Abstract
Despite intensive clinical and laboratory research and effort, Glioblastoma remains the most common and invariably lethal primary cancer of the central nervous system. The identification of stem cell and lineage-restricted progenitor cell populations within the adult human brain in conjunction with the discovery of stem-like cells derived from gliomas which are themselves tumorigenic and have been shown to have properties of self-renewal and multipotency, has led to the hypothesis that this population of cells may represent glioma initiating cells. Extensive research characterizing the anatomic distribution and phenotype of neural stem cells in the adult brain, and the genetic underpinnings needed for malignant transformation may ultimately lead to the identification of the cellular origin for glioblastoma. Defining the cellular origin of this lethal disease may ultimately provide new therapeutic targets and modalities finally altering an otherwise bleak outcome for patients with glioblastoma.
Keywords: Glioma, Neuronal Stem Cell, Glioma Initiating Cell, Review
2. INTRODUCTION
Glioblastoma is the most common and lethal primary malignancy of the central nervous system (CNS) representing approximately 50% of all gliomas (1). With a median survival of 14–16 months, and 2-year survival rate of less than 10–15% using today’s best treatment modalities, improvements in survival over the past 100 years can be measured in weeks (2). These tumors represent a relentless disease state with a clear propensity towards migration and invasion within and into the neural parenchyma along with recurrence at both local and distant sites (3).
Traditional views have held that these tumors originate from the malignant transformation of differentiated glial components of the CNS (i.e. astrocytes and oligodendrocytes) (4). The discovery of multipotent stem cell and lineage-restricted progenitor cell populations in the CNS of the postnatal mammalian brain, and subsequent identification of stem-like cells in several primary brain tumors including glioblastoma has led to the development of the Cancer Stem Cell (CSC) theory of gliomas challenging this hypothesis (4–7). This theory posits that rather than the de-differentiation of mature cell populations being the mechanism behind gliomagenesis, gliomas are instead derived from the malignant transformation of cells derived from this newly defined neural stem cell or lineage-restricted progenitor population. Moreover, accumulating evidence suggests that these glioblastoma-derived stem-like cells are responsible for the invasiveness and resistance to treatment that characterizes glioblastoma (8–15).
Based on the cancer stem cell theory, extensive research defining the phenotypic as well as genetic similarities between adult neural stem cells (NSCs) and glioma initiating cells (GICs) is on going in the hopes of decisively identifying the cellular origin of this tumor, potentially opening the door to new therapeutic targets and modalities. In this article, we discuss evidence supporting the neural stem cells and lineage restricted progenitor cells as the origin of glioblastomas, and specifically as a source of glioma initiating cells (GICs).
3. HYPOTHESES REGARDING THE CELLULAR ONTOLOGY OF GLIOBLASTOMA: CANCER STEM CELL (CSC) MODEL
Traditionally it was thought that mature cells of astroglial or oligodendroglial lineage, through a number of genetic changes, underwent neoplastic transformation to yield gliomas (16). According to this theory, mutations permitting mature, differentiated cells to re-enter a relatively undifferentiated state through the removal of cell-cycle check points, blockade of apoptotic pathways, and the re-activation of developmental pathways were required for the formation of gliomas (16). This theory was in large part based on the widely held belief that the brain was a quiescent organ, incapable of post-natal neurogenesis and the idea that astrocytes and oligodendrocytes were the only cell populations capable of division in the mature brain (17).
Beginning with the findings of Altman and Das demonstrating neurogenesis in the adult rat brain (18, 19) and the subsequent findings in the canary by Goldman and Nottebohm (20), this dogma has been increasingly challenged. In the years following these discoveries, numerous studies have demonstrated that adult mammalian brain is capable of continued neurogenesis; a finding that has been confirmed in the adult human brain (21, 22). This process of neurogenesis in the adult brain has been primarily confined to the forebrain subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus (21–32). Within these locations in both rodents and humans, a population of astrocytes has been described which have the properties of self-renewal and multipotentiality consistent with a neural stem cell identity (22, 27, 33, 34). Subsequent to the discovery of stem cell populations residing within the SVZ and SGZ of the central nervous system, several groups reported the presence of stem cell-like populations isolated from glioblastoma tissue (6, 7, 35). In the appropriate culture conditions, it was found that a subset of tumor cells from surgical explants could generate neurospheres which could give rise to neurons, astrocytes and oligodendrocytes, a previously described property of NSCs (6, 35). Additionally, glioblastoma-derived neurosphere cells could recapitulate histologically and cytologically, a tumor resembling the initial glioblastoma in an immunocompromised murine xenograft model (6, 7).
Growing evidence from a number of cancers has supported the theory that most if not all tumors in humans are comprised of a heterogeneous population of cells which have varying tumorigenic potential (36–38). Initially demonstrated in leukemia and subsequently in several solid tumors including those from breast, head and neck, colon, pancreatic, and prostatic cancer, it was shown that only a subset of cells isolated from patients possessed the ability to proliferate in vitro, differentiate into mature forms under the proper conditions, and form tumors in xenograft models having characteristics resembling the parent malignancy (39–44). These findings led to the hypothesis that within a tumor, a stem cell-like population is primarily responsible for its tumorgenicity; hence they are referred to as Cancer Stem Cells. The discovery of cell populations derived from glioblastoma which demonstrated these properties has led to the hypothesis being extended to include glioblastoma. Taken together the hypothesis has been made that GICs are derived from NSCs and by extension, arise from the SVZ (4, 7, 21, 45–50).
4. SOURCES OF NSCs AND LINEAGE-RESTRICTED PROGENITOR CELLS IN THE POST-NATAL MAMMALIAN BRAIN
As mentioned earlier, NSCs and lineage-restricted progenitor cells, which are defined as cell subpopulations capable of self-renewal and differentiation into multiple lineages (51), have been described in several regions of the adult mammalian brain including the SVZ, SGZ of the hippocampal dentate gyrus, striatum, frontal and temporal cortex, as well as the subcortical white matter (24, 51–55). Of these regions, the SVZ has been found to be the largest reservoirs of these cells in both the adult rodent and human brain.
4.1. Organization of the Rodent Subventricular Zone
In the adult rodent brain, the SVZ lines the lateral wall of the lateral ventricles bilaterally underlying the ependymal layer (Figure 1). Within this region, resides a population of slowly dividing astrocyte-like NSCs termed type B1 cells (27, 56). These cells, believed to be derived from a neuroepithelial lineage that has also been shown to be the source of the ependymal lining of the ventricular wall, is thought to be responsible for neurogenesis throughout the adult life (57).
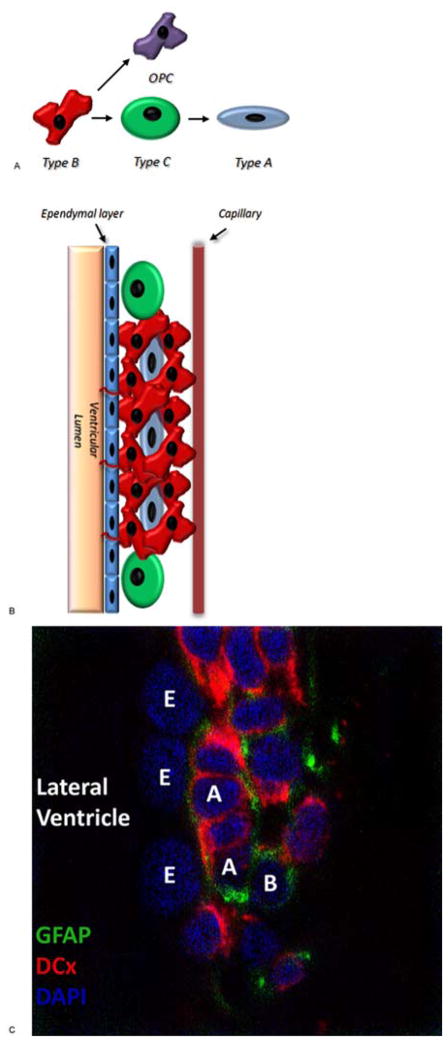
Figure 1.
Structure of the Rodent SVZ. (A) Drawing depicting the progression of neural stem cell (type B) to oligodendroglial precursors (OPC) or transit amplifying cells (type C) to immature neuroblasts (type A). (B) Drawing depicting the cytoarchitecture and cellular composition of the rodent SVZ. (C) Immunohistochemistry (40x) of the rodent SVZ. GFAP – glial fibrillary acidic protein; DCx – doublecortin; DAPI – 4′,6-diamidino-2-phenylindole; E—ependymal cells; A—type A cells; B—type B cells.
Through asymmetrical divisions, type B1 cells within the SVZ give rise to type C cells, a rapidly proliferating cell population, alternatively referred to as transit-amplifying cells. Immature neuroblasts or type A cells, derived from this pool of transit-amplifying cells subsequently migrate from the SVZ in chains surrounded by the glial processes of type B2 cells towards the olfactory bulb comprising the rostral migratory stream (RMS). These migrating neuroblasts ultimately contribute to various populations of interneurons of the granule layers of the olfactory bulb (26, 58–60).
Interestingly, the SVZ has also been shown to be a source of oligodendrocytes both during development and in response to demyelinating disease (61, 62). Menn et al. found that the source of these SVZ-derived oligodendrocytes was a subpopulation of Olig2 expressing type B cells which migrate into the subcortical white matter in a manner orthogonal to the RMS where they became local oligodendrocyte progenitor cells (OPCs) (63). Though the majority of research is focused on cells within the SVZ, this population of subcortical progenitor cells cannot be excluded as a possible source of GICs. Consistent with this hypothesis, using a murine model of oligodendroglioma, Persson et al. recently found that OPCs, and not NSCs, enriched for a tumor-forming cell population (64).
4.2. Organization of the Human Subventricular Zone
In contrast to the structure of the rodent SVZ where astrocyte-like stem cells directly oppose the ventricular ependyma, a feature also described in the SVZ of canines and non-human primates, the human SVZ possesses a more complex organization comprised of four distinct layers including a hypocellular gap separating the presumed stem cell population from the ependyma (Figure 2; (12, 21, 22, 65–67)). From innermost to outermost layers these are: layer I is a monolayer of ependymal cells lining the ventricular cavity; layer II is an immediately adjacent is the hypocellular gap; layer III is a ribbon of astrocytes in which a population of neural stem cells is felt to reside; and layer IV is referred to as the transitional zone comprised primarily of myelinated fibers.
Figure 2.
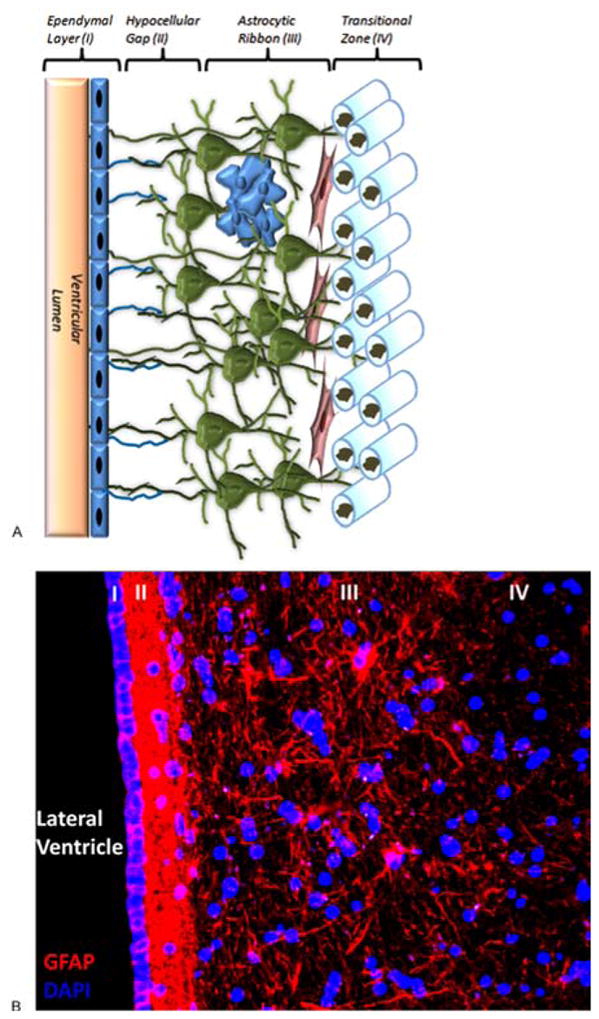
Structure of the Human SVZ. (A) Drawing depicting the cellular composition and cytoarchitecture of the human SVZ. Green cells represent layer III astrocytes, blue cells are a trapped ependymal cell rest, and elongated red cells are migratory neuron-like cells characterized at the layer III-IV interface (see text for more information). (B) Immunohistochemistry (20x) depicting the four layers that make up the human SVZ (Layer I – Ependymal layer, Layer II – Hypocellular gap, Layer III – Astrocytic ribbon, Layer IV – Transitional zone). GFAP – glial fibrillary acidic protein; DAPI – 4′,6-diamidino-2-phenylindole.
Unique to the adult human SVZ, the hypocellular gap is rich in glial fibrillary acidic protein (GFAP) expressing processes with ependymal expansions and an abundant network of astrocyte-astrocyte and astrocyte-ependymal interconnections. The function of these interconnections is unclear though it has been hypothesized that they may regulate neuronal function, play a role in metabolic homeostasis, or control NSC proliferation and differentiation (21, 68–70). Layer III, comprised principally of large astrocyte-like cells is the layer in which mitotic bodies consistent with dividing stem cells have been identified, although not to the degree seen in the SVZ of other mammals such as the rodent and non-human primates (21, 22). While neuron-like cells have been identified between layers III and IV which appear to be migratory based on their morphology, the presence of definitive migrating chains of neuroblasts has not been shown (21, 22, 71). Curtis et al. however have argued that an RMS-like structure in fact does exist in the adult human brain with chains of neuroblasts arranged around a ventricular remnant extending from the anterior horn of the lateral ventricle to the olfactory bulb. Subsequent studies by Bradford et al. recently provided further support of this finding demonstrating expression of Neogenin, a Netrin/RGMa receptor thought to be a marker of neurogenesis descriptive of the rodent RMS, in basal forebrain of humans along what is purported to represent the human equivalent of the RMS (71). Research in our laboratory has confirmed the presence of a structure analogous to the RMS of rodents in human tissues of fetal origin though their applicability to the adult human cortex remains controversial (71–75).
Given the fundamental differences in SVZ anatomy noted between the rodent and human brain, it is not unreasonable to expect that different developmental pathways and structures are at work explaining the failure to definitively demonstrate populations of stem cells and progenitor cells having the same or similar properties in both. In rodents, robust neurogenesis can be seen in the SVZ extending out through the RMS which experiences a decline as the animal ages (76). In contrast with this finding, neurogenesis in the human SVZ, both in the fetal and adult brain is of relatively smaller magnitude though clearly still present (21). This noted disparity should however be interpreted with caution; studies characterizing the anatomy of the rodent SVZ and its associated patterns of neurogenesis have been performed in both embryonic and relatively young mice in comparison to investigations in humans where though fetal tissues have been examined, adult neurogenesis has largely been evaluated in the aged brain which is unlikely to be equivalent to that of the young rodent.
Further illustrating the disparities between rodent and human SVZ architecture, Hansen et al. recently described a population of radial glia-like cells populating the outer layers of the SVZ (OSVZ) of the human neocortex, not seen in the rodent brain, which are capable of undergoing both proliferative divisions and self-renewing asymmetric divisions giving rise to neuronal progenitor cells which are themselves capable of further proliferation indicating yet another potential source of stem cells associated with the SVZ (77). These radial glia cells are distinctly different from the traditionally defined population in that while they project apical processes toward the pial surface, the majority fail to show basal processes extending to the ventricular wall (77). Though of unclear significance, this population of radial glia-like cells is thought to represent in part, a mechanism allowing for the neocortical expansion defining of humans and higher primates (77). Furthermore, it is not unreasonable to consider the possibility that these cells may represent yet another potential source of GICs.
Despite the controversies surrounding the functional anatomy of the human SVZ and the striking differences seen between it and other mammalian SVZs, it is clear that neural stem cell-like populations can be isolated from SVZ explants though the exact in situ localization of these populations remains elusive for now (22, 77).
5. PUTATIVE MALIGNANT TRANSFORMATION OF NSCs AND LINEAGE-RESTRICTED PROGENITOR CELLS
Unlike mature, differentiated components of the CNS such as astrocytes and neurons, stem cells and lineage-restricted progenitor cells remain highly proliferative resulting in increased susceptibility to malignant transformation of neural stem cells. Exposure to N-ethyl-N-nitrosourea (ENU) or avian sarcoma virus has been shown to result in tumor formation preferentially within the SVZ of the canine and rodent brain rather than in areas with less proliferative capacity (78–81). Feldkamp et al. found in a murine model that exposure of progenitor cells to high levels of epithelial derived growth factor (EGF) or platelet derived growth factor (PDGF) resulted in the formation of tumors histologically resembling human gliomas (82). Furthermore, ventricular infusion of EGF or PDGF results in increased proliferation of SVZ progenitor cells and the development of highly invasive glioma-like masses (46, 83). Agreeing with these findings, constitutively activating mutations and/or increased expression of EGF and PDGF receptors as well as autocrine PDGF/PDGFR signaling are known to be present in malignant gliomas (84–87). Both PDGF and EGF signaling can feed through the Ras/Akt pathways, widely known to be significant in oncogenesis, and retroviral delivery of activated Ras and Akt to mouse progenitor cells induces high-grade glioma formation (88). As with other malignancies, disruption of tumor suppressor function has also been found to result in glioma formation; p53−/−/NF1−/− dual knockout mice develop spontaneous lesions associated with the SVZ closely mimicking human glioblastoma in appearance and behavior (Figure 3; (89)).
Figure 3.
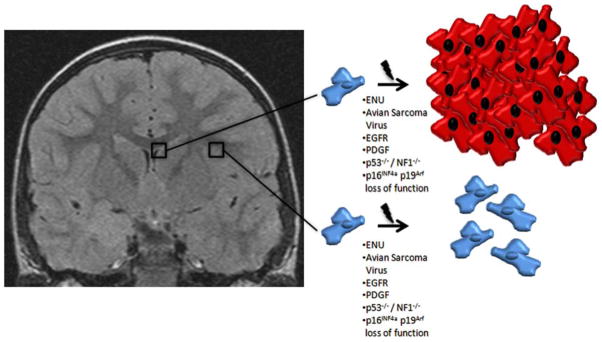
Differential oncologic effects upon cells of the human brain with varying proliferative potential. Left: T1-weighted coronal MRI of the human brain. Top right: Drawing depicting the putative effect of chemical and mutational insults experimentally proposed to be involved in glioblastoma formation upon SVZ derived cells with proliferative capacity. Bottom right: Drawing depicting the absent or reduced effect upon subcortical/cortical cells known to have decreased proliferative capacity compared with the SVZ of chemical and insults experimentally proposed to be involved in glioblastoma formation. ENU - N-ethyl-N-nitrosourea; EGF - epithelial derived growth factor; PDGF - platelet derived growth factor, NF – neurofibromatosis.
Interestingly but not necessarily surprising is the decline noted in the size of the stem cell population as the brain ages (90). Central to this decline is the upregulation of p16INK4a and p19Arf expression which are involved in cellular senescence. Deletion of p16INK4a partially rescues the age-related decline in the stem cell population of the mouse SVZ (90). p16INK4a/p19Arf loss of function mutations are one of the most prevalent genetic alterations in glioblastoma (91); these changes however are not sufficient to initiate gliomagenesis on their own, but can in conjunction with EGFR activation, stimulate glioma formation in both murine NSCs and differentiated astrocytes (92).
The Notch signaling pathway represents another developmentally important transcriptional pathway implicated in glioma propagation. Under normal circumstances, Notch is involved in the maintenance, but not the generation of the neural stem cell population. Mice with homozygous deletion of the Notch1 gene have rapidly depleted neural stem cell populations during embryonic development (93, 94). In vitro, blockade of Notch by gamma secretase inhibitors (GSIs) reduced neurosphere formation through reduced proliferation and increased apoptosis where in contrast, expression of active Notch1 increased tumorgenicity (95). In vivo, GICs pretreated with GSIs failed to form tumors upon subcutaneous injection into nude mice. Similarly, delivery of GSIs via drug impregnanted polymer beads blocked tumor growth and promoted survival (95).
6. SIMILARTIES BETWEEN NSCs AND GLIOMA INITIATING CELLS
NSCs and lineage-restricted progenitor cells of the SVZ have unique expression patterns of tumor suppressor genes, cell surface markers, cytoskeletal proteins, transcription factors and growth factors/growth factor receptors which are largely shared by glioma stem cell-like populations. Additionally, glioblastomas have been shown to orchestrate a vascular niche mimicking the normal neural stem cell niche thereby helping to maintain the glioma initiating cell pool (45).
6.1. Transcription factors
One of the arguments for NSCs being a source of GICs is the expression of early developmental genes which persist in GICs contributing to proliferation and self-renewal. The background upon which these genes act in conjunction with possible changes in the regulation of a given pathway likely makes the distinction between the normal, and the tumorigenic state seen in glioblastoma.
For example, Sonic Hedgehog (Shh) is a central regulator of patterning and proliferation in the cerebellum during development (96–98). Dysregulation of the Shh pathway in neural progenitors in the external granular layer of the cerebellum by mutations of Patched, the Shh receptor, constitutive activation of Smoothened, or over expression of N-myc lead to the development of medulloblastoma (99–102). Similar to medulloblastoma, Shh signaling is also implicated in the pathogenesis of glioblastoma. Shh modulates the activity of three zinc-finger transcription factors, Gli1, Gli2, and Gli3, which are involved in proliferation and self-renewal by promoting cell-cycle entry and DNA replication (103, 104). Gli1 is normally expressed by progenitor cells in the SVZ where they are help to maintain the stem cell population (105–107). Gli1 expression has also been found in both low- and high-grade gliomas where the Shh-Gli1 axis is thought to be involved in maintenance of GICs and tumorigenesis (96). Supporting this theory, administration of cyclopamine, a Shh inhibitor inhibits the in vitro growth of some glioma stem cell-like lines (108).
6.2. Tumor suppressor genes
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a tumor suppressor gene which lowers phosphatidylinositol phosphate (PIP3) levels enhancing rates of apoptosis and also limits cell motility through a G protein-coupled mechanism (109, 110). PTEN expression in the SVZ is involved in control of stem cell and precursor cell proliferation, precursor cell migration, as well as being required for neuronal differentiation from precursor cells of the SVZ (111–114). Deletion of PTEN leads to persistent neural stem cell self-renewal and expansion of the SVZ while decreasing apoptosis (115, 116). Similarly, deletion of PTEN in gliomas increases the size of the side population and hence ABCG2 transporter expression and activity, which are thought to be mainly associated with stem cell populations (9). In primary or de novo glioblastoma, PTEN mutations are frequently described; interestingly epigenetic silencing of PTEN through promoter methylation is described in low-grade tumors and secondary glioblastoma (117).
p53, another tumor suppressor gene, with a key role in regulating DNA repair pathways, is also frequently deleted or mutated in glioblastoma (4, 118). By itself, p53 mutation is insufficient to induce glioma formation requiring additional carcinogenic or genetic insults such as exposure to ENU, constitutive activation of the Ras pathway, or increased PDGF signaling (89, 119, 120). Co-deletion of the neurofibromatosis (NF)-1 tumor suppressor has been shown to cooperate with p53 deletions, inducing glioma formation in mouse models (89). Additionally, NF-1 patients are characterized by an increased frequency of glioblastoma occurrence (121, 122).
6.3. Growth factors / cytokines and their receptors
As was described above, EGF and PDGF have a pronounced effect upon NSCs, capable under the right circumstances of causing hyperplasia and proliferation resulting in the development of periventricular neoplasms akin to early gliomas (46, 83–87). In human gliomas, EGFR amplification or expression of EGFRvIII, a constitutively active mutation, is seen in nearly half of all high-grade tumors (123–125). In the rodent SVZ, EGF prevents C cell differentiation and encourages infiltrative behavior similar to that seen in high-grade gliomas (83). Similarly for PDGF, a role has been defined in the normal stem cells niche of the adult SVZ; a population of type B cells expressing PDGF can give rise in vivo to both oligodendrocytes and neurons (46). Experimental over-expression of PDGF induces areas of proliferation and hyperplasia within the SVZ similar to those seen in early gliomagenesis (46).
Transforming growth factor (TGF)-beta has also been shown to have a place in normal SVZ function, having a role in progenitor cell differentiation (126, 127). In glioblastoma, TGF-beta expression has been demonstrated by several groups including our own (unpublished observation). In glioblastoma, TGF-beta expression has been described in both whole tumor samples and specifically in GIC populations in vitro and in in vivo experimental models. Under these circumstances, TGF-beta likely contributes to the development of the immunosuppressive phenotype described in glioblastoma involving both the Treg subset of T cells and macrophage/microglial populations (128–132).
6.4. Vascular niche
Stem cells of all tissues and organisms reside in unique microenvironments or niches (133, 134). Within the SVZ of the mammalian brain, clusters of capillaries, associated growth factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and extracellular matrix components such as tenascin-C and chondroitin sulfate, create an environment which fosters stem cell growth and regulates proliferation and cell fate through cross talk and maintenance of local environmental factors such as pH and oxygen tension (135–142). Similarly, gliomas are typically highly vascularized tumors often with extensive capillary beds that can provide a similar vascular niche for GICs to reside in. Calabrese et al found that CD133+/Nestin+ glioma-derived stem cells were closely associated with capillary associated endothelial cells; experimentally the number of blood vessels or endothelial cells in an orthotopic brain tumor model increased the fraction of self-renewing cells within the tumor and accelerated tumor growth (45). Expectedly, depleting blood vessels in the same model arrested tumor growth and depleted the self-renewing cell populations in these tumors. Recently, using a mouse model of PDGF-induced glioma, Charles et al. found that nitric oxide (NO) production via endothelial Nitric Oxide Synthase (eNOS) expressed by the endothelium within the perivascular niche activated Notch activity in GICs (143) . Activated Notch increased their capacity to form neurospheres in vitro, and enhanced their tumorigencity in vivo, tying together the importance of dysregulated developmental pathways and, the association of GICs and the perivascular niche in gliomagenesis (143).
6.5. Cytoskeletal proteins
Nestin, a type IV intermediate filament protein, is expressed during development by neural progenitors throughout the brain but becomes restricted to the SVZ in the adult brain (144). Nestin+ cells are particularly sensitive to ENU exposure persisting throughout tumor progression (145, 146). Nestin+ cells have also been described in human gliomas, which while not an adequate marker of GICs suggests a link between stem cell populations and glioma formation (147). The microtubule-associated protein (MAP), doublecortin, is preferentially expressed in high-grade human gliomas (148). In a non-tumorigenic context, doublecortin is encoded by a locus on the X chromosome associated with lissencephaly, contains two actin-binding domains and is involved in neuronal migration (149). In the adult brain, it is primarily expressed in migrating neuroblasts such as the type A cells of the rodent RMS (149, 150). Additionally, over expression of doublecortin in vitro is protective against hypoxia and glucose deprivation in glioma lines (151).
7. CLINICAL EVIDENCE FOR THE SVZ AS A SOURCE OF GLIOMA INITIATING CELLS
Although populations of cells from both glioblastoma and the SVZ can be shown to possess stem cell-like properties in vitro, this in and of itself does not provide for the suspected connection between stem or progenitor cells of the SVZ and glioblastoma. Several clinical observations from glioma patients do however provide indirect support for this hypothesis. It has been noted by several groups that a large proportion of gliomas are found to associate with and involve the SVZ at the time of diagnosis; this relationship appears to have a negative impact on outcome (47, 152). In 2007, Lim et al. defined an MRI-based classification scheme for glioblastoma correlating in a limited series with predicted tumor recurrence pattern (47). In their schema, glioblastomas were stratified into four groups based on the association of the identified contrast enhancing lesion (CEL) with the SVZ and the cortex. CELs involving both the SVZ and cortex were most likely to present with multifocal disease and have noncontiguous recurrences. Where in contrast, those tumors which spared both the cortex and SVZ were never multifocal and recurrences were always adjacent to the initial site of diagnosis. Consistent with this classification scheme, Chaichana et al. in an observational study, found that median survival for patients with lateral ventricle associated glioblastoma was significantly decreased compared with glioblastoma patients in whom the tumor was not associated with the lateral ventricle (8 months vs 11 months; (152)). Further supporting a central role for the SVZ in glioblastoma, Evers et al. retrospectively evaluated a cohort of 55 patients previously diagnosed with WHO Grade III (anaplastic astrocytoma) or Grade IV gliomas (glioblastoma), examining the correlation between the amount of radiation delivered to the periventricular tissues and progression-free survival (PFS) (153). Within their cohort, PFS was significantly increased and relative risk of progression (RR) significantly reduced in those receiving a high dose of radiation to the bilateral periventricular regions (>59Gy) compared to the groups receiving a lower dose (<59Gy). This effect was not seen when comparing high versus low doses of radiation to the SGZ nor was the effect seen when stratifying groups by total dose delivered indicating radiation specifically delivered to the periventricular region and hence the SVZ as important(153).
8. ASSIGNING A SOURCE FOR GLIOMA INITIATING CELLS
A substantial amount of clinical and experimental results suggest that the cell of origin likely derives from the NSCs and/or progenitor cells of the SVZ. Clinically, most though not all glioblastomas are found to be associated with the periventricular regions of the lateral ventricles in humans (21, 47). Additionally, delivery of higher than average doses of radiation to the bilateral periventricular regions correlates with longer PFS compared with those receiving lower doses to the same regions suggesting that cell populations arising from or residing in this region are important in tumor recurrence (153). Experimentally, manipulation of pathways known to be abnormal in glioblastoma such as EGF/EGFR, PDGF/PDGFR, SHH, and Notch signaling in NSCs can cause phenotypic changes closely mimicking the human disease.
Several difficulties are readily apparent when trying to assign a specific origin to GICs. Much of our understanding of neural stem cell biology comes from studies of the rodent brain. Within the brain of postnatal rodents, several distinct populations of neural stem cell and lineage-restricted progenitors have been described. Both EGF and PDGF have been implicated as important growth factors in gliomagenesis with amplification or activating mutations of the EGFR being described in almost half of all malignant gliomas, similarly PDGF and PDGFR are upregulated in glioblastoma (84, 87, 154). EGF responsive type C cells of the SVZ are a sizable population of migratory, rapidly dividing transit-amplifying cells. Stimulation with EGF blocks type C cell differentiation, promotes neurosphere formation in vitro, and enhances their migration along white matter tracts and blood vessels similar to gliomas. B cells of the SVZ have been shown to express PDGF which in the rodent SVZ causes progenitor cell proliferation and hyperplasia similar to that seen in early gliomagenesis (46). Moreover, treatment with antimitotic agents (e.g cytosine-b-D-arabino-furanoside) kill type C cells while sparing type B cells. Given that the GIC population is hypothesized to be responsible for both invasive spread and recurrence characteristic of glioblastomas, this finding can suggest that type B cells are the origin of GICs explaining in part their resistance to chemotherapy agents (4, 155). Limited evidence supports the possibility of OPCs being a source of GICs; Kondo and Raff demonstrated that OPCs manipulated in vitro could acquire stem-like properties along with the re-expression of Sox2, an immature neuro-epithelial marker associated with glioblastomas (156, 157). Additionally, in a murine model of oligodendroglioma, Persson et al. demonstrated that OPC-like rather than NSC-like cells populations enriched for tumor-initiating cells; a finding further supported by the finding that NG2+-enriched populations from human oligodendroglioma explants could recapitulate oligodendrogliomas in a xenograft model (64). In some cases glioblastomas fail to contact the ventricle and hence the SVZ, suggesting that for a subset of glioblastomas, OPCs or perhaps OSVZ associated radial glia may be the origin of GICs (47, 77).
Though evidence from rodent models can be cited in favor of multiple cellular origins for GICs, fundamentally the ability to generalize these results to the human disease is limited. The structure and function of the human SVZ and associated NSCs are disparately different from those of the rodent. The presence of a hypocellular gap, lack of a clearly defined RMS, absence of human equivalents to types A, B, and C cells, and the presence of outer SVZ layer radial glia that fail to contact the ependyma all stand in clear contrast to the rodent SVZ.
Additionally, the initial supposition that CD133+ cells represented the GIC subpopulation of gliomas is increasingly being called into question (4, 7, 38, 50). Initial studies suggested that the CD133+ population represented GICs due to the observation that neurospheres were more readily established from these sorted populations compared with CD133− cells, and as few as 100 CD133+ cells were sufficient to establish tumors in xenograft models (7). Recently however data is accumulating to suggest while more rare, CD133− populations can demonstrate a stem-like phenotype and propagate tumors in xenograft models. It is unclear however if CD133+ and CD133− cells represent entities along a developmental spectrum or instead reflect distinct stem-like populations based on transcriptional profiles, both able to act as GICs (158, 159).
9. CLINICAL IMPLICATIONS OF GLIOMA INITIATING CELL IDENTITY
As previously discussed, the CSC population within glioblastomas has a pronounced resistance to both radiation and chemotherapy suggesting in the context of the CSC hypothesis that these populations are a likely source of resistance to treatment and recurrence inherent in this disease (8, 10). Not surprisingly, numerous groups are studying various molecular pathways involved in glioma-associated CSC biology which may ultimately find their way into the clinical armamentarium. Heimberger’s group at MD Anderson for example has shown that constitutive activation of the Signal Transducer and Activator of Transcription Protein (STAT)-3 in CSCs is involved in the establishment of an immunosuppressive state; pharmacologic inhibition of STAT3 with WP1066 was found to partially restore glioma-specific immune responses in allogeneic T cells suggesting a possible role in the treatment of glioblastoma by aiding the host anti-tumor response (14). Modulation of the SHH and Notch pathways has been shown in vitro and in vivo to deplete stem cell-like populations derived from gliomas and inhibit tumor growth in xenograft models; in pediatric populations, a phase I study for medulloblastoma using the small molecule inhibitor of SHH, GDC-0449, is currently underway (95, 108, 160). Driving the differentiation of glioma-associated CSCs through bone morphogenic protein (BMP)-4 treatment similarly inhibited CSC proliferation and tumor growth (161). Furthermore, radiation directed at the bilateral periventricular regions of glioblastoma patients has correlated with improved PFS and reduced RR of progression (153).
These experimental and clinical observations represent an attractive target for future treatment modalities. Though like most oncologic treatments, need be considered carefully. Radiation and chemotherapy most often represent a balancing act between efficacy against tumor and normal tissues, a concept known as the therapeutic index. Targeting STAT3 in stem-cell populations is an attractive avenue for aiding the host anti-tumor immune response; STAT3 however is a key signaling molecule in the response to leukemia inhibitory factor (LIF), important in neural stem cell maintenance (14, 162). Similarly, radiation targeted at the periventricular regions, a proposed source of GICs and known location of NSCs, improves PFS (153). Acharya et al. however, have recently demonstrated low dose radiation exposure (1–2 Gy) can have detrimental effects on NSCs of the SGZ promoting apoptosis and increased oxidative and nitrosative stress in surviving NSC populations suggesting a mechanism for radiation-associated cognitive decline (163). Thus while, treatments targeting the GIC population may provide a new avenue for the treatment of glioblastoma, the effects on the normal parenchyma will have to be weighed.
10. CONCLUSIONS
Starting with the initial identification of a CD133+-expressing cell subpopulation derived from gliomas which demonstrated properties of self-renewal, multipotentiality, and recapitulation of glioblastomas in a xenograft model, the role of stem-like cells in the ontology of glioblastoma has been a central topic of interest and investigation among glioma researchers and clinicians (7). The cancer stem cell hypothesis represents an important advancement in our understanding of the origin of glioblastoma, opening a new avenue for therapeutic modalities to target a currently incurable and invariably lethal disease. Despite intensive research by a number of groups, the definitive identity of GICs remains elusive.
Glioblastoma is a highly heterogeneous disease entity with a spectrum of pathologic features which can vary from patient to patient. Numerous genetic derangements can be demonstrated in tumor samples from both primary and secondary glioblastomas, which can recapitulate glioblastomas in experimental models. Differences in anatomic locations can also be shown to have prognostic significance. Furthermore, experimental evidence can be provided that implicates several different stem cell and lineage-restricted progenitor populations as the source of GICs. While research continues to hunt for the true origin of GICs, the possibility that glioblastomas are in fact several different disease entities which differ only in relatively subtle ways as evidenced by the apparent differences in anatomic origins (periventricular vs subcortical vs cortical), genetic derangements (p53 mutations, PTEN mutations, EGFR amplification and EGFRvIII mutations, etc), as well as pathologic subtypes (glioblastoma, gliosarcoma, giant cell glioblastoma) might be considered. Only continued investigation will shed light on this dilemma.
Acknowledgments
We would like to thank Drs. Pragathi Achanta and Candice Shaifer for their immense help in reviewing and editing this manuscript. Work in our laboratory is supported through an NIH T32 Neurooncology Training Grant (DC), NIH R01 (R01NS070024 to AQH), NIH KO8 (1K08NS055851 to AQH), Robert Wood Johnson Foundation/Harold Amos Medical Faculty Development Program (102690 to AQH), Howard Hughes Medical Institute Physician-Scientist Award (905675 to AQH).
Abbreviations
- CNS
central nervous system
- CSC
cancer stem cell
- NSC
neural stem cell
- GIC
glioma initiating cell
- SVZ
subventricular zone
- SGZ
subgranular zone
- RMS
rostral migratory stream
- OPC
oligodendrocyte precursor cell
- GFAP
glial fibrillary acidic protein
- ENU
N-ethyl-N-nitrosourea
- EGF
epithelial growth factor
- PDGF
platelet-derived growth factor
- GSI
gamma secretase inhibitor
- Shh
sonic hedgehog
- PTEN
phosphatase and tensin homologue deleted on chromosome
- PIP3
phosphatidylinositol phosphate
- NF
neurofibromatosis
- TGF
transforming growth factor
- BDNF
brain-derived neurotrophic factor
- VEGF
vascular endothelial growth factor
- NO
nitric oxide
- eNOS
endothelial-derived nitric oxide synthase
- MAP
microtubule associated protein
- CEL
contrast enhancing lesion
- PFS
progression-free survival
- RR
relative risk
- DAPI
4′,6-diamidino-2-phenylindole
- STAT
signal transducer and activator of transcription
- LIF
leukemia inhibitory factor
- BMP
bone morphogenic protein
References
- 1.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–9. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes Aa, Marosi C, Bogdahn U, Jr, Curschmann, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Chaichana KL, Garzon-Muvdi T, Parker S, Weingart JD, Olivi A, Bennett R, Brem H, Quinones-Hinojosa A. Supratentorial Glioblastoma Multiforme: The Role of Surgical Resection Versus Biopsy Among Older Patients. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. The New England journal of medicine. 2005;353:811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 5.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–34. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 6.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell stem cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eramo a, Ricci-Vitiani L, Zeuner a, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, {De Maria} R. Chemotherapy resistance of glioblastoma stem cells. Cell death and differentiation. 2006;13:1238–41. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 11.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou M-F, de Tribolet N, Regli L, Wick W, Kouwenhoven MCM, Hainfellner Ja, Heppner FL, Dietrich P-Y, Zimmer Y, Cairncross JG, Janzer R-C, Domany E, Delorenzi M, Stupp R, Hegi ME. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 12.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Experimental neurology. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF, Heimberger AB. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Molecular cancer therapeutics. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie TX, Aldape KD, Gong W, Kanzawa T, Suki D, Kondo S, Lang F, Ali-Osman F, Sawaya R, Huang S. Aberrant NF-kappaB activity is critical in focal necrosis formation of human glioblastoma by regulation of the expression of tissue factor. Int J Oncol. 2008;33:5–15. [PubMed] [Google Scholar]
- 16.Holland EC. Progenitor cells and glioma formation. Current opinion in neurology. 2001;14:683–8. doi: 10.1097/00019052-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J, Jones EJ. Cajal’s Degeneration and Regeneration of the Nervous System. Oxford University Press; 1991. [Google Scholar]
- 18.Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302–18. doi: 10.1016/0014-4886(62)90040-7. [DOI] [PubMed] [Google Scholar]
- 19.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 20.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–4. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–10. [PubMed] [Google Scholar]
- 24.Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–8. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 25.Blakemore WF. The ultrastructure of the subependymal plate in the rat. J Anat. 1969;104:423–33. [PMC free article] [PubMed] [Google Scholar]
- 26.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 29.Gould E, Frankfurt M, Westlind-Danielsson A, McEwen BS. Developing forebrain astrocytes are sensitive to thyroid hormone. Glia. 1990;3:283–92. doi: 10.1002/glia.440030408. [DOI] [PubMed] [Google Scholar]
- 30.Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–50. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott KW, Lantos PL. Cell proliferation in the subependymal layer of the postnatal marmoset, Callithrix jacchus. Brain Res Dev Brain Res. 1990;57:269–77. doi: 10.1016/0165-3806(90)90053-2. [DOI] [PubMed] [Google Scholar]
- 32.Reznikov KY. Cell proliferation and cytogenesis in the mouse hippocampus. Adv Anat Embryol Cell Biol. 1991;122:1–74. doi: 10.1007/978-3-642-76447-9. [DOI] [PubMed] [Google Scholar]
- 33.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 34.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–44. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 35.Ignatova TN, V, Kukekov G, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 36.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi N, Ishii H, Sekimoto M, Haraguchi N, Doki Y, Mori M. Properties and identification of cancer stem cells: a changing insight into intractable cancer. Surgery today. 2010;40:608–13. doi: 10.1007/s00595-009-4106-6. [DOI] [PubMed] [Google Scholar]
- 38.Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England journal of medicine. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlstrand J, V, Collins P, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–41. [PubMed] [Google Scholar]
- 41.Esposito I, Kleeff J, Bischoff SC, Fischer L, Collecchi P, Iorio M, Bevilacqua G, Buchler MW, Friess H. The stem cell factor-c-kit system and mast cells in human pancreatic cancer. Lab Invest. 2002;82:1481–92. doi: 10.1097/01.lab.0000036875.21209.f9. [DOI] [PubMed] [Google Scholar]
- 42.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 43.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 45.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–9. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchida K, Yonezawa M, Nakamura S, Kobayashi T, Machida T. Impaired neurogenesis in the growth-retarded mouse is reversed by T3 treatment. Neuroreport. 2005;16:103–6. doi: 10.1097/00001756-200502080-00005. [DOI] [PubMed] [Google Scholar]
- 50.Vescovi AL, Galli R, Reynolds Ba. Brain tumour stem cells. Nature reviews Cancer. 2006;6:425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 52.Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 53.Bedard A, Cossette M, Levesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002;328:213–6. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- 54.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–47. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 55.Kempermann G. Neuronal stem cells and adult neurogenesis. Ernst Schering Res Found Workshop; 2002. pp. 17–28. [DOI] [PubMed] [Google Scholar]
- 56.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–32. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–8. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–81. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 60.Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull. 1997;42:9–21. doi: 10.1016/s0361-9230(96)00116-5. [DOI] [PubMed] [Google Scholar]
- 61.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–12. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 62.Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–6. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, Ayers-Ringler J, Nishiyama A, Stallcup WB, Berger MS, Bergers G, McKnight TR, Goldman SA, Weiss WA. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18:669–82. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blakemore WF, Jolly RD. The subependymal plate and associated ependyma in the dog. An ultrastructural study. J Neurocytol. 1972;1:69–84. doi: 10.1007/BF01098647. [DOI] [PubMed] [Google Scholar]
- 66.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–7. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- 68.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 69.Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol. 1995;73:333–46. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- 70.Pascual O, Haydon PG. Synaptic inhibition mediated by glia. Neuron. 2003;40:873–5. doi: 10.1016/s0896-6273(03)00760-8. [DOI] [PubMed] [Google Scholar]
- 71.Guerrero-Cazares H, Gonzalez-Perez O, Soriano-Navarro M, Zamora-Berridi G, Garcia-Verdugo JM, Quinones-Hinojosa A. Cytoarchitecture of the Lateral Ganglionic Eminence and Rostral Extension of the Lateral Ventricle in the Human Fetal Brain. J Comp Neurol. 2010 doi: 10.1002/cne.22566. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradford D, Faull MaCurtis RLM, Cooper HM. Characterization of the netrin/RGMa receptor neogenin in neurogenic regions of the mouse and human adult forebrain. The Journal of comparative neurology. 2010;518:3237–53. doi: 10.1002/cne.22397. [DOI] [PubMed] [Google Scholar]
- 73.Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007;8:712–23. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- 74.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–9. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 75.Kam M, Curtis MA, McGlashan SR, Connor B, Nannmark U, Faull RL. The cellular composition and morphological organization of the rostral migratory stream in the adult human brain. J Chem Neuroanat. 2009;37:196–205. doi: 10.1016/j.jchemneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–65. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- 77.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 78.Hopewell JW. The subependymal plate and the genesis of gliomas. J Pathol. 1975;117:101–3. doi: 10.1002/path.1711170208. [DOI] [PubMed] [Google Scholar]
- 79.Lantos PL, Pilkington GJ. Neuroblasts in cerebral tumors induced by ethylnitrosourea in rats. A fine structrual study. Virchows Arch B Cell Pathol. 1977;25:243–59. doi: 10.1007/BF02889437. [DOI] [PubMed] [Google Scholar]
- 80.Lantos PL, Pilkington GJ. The development of experimental brain tumours. A sequential light and electron microscope study of the subependymal plate. I. Early lesions (abnormal cell clusters) Acta Neuropathol. 1979;45:167–75. doi: 10.1007/BF00702668. [DOI] [PubMed] [Google Scholar]
- 81.Pilkington GJ, Lantos PL. The development of experimental brain tumours a sequential light and electron microscope study of the subependymal plate. II. Microtumours. Acta Neuropathol. 1979;45:177–85. doi: 10.1007/BF00702669. [DOI] [PubMed] [Google Scholar]
- 82.Feldkamp MM, Lau N, Guha A. Signal transduction pathways and their relevance in human astrocytomas. J Neurooncol. 1997;35:223–48. doi: 10.1023/a:1005800114912. [DOI] [PubMed] [Google Scholar]
- 83.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 84.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60:168–73. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 85.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–9. [PubMed] [Google Scholar]
- 86.Kesari S, Stiles CD. The bad seed: PDGF receptors link adult neural progenitors to glioma stem cells. Neuron. 2006;51:151–3. doi: 10.1016/j.neuron.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–35. [PubMed] [Google Scholar]
- 88.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–30. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–52. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ivanchuk SM, Mondal S, Dirks PB, Rutka JT. The INK4A/ARF locus: role in cell cycle control and apoptosis and implications for glioma growth. J Neurooncol. 2001;51:219–29. doi: 10.1023/a:1010632309113. [DOI] [PubMed] [Google Scholar]
- 92.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 93.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 94.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–58. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 97.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 98.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 99.Browd SR, Kenney AM, Gottfried ON, Yoon JW, Walterhouse D, Pedone CA, Fults DW. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66:2666–72. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 100.Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100:7331–6. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–62. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 102.Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, Stephen D, Zagzag D, Joyner AL, Turnbull DH. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–9. [PubMed] [Google Scholar]
- 103.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 104.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 105.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–50. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 106.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–44. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur J Neurosci. 1999;11:3199–214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- 108.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–96. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 111.Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–13. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem. 2003;88:24–8. doi: 10.1002/jcb.10312. [DOI] [PubMed] [Google Scholar]
- 113.Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, Mendez-Gomez HR, Carrera AC, Abad JL, Gonzalez M, de la Rosa EJ, Vicario-Abejon C, de Pablo F. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119:2739–48. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 114.Zheng H, Ying HH, Yan H, Kimmelman AC, Hiller DJ, Chen A-j, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, Depinho RA. p53 and Pten control neural and glioma stem/ progenitor cell renewal and differentiation. October. 2008;455:1129–1134. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael ST, Kornblum HI, Liu X, Wu H. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–86. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–9. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 117.Wiencke JK, Zheng S, Jelluma N, Tihan T, Vandenberg S, Tamguney T, Baumber R, Parsons R, Lamborn KR, Berger MS, Wrensch MR, Haas-Kogan DA, Stokoe D. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol. 2007;9:271–9. doi: 10.1215/15228517-2007-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newcomb EW, Madonia WJ, Pisharody S, Lang FF, Koslow M, Miller DC. A correlative study of p53 protein alteration and p53 gene mutation in glioblastoma multiforme. Brain Pathol. 1993;3:229–35. doi: 10.1111/j.1750-3639.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 119.Hesselager G, Uhrbom L, Westermark B, Nister M. Complementary effects of platelet-derived growth factor autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res. 2003;63:4305–9. [PubMed] [Google Scholar]
- 120.Katayama K, Ueno M, Yamauchi H, Nagata T, Nakayama H, Doi K. Ethylnitrosourea induces neural progenitor cell apoptosis after S-phase accumulation in a p53-dependent manner. Neurobiol Dis. 2005;18:218–25. doi: 10.1016/j.nbd.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 121.Rodriguez HA, Berthrong M. Multiple primary intracranial tumors in von Recklinghausen’s neurofibromatosis. Arch Neurol. 1966;14:467–75. doi: 10.1001/archneur.1966.00470110011002. [DOI] [PubMed] [Google Scholar]
- 122.Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–88. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, Scheithauer BW, Jenkins RB, James CD. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–7. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 124.Humphrey PA, Gangarosa LM, Wong AJ, Archer GE, Lund-Johansen M, Bjerkvig R, Laerum OD, Friedman HS, Bigner DD. Deletion-mutant epidermal growth factor receptor in human gliomas: effects of type II mutation on receptor function. Biochem Biophys Res Commun. 1991;178:1413–20. doi: 10.1016/0006-291x(91)91051-d. [DOI] [PubMed] [Google Scholar]
- 125.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–40. [PubMed] [Google Scholar]
- 126.Bonnert TP, Bilsland JG, Guest PC, Heavens R, McLaren D, Dale C, Thakur M, McAllister G, Munoz-Sanjuan I. Molecular characterization of adult mouse subventricular zone progenitor cells during the onset of differentiation. Eur J Neurosci. 2006;24:661–75. doi: 10.1111/j.1460-9568.2006.04912.x. [DOI] [PubMed] [Google Scholar]
- 127.Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor beta1 and beta2 in the plasma of patients with glioblastoma. J Neurooncol. 2006;79:61–5. doi: 10.1007/s11060-005-9116-7. [DOI] [PubMed] [Google Scholar]
- 128.Dey M, Farzana Hussain S, Heimberger AB. The Role of Glioma Microenvironment in Immune Modulation: Potential Targets for Intervention. Letters in Drug Design & Discovery. 2006;3:443–453. [Google Scholar]
- 129.Golestaneh N, Mishra B. TGF-beta, neuronal stem cells and glioblastoma. Oncogene. 2005;24:5722–30. doi: 10.1038/sj.onc.1208925. [DOI] [PubMed] [Google Scholar]
- 130.Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, Fuller GN, Sawaya R. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clinical neurosurgery. 2009;56:98–106. [PubMed] [Google Scholar]
- 131.Kong DS, Song SY, Kim DH, Joo KM, Yoo JS, Koh JS, Dong SM, Suh YL, Lee JI, Park K, Kim JH, Nam DH. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–8. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 132.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010 doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 134.Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol. 2007;8:345–54. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- 135.Garcion E, Halilagic A, Faissner A. Cffrench-Constant Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 136.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–68. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 137.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 138.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell stem cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 139.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 140.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–9. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 141.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 142.Sirko S, von Holst A, Wizenmann A, Gotz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–38. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 143.Charles Na, Holland EC. TRRAP and the maintenance of stemness in gliomas. Cell stem cell. 2010;6:6–7. doi: 10.1016/j.stem.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 144.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–22. doi: 10.1007/s00018-004-4144-6. [DOI] [PubMed] [Google Scholar]
- 145.Jang T, Litofsky NS, Smith TW, Ross AH, Recht LD. Aberrant nestin expression during ethylnitrosourea-(ENU)-induced neurocarcinogenesis. Neurobiol Dis. 2004;15:544–52. doi: 10.1016/j.nbd.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 146.Savarese TM, Jang T, Low HP, Salmonsen R, Litofsky NS, Matuasevic Z, Ross AH, Recht LD. Isolation of immortalized, INK4a/ARF-deficient cells from the subventricular zone after in utero N-ethyl-N-nitrosourea exposure. J Neurosurg. 2005;102:98–108. doi: 10.3171/jns.2005.102.1.0098. [DOI] [PubMed] [Google Scholar]
- 147.Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, Li SN, Lahn BT, Xiang AP. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61:467–73. doi: 10.1136/jcp.2007.047605. [DOI] [PubMed] [Google Scholar]
- 148.Daou MC, Smith TW, Litofsky NS, Hsieh CC, Ross AH. Doublecortin is preferentially expressed in invasive human brain tumors. Acta Neuropathol. 2005;110:472–80. doi: 10.1007/s00401-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 149.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 150.Rich JN, Hans C, Jones B, Iversen ES, McLendon RE, Rasheed BK, Dobra A, Dressman HK, Bigner DD, Nevins JR, West M. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65:4051–8. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 151.Santra M, Liu XS, Santra S, Zhang J, Zhang RL, Zhang ZG, Chopp M. Ectopic expression of doublecortin protects adult rat progenitor cells and human glioma cells from severe oxygen and glucose deprivation. Neuroscience. 2006;142:739–52. doi: 10.1016/j.neuroscience.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 152.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–24. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 153.Evers P, Lee PP, Demarco J, Agazaryan N, Sayre JW, Selch M, Pajonk F. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–47. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 155.Stiles CD, Rowitch DH. Glioma Stem Cells: A Midterm Exam. Neuron. 2008:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 156.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–7. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 157.Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18:2963–72. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 159.Lottaz C, Beier D, Meyer K, Kumar P, Hermann A, Schwarz J, Junker M, Oefner PJ, Bogdahn U, Jr, Wischhusen, Spang R, Storch A, Beier CP. Transcriptional profiles of CD133+ and CD133− glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer research. 2010;70:2030–40. doi: 10.1158/0008-5472.CAN-09-1707. [DOI] [PubMed] [Google Scholar]
- 160.Gajjar AJ, Stewart CF, Ellison DW, Curran T, Phillips P, Goldman S, Packer RJ, Kun LE, Boyett JM, Gilbertson RJ. A phase I pharmacokinetic trial of sonic hedgehog (SHH) antagonist GDC-0449 in pediatric patients with recurrent or refractory medulloblastoma: A Pediatric Brain Tumor Consortium study (PBTC 25) Journal of Clinical Oncology. 2010;28 [Google Scholar]
- 161.Piccirillo SGM, Reynolds Ba, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi a, Dimeco FF, Vescovi aL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 162.Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci. 2004;27:255–66. doi: 10.1016/j.mcn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]