Abstract
The blood-brain barrier has been modeled in vitro in a number of species, including rat, cow and human. Coculture of multiple cell types is required for the correct expression of tight junction proteins by microvascular brain endothelial cells (MBEC). Markers of inflammation, especially MHC-II, and cell adhesion molecules, such as VCAM-1, are not expressed on the luminal surface of the barrier under resting conditions. The rhesus macaque model has been used to study early events of HIV-neuropathogenesis in vivo, but a suitable in vitro model has not been available for detailed mechanistic studies. Here we describe an in vitro rhesus macaque blood-brain barrier (BBB) that utilizes autologous MBEC and astrocytes. We believe that this model is highly relevant for examining immunological events at the blood-brain barrier and demonstrate its potential usefulness for examining early events in AIDS neuropathogenesis.
Keywords: 3-D model, rhesus macaque, activation markers
1 Introduction
Neurological disease is a common side effect of AIDS. The clinical syndrome, referred to as AIDS dementia complex, is mirrored in the SIV-macaque model of AIDS, and is characterized by perivascular accumulations of macrophages and the presence of virus within the brain. In the absence of in vivo data for early HIV infection, the macaque model has proved invaluable (Persidsky et al 1995; Wykrzykowska et al 1998; Zink et al 1998). In vitro models have been developed using human endothelial cells derived from a variety of tissues which allows modeling of early time points in the neuropathogenesis of AIDS (Collins et al 2000; Persidsky & Gendelman 1997; Shaw & Greig 1999). Because these models are used for the initial stages of disease, and the ability to obtain brain tissue soon after HIV infection is severely limited, there can be no direct correlation between in vitro and in vivo conditions.
Some of these in vitro models are as simple as a monolayer of endothelial cells cultured on either glass coverslips or tissue culture plastic (Dorovini-Zis et al 1991; Omari & Dorovini-Zis 2001; Strelow et al 1998). Others extend to cultures under flow conditions using astrocytes cocultured with microvascular brain endothelial cells (MBEC) (Janigro et al 1998). While useful, many of these models use brain cells derived from different species (Janigro et al 1998) and thus their relevance is questionable, particularly with regard to experiments examining cell-to-cell interactions and immune effects of the barrier. While inbred rodents have been used to construct acceptable models of the BBB (Stanness et al 1999), none of these models use autologous MBEC and astrocytes from a species suitable for the study of AIDS neuropathogenesis. Models have been constructed using human-derived tissue, but few are optimal for modeling the BBB: some use cells from the same individual (Hurwitz et al 1993), but most models do not use brain-derived cells to model the BBB (Weiss et al 1998).
Thus, these in vitro models have been used to attempt to model the initial events of HIV neuropathogenesis. Several groups have developed systems for analyzing chemotaxis of peripheral blood mononuclear cells (PBMCs) using in vitro models of HIV infection (Hofman et al 1999; Schmidtmayerova et al 1996; Weiss et al 1998). Proteins found in brains of individuals with neuroAIDS, such as tat (Weiss et al 1999), CC-chemokines (Persidsky et al 1999) and tumor necrosis factor-α (TNF-α)(Weiss et al 1998) have been shown to induce the transmigration of PBMCs. However, the migration of these cells appears to be complete within 2.5hrs, suggesting less than optimal barrier function of the umbilical-cord-derived cells used in these models. In some model systems, this may be related to the absence of attempts to ensure that the endothelial component of the model was confluent before astrocytes were added to the culture (Hurwitz et al 1993). Persidsky et. al. (1997) have shown that, with care, a model can be constructed whereby migration of PBMCs can be qualitatively and quantitatively analyzed in a more realistic time frame (2 days versus 2 hours).
We have developed a rhesus macaque BBB in vitro using autologous MBEC and astrocytes. We describe in detail the isolation and culture of each cell type, and its integration into the in vitro model of the BBB. Data derived using this in vitro model can be directly correlated with in vivo data during the acute stages of SIV infection. We believe this model will prove invaluable for in vitro investigations of events in the neuropathogenesis of AIDS that occur at the level of the BBB.
2 Materials and Methods
Unless otherwise stated, all culture media and reagents were obtained from Gibco BRL (Grand Island, NY).
Cell culture
MBEC were isolated from normal rhesus macaques at necropsy (within 4 hours of death) as previously described (MacLean et al 2001). MBEC media consisted of: M199, 10% FCS, 5% human serum, 15μg/ml endothelial cell growth supplement (ECGS, ICN Flow, Costa Mesa, CA), 1x insulin-transferrin-selenium premix and antibiotics (1x penicillin-streptomycin solution). After 7–10 days distinct colonies were visible and these were passaged with the aid of cloning rings to 2% gelatin coated tissue culture flasks.
Autologous a strocytes were cultured by methods previously described by others (Guillemin et al 1997; Yong 1992). Brains were prepared as for MBEC isolation, with meninges removed before dissection. Small pieces of brain (normally frontal cortex) were incubated in the presence of trypsin and DNAse for 30 minutes. Trypsin was then inhibited by the addition of fetal calf serum. Cells were then passed through a 120μm nylon mesh, pelleted at 1000 rpm and plated at 105/ml in M199, 5% FCS. On days 2 and 3, medium was replaced. Vigorous washing of the flasks removed loosely adherent cells (identified as neurons by MAP-2 staining). At first passage, EDTA washing and quick trypsinization (See MacLean et al 2001) removed astrocytes, leaving cells consistent with microglia (identified as such by CD11b staining) attached to the flask. Astrocytes were subcultured at a ratio of 1:4.
Cells were grown to confluency and phenotyped immunocytochemically using antibodies to identify MBEC (CD147, GLUT1 and von Willebrands factor related antigen), astrocytes (GFAP), neurons (MAP2), and microglia (CD11b). Only astrocyte and MBEC cultures that were in excess of 95% immunopositive for GLUT-1 and CD147 (MBEC) and GFAP (astrocytes) were used for these experiments.
At passages 3 and 4, MBEC were seeded at a density of 106/ml onto 3μm PET filter inserts (Falcon, Franklin Lakes, NJ, 24 well) precoated with 50μg/ml fibronectin (Sigma, St. Louis, MO) in 2% gelatin solution. After 5 days in culture, monolayers were checked for confluence by staining with hematoxylin and eosin by standard techniques (Wilkinson 1988). If monolayers were confluent, astrocytes were seeded onto the lower layer of the filter at a density of 105/ml. This procedure required the removal of culture media from the upper well of the assembly, and the inversion of the entire multiwell dish. Astrocytes were allowed to adhere for two hours in a humidified atmosphere before reversion of the dish and addition of MBEC media to the upper well, and astrocyte media to the lower well.
Immunocytochemical analysis of BBB modeled in vitro
After at least 5 days in culture, the filter assemblies were fixed in ice-cold acetone/methanol (1:1 v/v) and processed for immunocytochemistry. Nonspecific binding of antibody was diminished by blocking with PBS containing 1% BSA for 1 hour. Primary antibody (see Table 1) was diluted in blocking buffer, incubated at room temperature for 2 hours in a humidified atmosphere, then washed, and secondary antibody (conjugated with AlexaFluor 568 – Molecular Probes, Eugene, OR) was applied for 45 minutes. Controls consisted of substitution of the primary antibody by an equivalent or greater concentration of an isotype matched antibody or normal rabbit serum (for Glut-1). Filters were thoroughly washed and mounted using MOWIOL 4–88 / Glycerol / DABCO (Calbiochem, La Jolla, CA/ Sigma/ Sigma). Images were captured for confocal microscopy using a Leica TCS confocal microcsope (Leica Microsystems, Exton, PA) and analyzed with NIH Image (v. 1.62) and Adobe Photoshop (v. 4), as described previously (MacLean et al 2001). For conventional immunocytochemistry, biotinylated horse anti-mouse (or goat anti-rabbit for GLUT-1, GFAP and vWf:related antigen) was used for the secondary antibody. Immunopositive cells were identified with avidin-biotin complex and developed with diaminobenzidine. Filters were counterstained with hematoxylin.
Table 1.
Antibodies used for immunofluorescence.
| Antigen / Specificity | Clone | Source | Concentration |
|---|---|---|---|
| VCAM-1 / CD106 | 1G11 | Immunotech | 4μg/ml |
| E-Selectin / CD62e | 1.2B6 | Immunotech | 4μg/ml |
| Glut-1 / Glucose transporter-1 | polyclonal | Chemicon | 0.45μg/ml |
| HT-7 / CD147 | HT-7 | Sigma | 1μg/ml |
| CD11b-FITC | Bear1 | Immunotech | As supplied |
| GFAP-cy3 | G-A-5 | Sigma | 1:2000 |
| Von Willebrand factor:related antigen | polyclonal | Dako | 12μg/ml |
| MAP-2 | HM-2 | Sigma | 1:500 |
Antibodies used in this study, and their antigens. All antibodies were diluted in PBS containing 0.5 % bovine serum albumin, the optimal concentration having been predetermined.
Transmission electron microscopy
For electron microscopy, filters were fixed in 3% glutaraldehyde for 30 minutes before washing in phosphate buffer and fixed in osmium tetroxide. Transwell membranes were kept intact throughout fixation, dehydration through graded series of ethanols to 90%. After 90% ethanol, a series of increasing concentrations of 2-hydroxypropyl methacrylate (HPMA) was used as a transitional solvent during drying to maintain the integrity of the plastic well and membrane as described previously (Brinkley et al 1967). In brief, three 15 minute incubations with 90% HPMA were followed by 95% and 97% HPMA incubations (each for 15 minutes). To transition to eponate, the assemblies were treated for 15 minutes each with the following ratios of HPMA to eponate; 2:1, 1:1. 1:2 and finally pure eponate 12. The transwell unit was filled and suspended in eponate 12 (Ted Pella Inc., Redding, CA) resin. The transwell insert was embedded in a mold fashioned from a 3ml plastic disposable transfer pipet that had an inch cut off the sealed top of the bulb. The mold was filled half way with resin and the transwell insert placed inside and additional resin pipetted into the cup portion of the insert. The membrane at the bottom of the cup is covered above and below with resin and free of any air bubbles. The mold was placed inside a 60°C oven for 24-hours to speed polymerization of the resin. The mold was removed and the block placed in a vise. A jewelers saw was used to make two parallel cuts 4 mm above and below the membrane. Having made these cuts one is left with a disc approximately 1cm in diameter and 8–10mm thick with the membrane in its center, occupying its circumference. Multiple cuts were made perpendicular to the membrane and the blocks were super glued onto mounting cyclinders with the membrane oriented on edge. Sections were cut on a Sorvall MT-2 ultramicrotome and stained with uranyl acetate and Sato’s lead stain (Sato et al 1967). The sections were viewed on a Jeol 1010 electron microscope.
Activation of in vitro model
TNF-α (100U/ml) was added to the lower well of the BBB model and incubated overnight. TNF-α was used because of its ability to induce expression of chemokines and adhesion molecules relevant to the neuropathogenesis of AIDS. Filters were fixed with paraformaldehyde, and surface expression of adhesion markers on endothelial cells assessed as a marker of activation. E-selectin were chosen as it is recognized as a marker of early activation of endothelial cells. VCAM-1 was used as it has been shown to be an important modulator of SIV neuroinvasion (Sasseville et al 1994).
3 Results
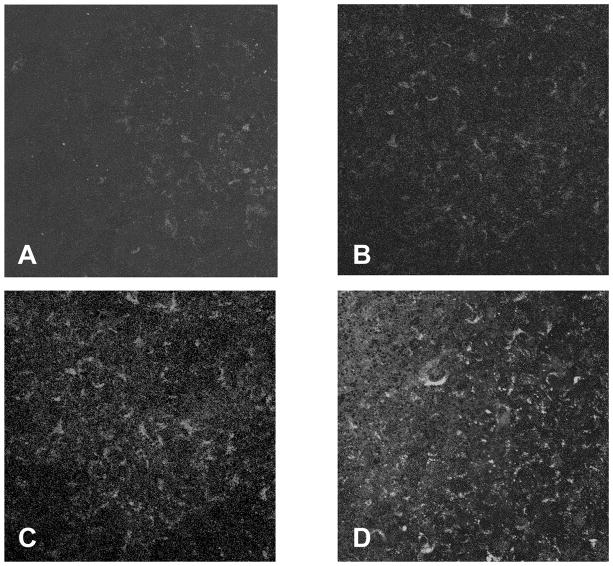
Monolayers of microvascular brain endothelial cells (MBEC), when cultured on the opposite side of a filter from autologous astrocytes, were found to be immunopositive for CD147 and GLUT-1 (Figure 1). CD147 (Fig. 1A) and GLUT-1 (Fig. 1B) immunostaining was diffusely present over the surface of the monolayers. Endothelial monolayers grown on top of 3μm PET filters were negative for glial fibrilliary acidic protein (GFAP) (Fig. 1C).
Figure 1.
Microvasculular brain endothelial cells (MBEC) cultured to confluency on 3μm PET filters were uniformly immunopositive for CD147 (A) and GLUT-1 (B). MBEC were negative for GFAP (an astrocyte-specific intermediate filament protein) (C). (Original magnigifications, x400 (Figs. A, B & C). The cells were stained while adhered to the PET filters.
Astrocytes cultured on the basal surface of PET filters were immunopositive for GFAP (Fig. 2A purple). MBEC cultured on the apical surface were immunopositive for GLUT-1 (Fig. 2A blue). Focal GFAP staining was noted on the apical surface of the filters (below the endothelial monolayer) and was thought to represent astrocyte foot processes extending from the basal surface. This was confirmed by electron microscopy (Fig. 2B). In areas where the two cell types met, electron dense areas consistent with tight junctions were present (Fig 2C and arrows in Fig 2B). Processes extending through the pores of the filters to make contact with the endothelial cells on the upper surface of the filter were confirmed to be GFAP-immunopositive astrocytes by dual color confocal microscopy (Fig. 2D). By confocal microscopy astrocyte foot processes were commonly observed to extend across the apical surface of the filter. As MBEC are cultured on this surface before astrocytes are added, this further illustrates the close association between MBEC and astrocytes that occur in this model. Neither confocal microscopy nor transmission electron microscopy demonstrated astrocyte processes extending between endothelial cells.
Figure 2.
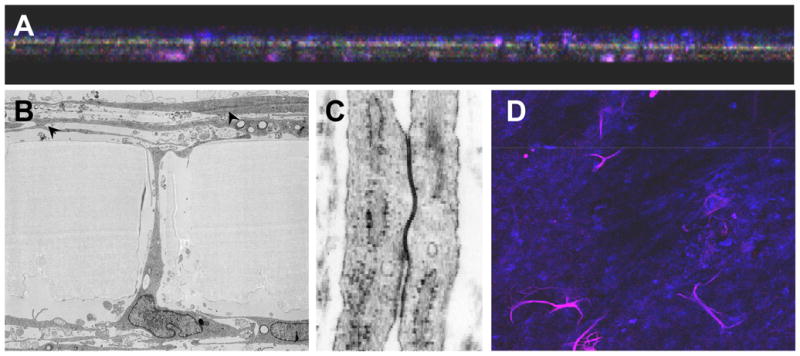
Cross section (x-z) view of the filters confirmed that microvascular brain endothelial cells (MBEC) were present on the upper surface of the filters (A, blue) and astrocytes predominately on the lower surface (A, purple). Focal GFAP staining was present on the apical surface of the filters, but below the endothelial monolayer (original magnification x200). Astrocyte processes were never observed to cross the endothelial monolayer. By low power transmission electron microscopy (x 8,500), astrocyte processes were observed to cross the filter within the pores (B). Electron dense areas, consistent with tight junction complexes between MBECs were noted (B, arrowheads, C). The morphology of the junctional complex are seen more clearly at higher magnification (x80,000) (C). Many of the astrocyte processes (GFAP immunopositive, purple) that crossed the filter were observed to be highly branched by confocal microscopy (D). Original magnification × 200).
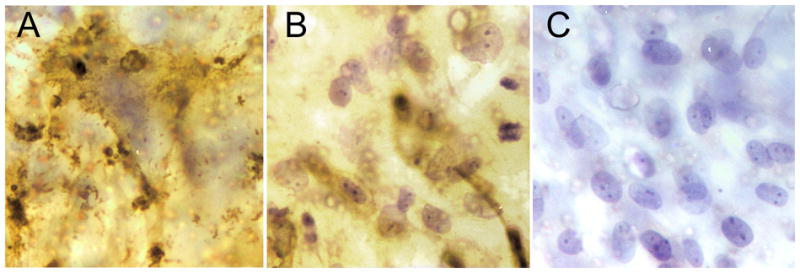
Similar to our prior observation with monolayer cultures of MBEC on gelatin coated coverslips MBEC cultured on the apical surface of 3μm PET filters, with astrocytes on the lower surface, were found to be immunonegative for many markers of inflammation, including VCAM-1 (Fig. 3A) and E-selectin (Fig. 3B).
Figure 3.
Under normal, resting, conditions, the microvascular brain endothelial cells (MBEC) had minimal expression of the inflammatory markers VCAM-1 (A) or E-selectin (B). When the lower well (astrocyte compartment) was exposed to 100 Units/ml TNF-α for 24hrs the levels of both of these markers was increased on the apical surface of MBECs (C, VCAM-1; D, E-selectin). (Original magnification x20).
We have previously shown that MBEC in culture are activated by addition of TNF-α to the culture medium bathing the cells. MBEC have never been reported to be activated by addition of stimulant only to the basolateral medium. We believe that this method of stimulation would be more physiological than activation from the apical surface. Addition of 100 Units/ml TNF-α for 24 hours to the lower well of the BBB model led to an increase in the levels of VCAM-1 and E-selectin (Figs. 3C, D, respectively).
4. Discusssion
Leukocyte-endothelial interactions are thought to be critical at multiple stages in the AIDS neuropathogenesis including neuroinvasion and development of HIV encephalitis. While in vitro models of the human BBB have been developed to examine these issues they have often used umbilical cord-derived cells rather than MBEC. The data obtained from these studies have been used to extrapolate to early events in HIV neuropathogenesis that cannot be studied in vivo. This study was undertaken because there has not been a suitable model for performing BBB experiments in vitro with rhesus macaque tissue. This is important because the rhesus macaque is the primary model for studying early events in AIDS neuropathogenesis.
Although coculturing non-brain, non-microvascular endothelial cells with astrocytes has been described as being capable of inducing certain markers of MBEC on the surface of nonbrain endothelium, e.g. GLUT-1, a glucose transporter specific to endothelial cells derived from brain) (Hurwitz et al 1993), other markers, such as CD147 (an activator of matrix metalloproteinases (MMPs) that, when present on endothelial cells, is only present on MBEC), have not been induced. These MMPs are thought to be vital in the breakdown of the BBB (Ghorpade et al 2001; Pagenstecher et al 1998) a well characterized terminal finding in neuroAIDS in humans (Blumberg et al 1994; Boven et al 2000) and rhesus macaques (Luabeya et al 2000).
The neuropathogenesis of AIDS has been shown to involve altered cytokine expression in the CNS of HIV-infected human and SIV-infected macaques (Orandle et al 2001; Tyor et al 1992; Wesselingh et al 1993). Among the cytokine changes, increased TNF-α may be particularly important due to its ability to activate endothelial cells and mononuclear cells. In addition, TNF-α has been shown to induce the production of the monocyte chemokine MIP-α (Schmidtmayerova et al 1996). Together, these data suggest that TNF-α plays a role in the increased leukocyte recruitment to the CNS. Therefore, it was important to determine if this cytokine could activate our in vitro model of the BBB. Preincubation of the BBB model with 100 U/ml TNF-α for 24 hours led to an activation phenotype of the endothelial cells. Thus, there now exists, a model to investigate very early events in the neuropathogensis of AIDS in vitro and in vivo in the same species.
The rhesus macaque has been used as the premier model of HIV-1 infection over the past decade. This novel in vitro model of macaque BBB will facilitate studies hitherto impossible to perform in this species. In addition to AIDS research, this new model of the macaque BBB will also be valuable for examining experimental allergic encephalitis (EAE) (Kerlero de Rosbo et al 2000), and may be adapted for research into Lyme disease (Roberts et al 1998), cerebral malaria (Davison et al 1998) and possibly West Nile virus associated encephalitis (Pogodina et al 1983).
Acknowledgments
We thank Kristen Toohey and Robin Rodriguez for excellent graphical assistance, and Douglas Pauley for editoral work. This work was supported by public health service grants NS35732, NS30769, NS37654, NS40237, MH61192, RR00164 and RR00168. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award.
References
- Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32:253–67. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Boven LA, Middel J, Verhoef J, De Groot CJ, Nottet HS. Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol. 2000;26:356–60. doi: 10.1046/j.1365-2990.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Murphy P, Richardson LC. Procedure for embedding in situ selected cells cultured in vitro. J Cell Biol. 1967;35:279–83. doi: 10.1083/jcb.35.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–9. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- Davison BB, Cogswell FB, Baskin GB, Falkenstein KP, Henson EW, et al. Plasmodium coatneyi in the rhesus monkey (Macaca mulatta) as a model of malaria in pregnancy. Am J Trop Med Hyg. 1998;59:189–201. doi: 10.4269/ajtmh.1998.59.189. [DOI] [PubMed] [Google Scholar]
- Dorovini-Zis K, Prameya R, Bowman PD. Culture and characterization of microvascular endothelial cells derived from human brain. Lab Invest. 1991;64:425–36. [PubMed] [Google Scholar]
- Ghorpade A, Persidskaia R, Suryadevara R, Che M, Liu XJ, et al. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 2001;75:6572–83. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin G, Boussin FD, Croitoru J, Franck-Duchenne M, Le Grand R, et al. Obtention and characterization of primary astrocyte and microglial cultures from adult monkey brains. J Neurosci Res. 1997;49:576–91. doi: 10.1002/(SICI)1097-4547(19970901)49:5<576::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Chen P, Incardona F, Zidovetzki R, Hinton DR. HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. J Neuroimmunol. 1999;94:28–39. doi: 10.1016/s0165-5728(98)00198-2. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood-brain barrier specific proteins by autologous endothelial cells. Brain Res. 1993;625:238–43. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- Janigro D, Strelow L, Grant G, Nelson JA. In vitro blood brain barrier model for HIV-induced CNS disease. NeuroAIDS. 1998;1 [Google Scholar]
- Kerlero de Rosbo N, Brok HP, Bauer J, Kaye JF, t Hart BA, Ben-Nun A. Rhesus monkeys are highly susceptible to experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein: characterisation of immunodominant T-and B-cell epitopes. J Neuroimmunol. 2000;110:83–96. doi: 10.1016/s0165-5728(00)00306-4. [DOI] [PubMed] [Google Scholar]
- Luabeya MK, Dallasta LM, Achim CL, Pauza CD, Hamilton RL. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol Appl Neurobiol. 2000;26:454–62. doi: 10.1046/j.1365-2990.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- MacLean AG, Orandle MS, Alvarez X, Williams KC, Lackner AA. Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells. J Neuroimmunol. 2001;118:223–32. doi: 10.1016/s0165-5728(01)00348-4. [DOI] [PubMed] [Google Scholar]
- Omari KI, Dorovini-Zis K. Expression and function of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) in an in vitro model of the human blood-brain barrier. J Neuroimmunol. 2001;113:129–141. doi: 10.1016/s0165-5728(00)00435-5. [DOI] [PubMed] [Google Scholar]
- Orandle MS, Williams KC, MacLean AG, Westmoreland SV, Lackner AA. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J Virol. 2001;75:4448–52. doi: 10.1128/JVI.75.9.4448-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–41. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997;62:100–6. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Nottet HS, Sasseville VG, Epstein LG, Gendelman HE. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J Neurovirol. 1995;1:229–43. doi: 10.3109/13550289509114019. [DOI] [PubMed] [Google Scholar]
- Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, et al. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75:71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- Roberts ED, Bohm RP, Jr, Lowrie RC, Jr, Habicht G, Katona L, et al. Pathogenesis of Lyme neuroborreliosis in the rhesus monkey: the early disseminated and chronic phases of disease in the peripheral nervous system. J Infect Dis. 1998;178:722–32. doi: 10.1086/515357. [DOI] [PubMed] [Google Scholar]
- Sasseville VG, Newman W, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ. Monocyte adhesion to endothelium in simian immunodeficiency virus- induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, et al. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–67. [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Imahashi T, Kagimoto A. Electron microscopic observation on a case of sideroblastic anemia. J Electron Microsc. 1967;16:283–4. [PubMed] [Google Scholar]
- Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996;93:700–4. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KT, Greig NH. Chemokine receptor mRNA expression at the in vitro blood-brain barrier during HIV infection. Neuroreport. 1999;10:53–6. doi: 10.1097/00001756-199901180-00010. [DOI] [PubMed] [Google Scholar]
- Stanness KA, Neumaier JF, Sexton TJ, Grant GA, Emmi A, et al. A new model of the blood-brain barrier: co-culture of neuronal, endothelial and glial cells under dynamic conditions. Neuroreport. 1999;10:3725–31. doi: 10.1097/00001756-199912160-00001. [DOI] [PubMed] [Google Scholar]
- Strelow LI, Watry DD, Fox HS, Nelson JA. Efficient infection of brain microvascular endothelial cells by an in vivo-selected neuroinvasive SIVmac variant. J Neurovirol. 1998;4:269–80. doi: 10.3109/13550289809114528. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, et al. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, et al. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–42. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–903. [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up- regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–9. [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, et al. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–82. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wilkinson PC. Micropore Filter Methods for Leukocyte Chemotaxis. Methods in Enzymology. 1988;162:38–50. doi: 10.1016/0076-6879(88)62061-1. [DOI] [PubMed] [Google Scholar]
- Wykrzykowska JJ, Rosenzweig M, Veazey RS, Simon MA, Halvorsen K, et al. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J Exp Med. 1998;187:1767–78. doi: 10.1084/jem.187.11.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VWA, JP . Culture of Glial Cels from Human Brain Biopsies. In: Federoff S, editor. Protocols for Neural Cell Culture. St. Louis: The Humana Press Inc; 1992. pp. 81–96. [Google Scholar]
- Zink MC, Spelman JP, Robinson RB, Clements JE. SIV infection of macaques--modeling the progression to AIDS dementia. J Neurovirol. 1998;4:249–59. doi: 10.3109/13550289809114526. [DOI] [PubMed] [Google Scholar]