it too was significantly growth inhibited. The most sensitive line, the non-small cell line, A549 was also the line that demonstrated the greatest depletion of intracellular polyamines and the greatest accumulation of the analogue. Similarly, the other non-small cell line, H157, also demonstrated greater overall polyamine pool depletion and analogue accumulation than the two representative small cell lines. This greater perturbation of polyamine pools in the non-small cell lines is consistent with previous analogue studies and is most likely the result of the significantly greater increase in polyamine catabolism induced by analogue treatment of the non-small cell lines as compared to the small cell lines, which demonstrate little or no induction of either SSAT or SMO in response to analogue treatment [11, 12, 25, 54]. Although analogue treatment of all cell types reduced ODC activity, most likely due to an increase in ODC antizyme production as previously demonstrated by Mitchell and colleagues [36, 37], it is clear that the combination of decreased polyamine synthesis and increased polyamine catabolism leads to the greatest depletion of intracellular polyamines. This phenotypic difference in total polyamine depletion may have more significance in vivo than in vitro, since in the in vivo setting the availability of circulating polyamines may be able to overcome the simple down regulation of ODC exhibited by the small cell lung cancer phenotype. The availability of circulating polyamines apparently caused decreased efficacy in the clinical trial of 2-difluoromethylornithine (DFMO) in small cell lung cancers [1, 2]. By contrast, in the non-small cell phenotype the increased polyamine catabolism may result in a greater growth inhibitory response in vivo by responding to analogue treatment with both a decrease in biosynthesis and an increase in catabolism. This may be particularly true for tumor types represented by the A549 cell line, in which nearly completed down regulation of ODC activity combined with highly induced catabolic enzymes, SSAT and SMO [25]. However, further in vivo testing in both phenotypes, with an analysis of the in vivo effects of CGC-11047 on polyamine metabolism, will be required to determine if differential sensitivity based on phenotype-specific changes in polyamine catabolism exists in vivo.
Although preclinical studies with the first generation, symmetrically substituted polyamine analogue BENSpm were encouraging [6, 7, 17, 27], clinical trial results were less so [24, 30, 48, 56]. As previously mentioned, the less than desirable clinical results may have been a result of the inherent properties of BENSpm, and/or the daily dosing schedule used in those trials that showed GI and CNS dose-limiting toxicities. We have previously demonstrated that daily or continuous administration of polyamine analogues is not necessary to achieve significant tumor growth inhibition in vivo [31]. Therefore, to reduce the potential for toxicity, we utilized a single weekly dose of 100 mg/kg CGC-11047. This treatment schedule demonstrated efficacy comparable to that reported for BENSpm given three times a day at 80 mg/kg for six consecutive days [7]. The single weekly dose of CGC-11047 significantly delayed tumor progression without observable toxicity. As the single dose schedule was highly effective in delaying tumor progression and even caused the complete regression of tumor in one of ten mice, and since there was no observable toxicity, alternative dosing schedules might be considered in future studies.
In summary, CGC-11047 represents a new conformationally restricted, short chain, symmetrically substituted polyamine analogue that demonstrates significant growth inhibitory activity in multiple human cells lines representative of the major forms of human lung cancer. Interestingly, although there is clearly a phenotype-specific response with respect to the induction of polyamine catabolism exhibited between the non-small cell lung cancer phenotype (high induction) and the small cell (not induced), this differential response did not translate into significant difference in growth response in vitro. However, as the non-small cell lines demonstrated greater polyamine depletion and greater accumulation of analogue, likely a result of increased catabolism, they may be expected to be more sensitive to analogue effects in the in vivo setting. Importantly, CGC-11047 demonstrated highly significant growth inhibition in a nude mouse model of human non-small cell lung demonstrating that further pre-clinical and, potentially, clinical trials in lung cancer are warranted.
Acknowledgments
These studies were supported by NIH grants CA051085 and CA098454, and by the Patrick C. Walsh Prostate Cancer Research Fund, for which RAC is the Schwatrz Scholar.
Abbreviations
- ODC
Ornithine decarboxylase
- SMO
Spermine oxides
- SSAT
Spermidine/spermine N1-acetyltransferase
Contributor Information
Amy Hacker, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans Street, Room 551-CRB1, Baltimore, MD 21231, USA.
Laurence J. Marton, Progen Pharmaceuticals, Redwood City, CA 94065, USA
Michelle Sobolewski, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans Street, Room 551-CRB1, Baltimore, MD 21231, USA.
Robert A. Casero, Jr., Email: rcasero@jhmi.edu, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans Street, Room 551-CRB1, Baltimore, MD 21231, USA
References
- 1.Abeloff MD, Slavik M, Luk GD, Griffin CA, Hermann J, Blanc O, Sjoerdsma A, Baylin SB. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. J Clin Oncol. 1984;2:124–130. doi: 10.1200/JCO.1984.2.2.124. [DOI] [PubMed] [Google Scholar]
- 2.Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase ii trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep. 1986;70:843–845. [PubMed] [Google Scholar]
- 3.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JR, Kramer D, et al. Antiproliferative properties of polyamine analogues: A structure-activity study. J Med Chem. 1994;37:3464–3476. doi: 10.1021/jm00047a004. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron RJ, Feng Y, Weimar WR, McManis JS, Dimova H, Porter C, Raisler B, Phanstiel O. A comparison of structure-activity relationships between spermidine and spermine analogue antineoplastics. J Med Chem. 1997;40:1475–1494. doi: 10.1021/jm960849j. [DOI] [PubMed] [Google Scholar]
- 6.Bernacki RJ, Bergeron RJ, Porter CW. Antitumor activity of N,N′-bis(ethyl)spermine homologues against human malme-3 melanoma xenografts. Cancer Res. 1992;52:2424–2430. [PubMed] [Google Scholar]
- 7.Bernacki RJ, Oberman EJ, Seweryniak KE, Atwood A, Bergeron RJ, Porter CW. Preclinical antitumor efficacy of the polyamine analogue N1, N11- diethylnorspermine administered by multiple injection or continuous infusion. Clin Cancer Res. 1995;1:847–857. [PubMed] [Google Scholar]
- 8.Boncher T, Bi X, Varghese S, Casero RA, Jr, Woster PM. Polyamine-based analogues as biochemical probes and potential therapeutics. Biochem Soc Trans. 2007;35:356–363. doi: 10.1042/BST0350356. [DOI] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Casero RA, Frydman B, Murray Stewart T, Woster PA. Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues. West Pharm Soc. 2005;289:C826–835. [PubMed] [Google Scholar]
- 11.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 12.Casero RA, Jr, Ervin SJ, Celano P, Baylin SB, Bergeron RJ. Differential response to treatment with the bis(ethyl)polyamine analogues between human small cell lung carcinoma and undifferentiated large cell lung carcinoma in culture. Cancer Res. 1989;49:639–643. [PubMed] [Google Scholar]
- 13.Casero RA, Jr, Celano P, Ervin SJ, Wiest L, Pegg AE. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem J. 1990;270:615–620. doi: 10.1042/bj2700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casero RA, Jr, Celano P, Ervin SJ, Applegren NB, Wiest L, Pegg AE. Isolation and characterization of a cdna clone that codes for human spermidine/spermine N1-acetyltransferase. J Biol Chem. 1991;266:810–814. [PubMed] [Google Scholar]
- 15.Casero RA, Jr, Mank AR, Xiao L, Smith J, Bergeron RJ, Celano P. Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res. 1992;52:5359–5363. [PubMed] [Google Scholar]
- 16.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. Faseb J. 1993;7:653–661. [PubMed] [Google Scholar]
- 17.Casero RA, Jr, Gabrielson EW, Pegg AE. Immunohistochemical staining of human spermidine/spermine N1-acetyltransferase superinduced in response to treatment with antitumor polyamine analogues. Cancer Res. 1994;54:3955–3958. [PubMed] [Google Scholar]
- 18.Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 19.Casero RA, Jr, Wang Y, Stewart TM, Devereux W, Hacker A, Smith R, Woster PM. The role of polyamine catabolism in anti-tumour drug response. Biochem Soc Trans. 2003;31:361–365. doi: 10.1042/bst0310361. [DOI] [PubMed] [Google Scholar]
- 20.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 21.Chang BK, Bergeron RJ, Porter CW, Liang Y. Antitumor effects of n-alkylated polyamine analogues in human pancreatic adenocarcinoma models. Cancer Chemother Pharmacol. 1992;30:179–182. doi: 10.1007/BF00686308. [DOI] [PubMed] [Google Scholar]
- 22.Chang BK, Bergeron RJ, Porter CW, Vinson JR, Liang Y, Libby PR. Regulatory and antiproliferative effects of N-alkylated polyamine analogues in human and hamster pancreatic adenocarcinoma cell lines. Cancer Chemother Pharmacol. 1992;30:183–188. doi: 10.1007/BF00686309. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- 24.Creaven PJ, Perez R, Pendyala L, Meropol NJ, Loewen G, Levine E, Berghorn E, Raghavan D. Unusual central nervous system toxicity in a phase I study of N1,N11 diethylnorspermine in patients with advanced malignancy. Invest New Drugs. 1997;15:227–234. doi: 10.1023/a:1005827231849. [DOI] [PubMed] [Google Scholar]
- 25.Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, et al. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells Cancer. Chemother Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 26.Frydman B, Porter CW, Maxuitenko Y, Sarkar A, Bhattacharya S, Valasinas A, Reddy VK, Kisiel N, Marton LJ, Basu HS. A novel polyamine analog (SL-11093) inhibits growth of human prostate tumor xenografts in nude mice. Cancer Chemother Pharmacol. 2003;51:488–492. doi: 10.1007/s00280-003-0598-8. [DOI] [PubMed] [Google Scholar]
- 27.Gabrielson E, Tully E, Hacker A, Pegg AE, Davidson NE, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase in breast cancer tissues treated with the polyamine analogue N1, N11-diethylnorspermine. Cancer Chemother Pharmacol. 2004;54:122–126. doi: 10.1007/s00280-004-0786-1. [DOI] [PubMed] [Google Scholar]
- 28.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 29.Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci USA. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, Casero RA., Jr Phase I study of N1,N11-diethylnorspermine in patients with non-small cell lung cancer. Clin Cancer Res. 2002;8:684–690. [PubMed] [Google Scholar]
- 31.Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ, et al. A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res. 2003;9:2769–2777. [PMC free article] [PubMed] [Google Scholar]
- 32.Kabra PM, Lee HK, Lubich WP, Marton LJ. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 33.Levin VA, Hess KR, Choucair A, Flynn PJ, Jaeckle KA, Kyritsis AP, Yung WK, Prados MD, Bruner JM, Ictech S, et al. Phase iii randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-pcv versus pcv for ana-plastic gliomas. Clin Cancer Res. 2003;9:981–990. [PubMed] [Google Scholar]
- 34.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 35.McCloskey DE, Casero RA, Jr, Woster PM, Davidson NE. Induction of programmed cell death in human breast cancer cells by an unsymmetrically alkylated polyamine analogue. Cancer Res. 1995;55:3233–3236. [PubMed] [Google Scholar]
- 36.Mitchell JL, Simkus CL, Thane TK, Tokarz P, Bonar MM, Frydman B, Valasinas AL, Reddy VK, Marton LJ. Antizyme induction mediates feedback limitation of the incorporation of specific polyamine analogues in tissue culture. Biochem J. 2004;384:271–279. doi: 10.1042/BJ20040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JL, Thane TK, Sequeira JM, Marton LJ, Thokala R. Antizyme and antizyme inhibitor activities influence cellular responses to polyamine analogs. Amino Acids. 2007;33:291–297. doi: 10.1007/s00726-007-0523-2. [DOI] [PubMed] [Google Scholar]
- 38.Pless M, Belhadj K, Menssen HD, Kern W, Coiffier B, Wolf J, Herrmann R, Thiel E, Bootle D, Sklenar I, et al. Clinical efficacy, tolerability, and safety of SAM486a, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: results from a phase II multicenter study. Clin Cancer Res. 2004;10:1299–1305. doi: 10.1158/1078-0432.ccr-0977-03. [DOI] [PubMed] [Google Scholar]
- 39.Porter CW, Sufrin JR. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986;6:525–542. [PubMed] [Google Scholar]
- 40.Porter CW, Bergeron RJ. Enzyme regulation as an approach to interference with polyamine biosynthesis—an alternative to enzyme inhibition. Adv Enzyme Regul. 1988;27:57–79. doi: 10.1016/0065-2571(88)90009-x. [DOI] [PubMed] [Google Scholar]
- 41.Porter CW, Ganis B, Libby PR, Bergeron RJ. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- 42.Porter CW, Bernacki RJ, Miller J, Bergeron RJ. Antitumor activity of N1,N11-bis(ethyl)norspermine against human melanoma xenografts and possible biochemical correlates of drug action. Cancer Res. 1993;53:581–586. [PubMed] [Google Scholar]
- 43.Reddy VK, Valasinas A, Sarkar A, Basu HS, Marton LJ, Frydman B. Conformationally restricted analogues of N1,N11-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem. 1998;41:4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 44.Reddy VK, Sarkar A, Valasinas A, Marton LJ, Basu HS, Frydman B. Cis-unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cell lines. J Med Chem. 2001;44:404–417. doi: 10.1021/jm000310s. [DOI] [PubMed] [Google Scholar]
- 45.Seely JE, Pegg AE. Ornithine decarboxylase (mouse kidney) Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- 46.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 2. Structural analogues and derivatives. Curr Drug Targets. 2003;4:565–585. doi: 10.2174/1389450033490876. [DOI] [PubMed] [Google Scholar]
- 47.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr Drug Targets. 2003;4:537–564. doi: 10.2174/1389450033490885. [DOI] [PubMed] [Google Scholar]
- 48.Streiff RR, Bender JF. Phase I study of N1-N11-diethylnorspermine (denspm) administered tid for 6 days in patients with advanced malignancies. Invest New Drugs. 2001;19:29–39. doi: 10.1023/a:1006448516938. [DOI] [PubMed] [Google Scholar]
- 49.Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B. Conformationally restricted analogues of N1,N14-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J Med Chem. 2001;44:390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 50.Wallace HM, Fraser AV. Polyamine analogues as anticancer drugs. Biochem Soc Trans. 2003;31:393–396. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 51.Wallace HM, Fraser AV. Inhibitors of polyamine metabolism: review article. Amino Acids. 2004;26:353–365. doi: 10.1007/s00726-004-0092-6. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 53.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Casero RA. Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem. 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 55.Waters WR, Frydman B, Marton LJ, Valasinas A, Reddy VK, Harp JA, Wannemuehler MJ, Yarlett N. [N1,N12]bis(ethyl)-cis-6,7-dehydrospermine: A new drug for treatment and prevention of cryptosporidium parvum infection of mice deficient in t-cell receptor alpha. Antimicrob Agents Chemother. 2000;44:2891–2894. doi: 10.1128/aac.44.10.2891-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, Casero RA, Jr, Davidson NE. A phase ii study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2003;9:5922–5928. [PubMed] [Google Scholar]