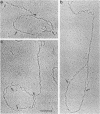
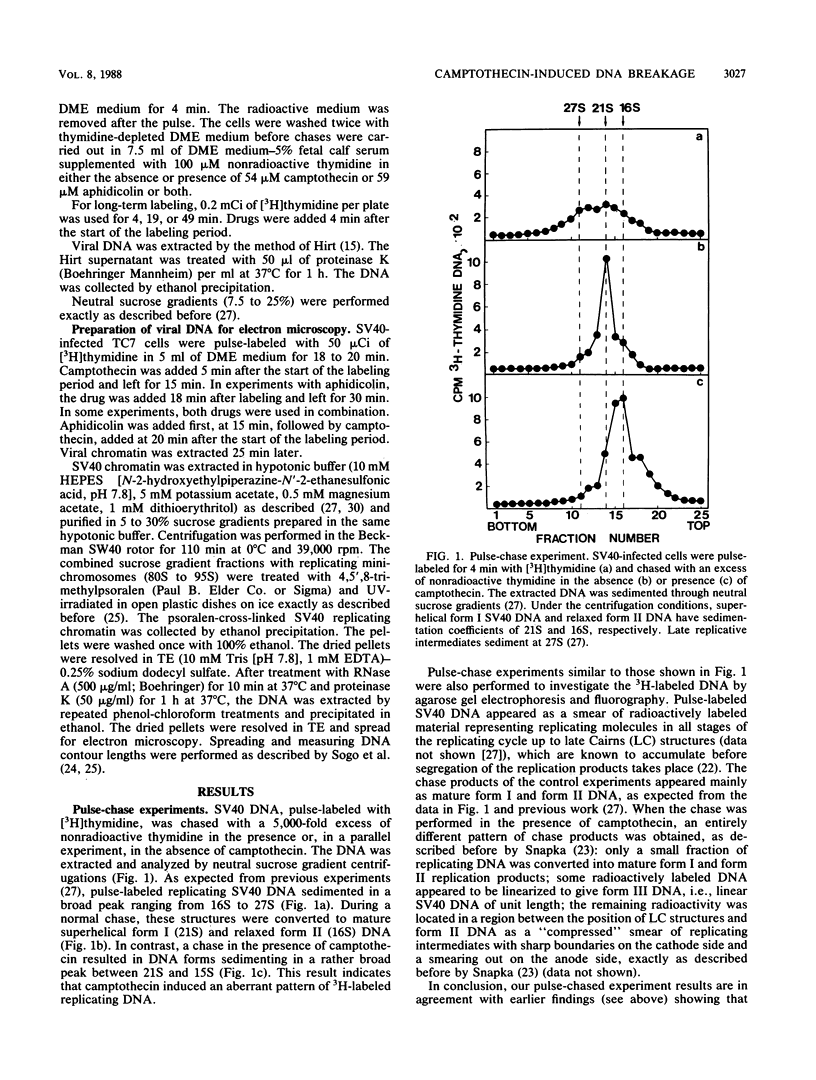
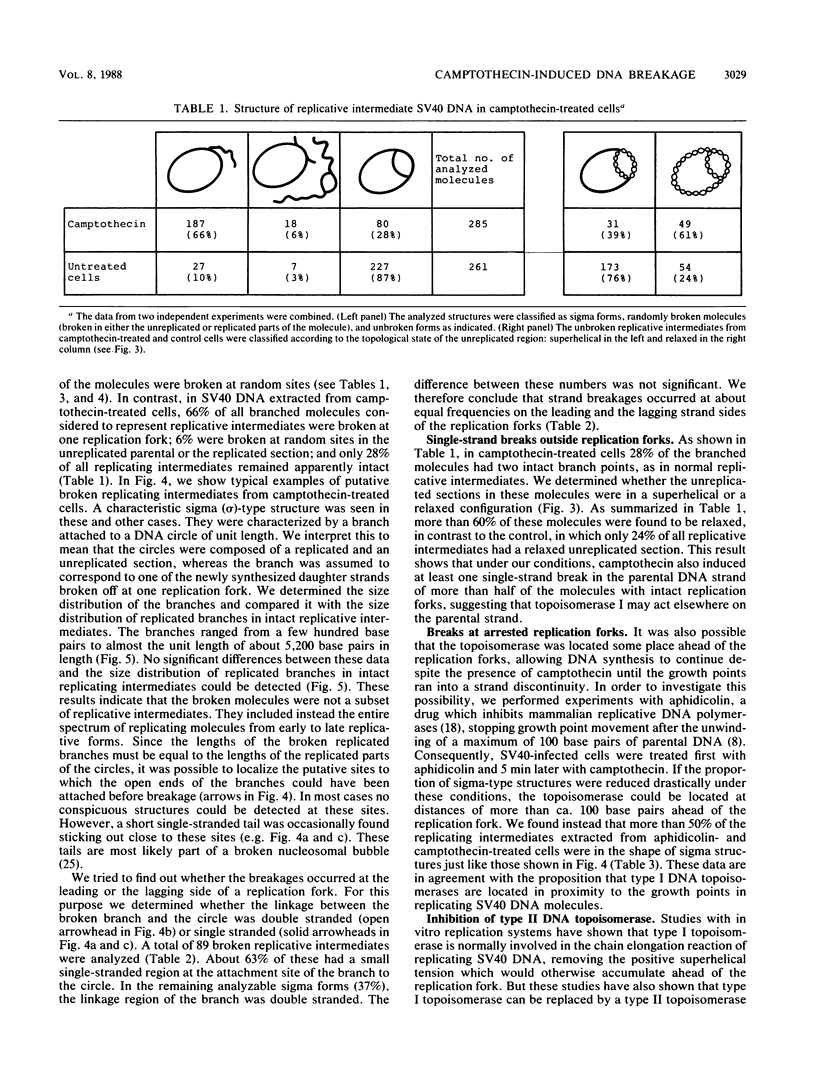
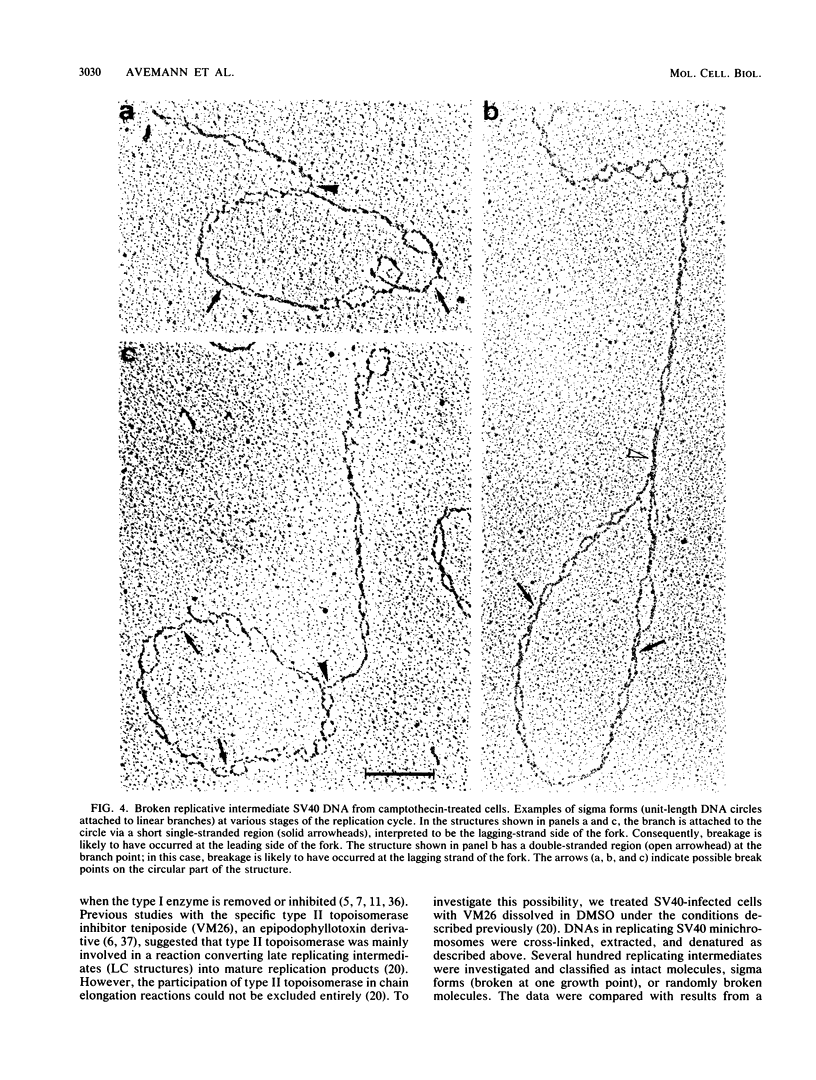
Abstract
The structure of replicating simian virus 40 minichromosomes, extracted from camptothecin-treated infected cells, was investigated by biochemical and electron microscopic methods. We found that camptothecin frequently induced breaks at replication forks close to the replicative growth points. Replication branches were disrupted at about equal frequencies at the leading and the lagging strand sides of the fork. Since camptothecin is known to be a specific inhibitor of type I DNA topoisomerase, we suggest that this enzyme is acting very near the replication forks. This conclusion was supported by experiments with aphidicolin, a drug that blocks replicative fork movement, but did not prevent the camptothecin-induced breakage of replication forks. The drug teniposide, an inhibitor of type II DNA topoisomerase, had only minor effects on the structure of these replicative intermediates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Penman S. Selective interruption of high molecular weight RNA synthesis in HeLa cells by camptothecin. Nat New Biol. 1972 May 31;237(74):144–146. doi: 10.1038/newbio237144a0. [DOI] [PubMed] [Google Scholar]
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonven B. J., Gocke E., Westergaard O. A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell. 1985 Jun;41(2):541–551. doi: 10.1016/s0092-8674(85)80027-1. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Camptothecin inhibits macromolecular synthesis in mammalian cells but not in isolated mitochondria of E. coli. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1412–1420. doi: 10.1016/0006-291x(70)90544-9. [DOI] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P., Sogo J. M., Stahl H. Inhibition of DNA synthesis by aphidicolin induces supercoiling in simian virus 40 replicative intermediates. EMBO J. 1985 Dec 1;4(12):3241–3246. doi: 10.1002/j.1460-2075.1985.tb04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Elgin S. C. Localization of specific topoisomerase I interactions within the transcribed region of active heat shock genes by using the inhibitor camptothecin. Mol Cell Biol. 1987 Jan;7(1):141–148. doi: 10.1128/mcb.7.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Pflugfelder G., Wang J. C., Lis J. T. Topoisomerase I interacts with transcribed regions in Drosophila cells. Cell. 1986 Feb 14;44(3):401–407. doi: 10.1016/0092-8674(86)90461-7. [DOI] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Cloning of yeast TOP1, the gene encoding DNA topoisomerase I, and construction of mutants defective in both DNA topoisomerase I and DNA topoisomerase II. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7178–7182. doi: 10.1073/pnas.82.21.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Krauss M. R., Buckler-White A. J., Benbow R. M., Pigiet V. Polyoma virus minichromosomes: a soluble in vitro replication system. J Virol. 1981 Jun;38(3):805–814. doi: 10.1128/jvi.38.3.805-814.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Pigiet V. P. Polyoma virus minichromosomes: characterization of the products of in vitro DNA synthesis. J Virol. 1983 Feb;45(2):585–593. doi: 10.1128/jvi.45.2.585-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin C., Yaniv M. Nicking-closing enzyme is associated with SV40 DNA in vivo as a sodium dodecyl sulfate-resistant complex. Nucleic Acids Res. 1979 Oct 10;7(3):679–687. doi: 10.1093/nar/7.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Horwitz S. B. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):723–727. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Richter A., Strausfeld U., Knippers R. Effects of VM26 (teniposide), a specific inhibitor of type II DNA topoisomerase, on SV40 DNA replication in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3455–3468. doi: 10.1093/nar/15.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Salzman N. P. Late replicative intermediates are accumulated during simian virus 40 DNA replication in vivo and in vitro. J Virol. 1979 May;30(2):600–609. doi: 10.1128/jvi.30.2.600-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M. Topoisomerase inhibitors can selectively interfere with different stages of simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4221–4227. doi: 10.1128/mcb.6.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Ness P. J., Widmer R. M., Parish R. W., Koller T. Psoralen-crosslinking of DNA as a probe for the structure of active nucleolar chromatin. J Mol Biol. 1984 Oct 5;178(4):897–919. doi: 10.1016/0022-2836(84)90318-8. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Zentgraf H., Knippers R. A large-tumor-antigen-specific monoclonal antibody inhibits DNA replication of simian virus 40 minichromosomes in an in vitro elongation system. J Virol. 1985 May;54(2):473–482. doi: 10.1128/jvi.54.2.473-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Schütz G. Camptothecin-induced in vivo topoisomerase I cleavages in the transcriptionally active tyrosine aminotransferase gene. Cell. 1987 Sep 25;50(7):1109–1117. doi: 10.1016/0092-8674(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Effects of cytosine arabinoside, daunomycin, mithramycin, azacytidine, adriamycin, and camptothecin on mammalian cell cycle traverse. Cancer Res. 1972 Dec;32(12):2720–2725. [PubMed] [Google Scholar]
- Vosberg H. P. DNA topoisomerases: enzymes that control DNA conformation. Curr Top Microbiol Immunol. 1985;114:19–102. doi: 10.1007/978-3-642-70227-3_2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Liu L. F. Identification of DNA topoisomerase II as an intracellular target of antitumor epipodophyllotoxins in simian virus 40-infected monkey cells. Cancer Res. 1985 Nov;45(11 Pt 2):5872–5876. [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]