Abstract
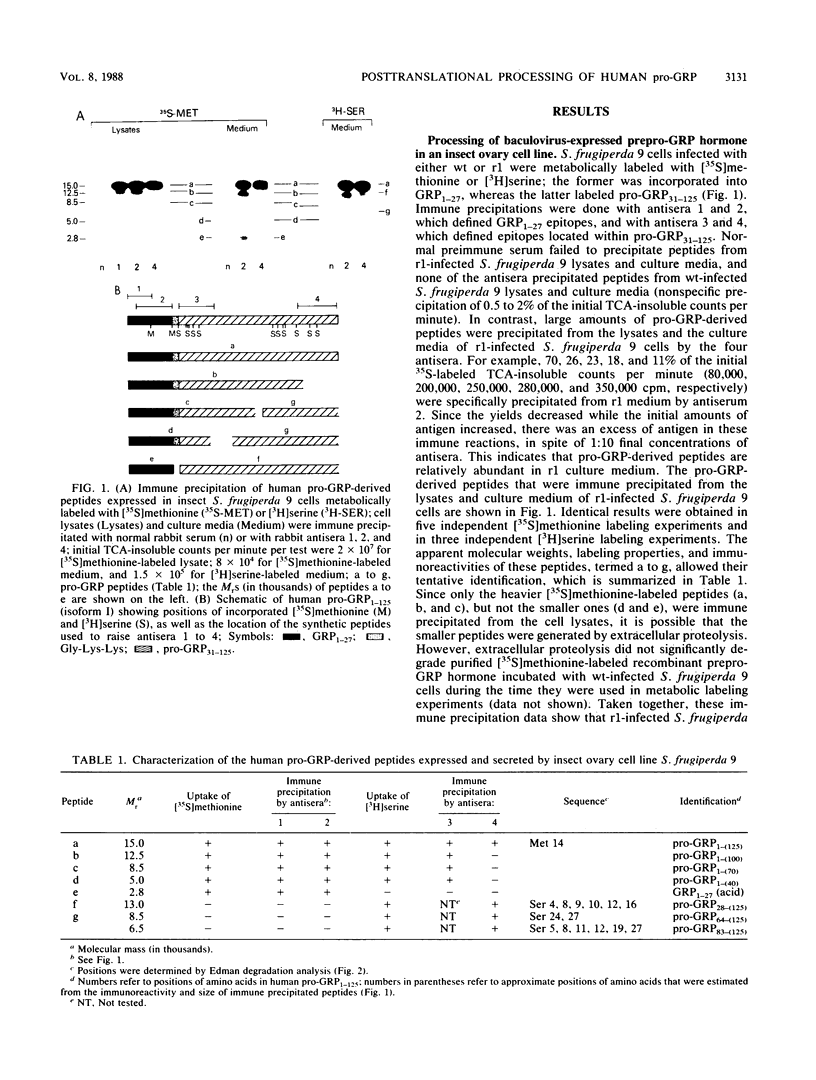
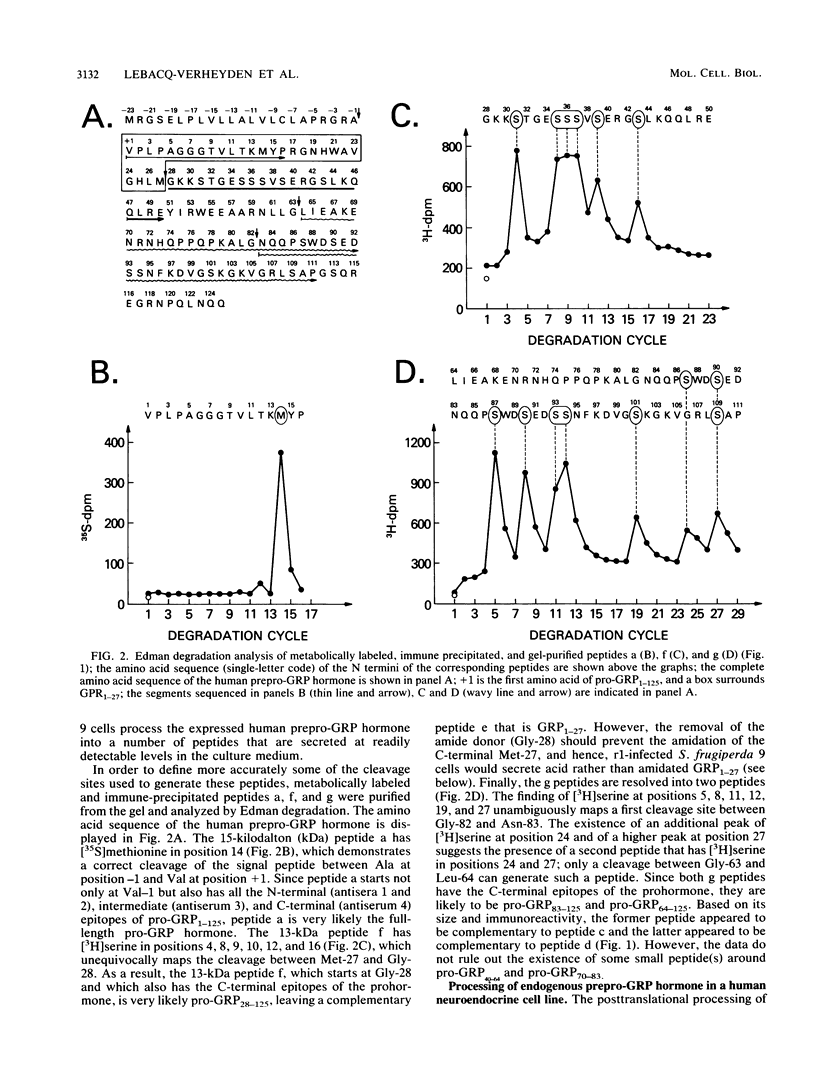
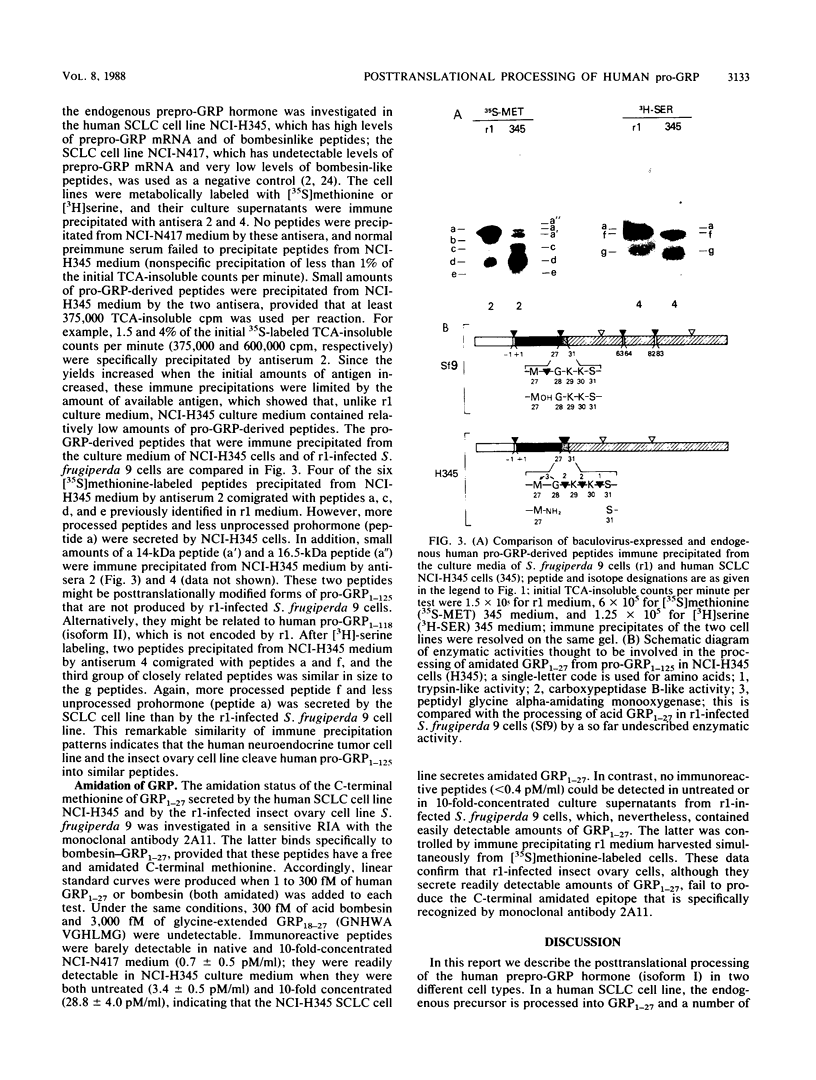
The 27-amino-acid gastrin-releasing peptide (GRP1-27) is a neuropeptide and growth factor that is synthesized by various neural and neuroendocrine cells. The major pro-GRP hormone (isoform I) contains both GRP1-27 and a novel C-terminal extension peptide termed pro-GRP31-125. In order to define potentially active neuropeptides that could be generated from this novel protein domain, we analyzed the posttranslational processing of endogenous human pro-GRP1-125 in a small-cell lung cancer cell line. Because such studies are much easier in an overexpression system, we investigated at the same time the posttranslational processing of baculovirus-expressed human pro-GRP1-125 in an insect ovary cell line. In the small-cell lung cancer cell line, GRP1-27 was cleaved as expected from the endogenous prohormone at a pair of basic amino acids (29 and 30) and alpha-amidated at its C-terminal methionine; however, a number of novel peptides were generated by additional cleavages in the pro-GRP31-125 domain. In the insect ovary cell line, GRP1-27 was cleaved from the expressed prohormone by a different mechanism, as were a number of other peptides that appeared to be similar in size to those produced by the human neuroendocrine tumor cell line. These data show for the first time that an insect ovary cell line that is widely used to overexpress proteins can process a human neuropeptide precursor. They also reveal the existence of novel pro-GRP-derived peptides that are candidates for biologically active ligands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971 Feb 15;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Coligan J. E., Gates F. T., 3rd, Kimball E. S., Maloy W. L. Radiochemical sequence analysis of biosynthetically labeled proteins. Methods Enzymol. 1983;91:413–434. doi: 10.1016/s0076-6879(83)91039-x. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Autocrine growth factors in human small cell lung cancer. Cancer Surv. 1985;4(4):707–727. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Cwikel B. J., Habener J. F. Provasopressin-neurophysin II processing is cell-specific in heterologous cell lines expressing a metallothionein-vasopressin fusion gene. J Biol Chem. 1987 Oct 15;262(29):14235–14240. [PubMed] [Google Scholar]
- Dickerson I. M., Dixon J. E., Mains R. E. Transfected human neuropeptide Y cDNA expression in mouse pituitary cells. Inducible high expression, peptide characterization, and secretion. J Biol Chem. 1987 Oct 5;262(28):13646–13653. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Drucker D. J., Mojsov S., Habener J. F. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J Biol Chem. 1986 Jul 25;261(21):9637–9643. [PubMed] [Google Scholar]
- Hellerman J. G., Cone R. C., Potts J. T., Jr, Rich A., Mulligan R. C., Kronenberg H. M. Secretion of human parathyroid hormone from rat pituitary cells infected with a recombinant retrovirus encoding preproparathyroid hormone. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5340–5344. doi: 10.1073/pnas.81.17.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Segal S., Cuttitta F., Battey J. F. Swiss 3T3 mouse embryo fibroblasts transfected with a human prepro-GRP gene synthesize and secrete pro-GRP rather than GRP. J Cell Biochem. 1988 Mar;36(3):237–248. doi: 10.1002/jcb.240360305. [DOI] [PubMed] [Google Scholar]
- Loh Y. P. Peptide precursor processing enzymes within secretory vesicles. Ann N Y Acad Sci. 1987;493:292–307. doi: 10.1111/j.1749-6632.1987.tb27214.x. [DOI] [PubMed] [Google Scholar]
- Lynch D. R., Snyder S. H. Neuropeptides: multiple molecular forms, metabolic pathways, and receptors. Annu Rev Biochem. 1986;55:773–799. doi: 10.1146/annurev.bi.55.070186.004013. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Cullen E. I., May V., Eipper B. A. The role of secretory granules in peptide biosynthesis. Ann N Y Acad Sci. 1987;493:278–291. doi: 10.1111/j.1749-6632.1987.tb27213.x. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1983 Jul 29;114(2):541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin C: a bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1984 Feb 29;119(1):14–20. doi: 10.1016/0006-291x(84)91611-5. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Walker M. D., Lee F., Kelly R. B. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell. Intracellular storage, proteolytic processing, and secretion on stimulation. Cell. 1983 Dec;35(2 Pt 1):531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Lebacq-Verheyden A. M., Spindel E. R., Cuttitta F., Gazdar A. F., Battey J. F. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986 Feb 15;261(5):2451–2457. [PubMed] [Google Scholar]
- Spindel E. R., Chin W. W., Price J., Rees L. H., Besser G. M., Habener J. F. Cloning and characterization of cDNAs encoding human gastrin-releasing peptide. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5699–5703. doi: 10.1073/pnas.81.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel E. R., Zilberberg M. D., Chin W. W. Analysis of the gene and multiple messenger ribonucleic acids (mRNAs) encoding human gastrin-releasing peptide: alternate RNA splicing occurs in neural and endocrine tissue. Mol Endocrinol. 1987 Mar;1(3):224–232. doi: 10.1210/mend-1-3-224. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Thomas G., Herbert E., Hruby D. E. Expression and cell type--specific processing of human preproenkephalin with a vaccinia recombinant. Science. 1986 Jun 27;232(4758):1641–1643. doi: 10.1126/science.3754979. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Schonbrunn A. Characterization of bombesin receptors in a rat pituitary cell line. J Biol Chem. 1983 Jun 25;258(12):7527–7535. [PubMed] [Google Scholar]
- Yoshizaki K., de Bock V., Solomon S. Origin of bombesin-like peptides in human fetal lung. Life Sci. 1984 Feb 27;34(9):835–843. doi: 10.1016/0024-3205(84)90200-5. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Murphy B. R., Collins P. L., Lebacq-Verheyden A. M., Battey J. F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987 Oct;160(2):465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]