Abstract
Aims
To present a statistical model for defining interindividual variation in response to morphine and to use this model in a preliminary hypothesis-generating multivariate genetic association study.
Methods
Two hundred and sixty-four cancer patients taking oral morphine were included in a prospective observational study. Pain and morphine side-effect scores were examined using principal components analysis. The resulting principal components were used in an exploratory genetic association study of single nucleotide polymorphisms across the genes coding for the three opioid receptors, OPRM1, OPRK1 and OPRD1. Associations in multivariate models, including potential clinical confounders, were explored.
Results
Two principal components corresponding to residual pain and central side-effects were identified. These components accounted for 42 and 18% of the variability in morphine response, respectively, were independent of each other and only mildly correlated. The genetic and clinical factors associated with these components were markedly different. Multivariate regression modelling, including clinical and genetic factors, accounted for only 12% of variability in residual pain on morphine and 3% of variability in central side-effects.
Conclusions
Although replication is required, this data-driven analysis suggests that pain and central side-effects on morphine may be two separate dimensions of morphine response. Larger study samples are necessary to investigate potential genetic and clinical associations comprehensively.
Keywords: analgesia, morphine, opioid, polymorphism, principal components
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
There is considerable interindividual variation in response to morphine for cancer pain in terms of analgesia and side-effects.
Different studies have used different outcomes and measurement scales to assess this variability.
It is likely that there are a number of clinical and genetic factors underlying this variation, but no definitive factors have been identified to date.
WHAT THIS STUDY ADDS
This is the first study in which multiple aspects of morphine response (including pain and side-effects) are analysed together to identify different elements of response.
Principal components analysis identifies residual pain on morphine and central side-effects as distinct dimensions of morphine response.
Sensitivity to the analgesic effects of morphine and development of morphine-related central side-effects appear to be associated with different clinical and genetic factors.
Introduction
The majority of patients taking morphine for cancer pain achieve good analgesia with minimal side-effects. There is, however, a significant proportion of patients (up to 30%) who experience either inadequate analgesia despite escalating doses and/or intolerable side-effects [1–4].
There is growing interest in the possibility that genetic factors might play a role in the variability of opioid response. Changes at a molecular level in the pharmacokinetic and pharmacodynamic pathways of an opioid may be involved [5].
Association studies in cancer patients on opioids have not as yet yielded any genetic or clinical marker that can be used to explain interindividual variation in response to opioids conclusively or to predict opioid response prospectively. One of the biggest challenges in this area is that there is little consensus with respect to definition of outcome measures in pain and analgesia studies [6]. Most studies have chosen one aspect of pain (often average pain intensity or pain relief) to determine the outcome [7–9]. Some studies have examined the level of pain sensation before and after administering the drug [10, 11]. Others have studied the dose of opioid required by individual patients [7, 8, 12–16]. Two studies have explored side-effects (central side-effects and nausea and vomiting, respectively) in cancer patients on morphine as outcome measures [17, 18]. Previous analysis of some of the patient data included in this study has used ‘morphine responder’ vs. ‘morphine nonresponder’ as the study outcome, a definition based on patients' subjective experience and the observations of the experienced clinical team [3].
The situation is made more complex by the fact that there is no standardized system for cancer pain assessment and classification or indeed choice of pain intensity scale [19–21]. Although the Brief Pain Inventory is a commonly used pain assessment tool, there is no consensus as to which of the five pain intensity variables is most clinically relevant. It is therefore difficult to compare results from different studies and is one of the reasons underlying nonreplication of genetic association studies in this field [22].
In this study, we carried out a two-stage, data-driven examination of interindividual variation in response to morphine. In the first stage, a comprehensive assessment of outcomes measuring morphine response was documented for each patient. Principal components analysis (PCA) was used as a hypothesis-generating technique to identify groups of morphine response outcome variables, called ‘components’, which explain a proportion of the interindividual variability seen in clinical practice. Principal components analysis (or a similar factor analysis approach) has been used previously to examine relationships between different pain scores and thresholds [23, 24] and between symptoms in patients with cancer [25–27]. This is the first time that it has been used to identify clusters of variables defining the underlying dimensions of morphine response. In the second stage of this study, the resulting principal components were used in an exploratory preliminary association study examining the effect of genetic polymorphisms in genes coding for the opioid receptors and other confounding clinical variables on morphine response.
Methods
Subjects
Two hundred and sixty-four white Caucasian patients on oral morphine for cancer pain were recruited to this prospective observational study, which was carried out at the Royal Marsden Hospital in London. Approval for this study was given by the Research and Ethics Committee, and all patients signed a written consent form before entering the study. These patients were under the care of the clinical palliative care team. Prior to recruitment into this study, each patient's morphine dose was titrated according to individual response, until they either achieved adequate analgesia without problematic side-effects or they failed to achieve adequate analgesia despite escalating morphine doses and/or experienced subjectively intolerable side-effects.
Clinical data were collected contemporaneously at the time of recruitment to the study. Pain severity was recorded using the modified Brief Pain Inventory [28]. This pain scoring system has been used in other studies in this area [7, 8]. The following five dimensions of pain were assessed: worst pain, least pain and average pain in the previous 24 h, current pain and percentage pain relief from morphine. For each of these pain modalities, study subjects recorded pain severity on an 11-point numerical rating scale (NRS) of either 0–10 (0 = no pain, 10 = worst pain imaginable) or 0−100 (0 = no pain relief and 100 = 100% or total pain relief). Morphine side-effects (drowsiness, confusion and hallucinations, constipation, dry mouth, myoclonus, itch and nausea) were recorded using the following four point Likert scale: grade 0, ‘not at all’; grade 1, ‘a little’; grade 2, ‘quite a bit’; and grade 3, ‘very much’. This scale is similar to those used in other studies of response to opioids [7, 18, 29, 30]. Demographic data (including age and sex), cancer diagnosis, current morphine dose, length of time on morphine and concomitant medications were documented. For those patients who had died at the time of analysis, the number of days between recruitment to the study and death were recorded. These data on patients who were still alive at the time of analysis were censored at this point. Blood samples were taken for haematological and biochemical analysis and genotyping.
Patients who were on subcutaneous, intramuscular or intravenous morphine or other regular opioids or those with renal impairment (plasma creatinine >1.5 times the upper limit of normal) were not included in this study.
Single nucleotide polymorphism selection and genotyping
DNA was extracted from whole blood samples (9 ml, trisodium citrate or disodium EDTA vacutainers) using a standard salting out method [31]. Single nucleotide polymorphisms (SNPs) were determined using sequence-specific primers and polymerase chain reaction [32, 33]. Seven polymorphisms in OPRM1 (rs621029, rs1799971, rs589046, rs563649, rs9479757, rs2075572 and rs533586), eight in OPRK1 (rs10504151, rs7836120, rs6473799, rs1365098, rs7016778, rs7824175, rs16918875 and rs963549) and five in OPRD1 (rs1042114, rs533123, rs419335, rs2236857 and rs2234918) were studied. The choice of candidate SNPs within each gene was based on location along the gene and the existence of published data for that polymorphism. Polymorphisms in regions most likely to have an impact on gene function were prioritized (promoter region, exons, intron–exon boundaries and 3′ untranslated region). The primer details are shown in Table S1.
Statistical analysis
Baseline patient characteristics were tabulated.
Stage one data analysis: principal components analysis
Principal components analysis is a variable reduction procedure. The purpose of PCA was to facilitate incorporation of a comprehensive number of outcome variables measuring response to morphine (including various pain intensity, pain relief and morphine side-effect scores) into a smaller number of artificial factors, called ‘components’, which would account for much of the variability in morphine response.
Although a formal prestudy power calculation was not performed because the patient data were originally collected for a different study [3], given that the reliability of PCA may be affected by sample size, the adequacy of the sample size and the suitability of the data for PCA were formally tested.
The morphine response outcome variables (subjective pain and side-effect scores) were entered into the PCA modelling as numerical data. Component scores were calculated using the regression method, and rotation (to improve the interpretability of the components) was carried out using an oblique method (direct oblimin). The PCA modelling produces eigenvalues for each component. These represent the variance in the data accounted for by that component. Traditionally, components with eigenvalues >1 are retained, because these account for a greater proportion of variance than is explained by one original variable [34, 35] (p. 649). Factor loadings, presented in the pattern matrix, represent the relationship between the variable and the component, i.e. the substantive importance of each variable to that component. Only factor loadings with an absolute value >0.4 (explaining at least 16% of variance in the component by that variable) were interpreted as being significant [36](p. 638).
Stage two data analysis: clinical and genetic association study
Multivariate linear regression analyses were conducted to examine the association between the clinical and genetic data and principal components, using the individual patient component scores as the outcome variable. The principal components were transformed because they were not normally distributed. Clinical and genetic factors significant at the level of 10% (P < 0.1) on univariate analysis were introduced simultaneously into the multivariate model using a stepwise approach. Candidate clinical variables included age, sex, time on morphine (in days), dose of morphine (in milligrams per 24 h) and time to death (in days), concomitant medications, biochemical and haematological parameters and tumour diagnosis. A co-dominant additive model was used, in which the genotypes were entered into regression analyses as noncategorical data. Only factors with P < 0.05 were retained in the final model. As part of the linear regression analysis, an anova was carried out to determine whether the multivariate model resulted in a significantly good predictor of the outcome variable. A Bonferroni adjustment for multiple testing was applied [37], dependent on the number of factors included in the final modelling.
All genotype frequencies were tested for Hardy–Weinberg equilibrium using χ2 goodness-of-fit tests. Statistical analysis and plots were performed using SPSS (SPSS Inc., Chicago, IL, USA) and GraphPad (GraphPad Prism version 4.02 for Windows; GraphPad Software, San Diego, CA, USA).
Results
Two hundred and sixty-four patients were included in this study. The characteristics of the study population are presented in Table 1.
Table 1.
Patient characteristics for total study population and univariate linear regression results
| Patient characteristic | Median (range) | P value | |
|---|---|---|---|
| Component 1 (residual pain) | Component 2 (central side-effects) | ||
| Age (years) | 60 (19–89) | 0.163 | 0.259 |
| Daily morphine dose [mg (24 h)−1] | 100 (10–1280) | 0.027 | 0.733 |
| White blood cell count (×109 l−1) | 8.5 (0.1–68.1) | 0.786 | 0.172 |
| Serum sodium (mmol l−1) | 136 (120–143) | 0.377 | 0.085 |
| Serum albumin (g l−1) | 30 (11–48) | 0.956 | 0.125 |
| Serum calcium* (mmol l−1) | 2.23 (1.5–2.9) | 0.633 | 0.759 |
| Days to death (days) | 113.5 (5–2590) | 0.985 | 0.032 |
| Days on morphine (days) | 81 (1–4455) | 0.615 | 0.017 |
| n (%) | |||
|---|---|---|---|
| Sex | |||
| Male | 131 (49.6) | 0.432 | 0.558 |
| Female | 133 (50.4) | ||
| Cancer diagnosis | |||
| Gynaecological | 24 (9.1) | 0.023 | 0.439 |
| Lung | 35 (13.3) | 0.546 | 0.598 |
| Breast | 46 (17.4) | 0.164 | 0.552 |
| Urogenital | 24 (9.1) | 0.694 | 0.332 |
| Upper gastrointestinal tract | 26 (9.8) | 0.925 | 0.897 |
| Lower gastrointestinal tract | 20 (7.6) | 0.955 | 0.96 |
| Head and neck | 22 (8.3) | 0.146 | 0.613 |
| Sarcoma | 30 (11.4) | 0.076 | 0.06 |
| Prostate | 17 (6.4) | 0.203 | 0.028 |
| Haematological | 15 (5.7) | 0.042 | 0.231 |
| Skin | 10 (3.8) | 0.066 | 0.414 |
| Concomitant medications | |||
| Antibiotic | 72 (27.3) | 0.607 | 0.775 |
| Anticonvulsant | 53 (20.1) | 0.292 | 0.708 |
| Anticoagulant | 59 (22.3) | 0.777 | 0.062 |
| Antiemetic | 122 (46.2) | 0.016 | 0.145 |
| Aspirin | 25 (9.5) | 0.999 | 0.913 |
| Benzodiazepine | 45 (17) | 0.745 | 0.305 |
| β-Blocker | 20 (7.6) | 0.096 | 0.124 |
| H2-blocker | 14 (5.3) | 0.77 | 0.037 |
| NSAID | 94 (35.6) | 0.416 | 0.458 |
| Paracetamol | 106 (40.2) | 0.227 | 0.707 |
| Proton pump inhibitor | 171 (64.8) | 0.554 | 0.18 |
| SSRI, SNRI antidepressants | 31 (11.7) | 0.153 | 0.019 |
| Steroid | 100 (37.9) | 0.768 | 0.27 |
| Tricyclic antidepressants | 43 (16.3) | 0.251 | 0.419 |
Corrected for albumin. NSAID, non-steroidal anti-inflammatory drug; SNRI, serotonin noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
The overall Kaiser–Meyer–Olkin measure of sampling adequacy was 0.822, and Bartlett's test of sphericity <0.001, which suggested that the sample size was statistically adequate for this type of analysis and that PCA was appropriate for this dataset.
Constipation, dry mouth and itch were excluded from analysis because the individual Kaiser–Meyer–Olkin values were 0.522, 0.554 and 0.534, which are only borderline for sampling adequacy [35](p. 620), [36](p. 642). Myoclonus was excluded from analysis because only 11 subjects experienced this symptom.
Two principal components emerged with eigenvalues >1 (Figure 1 and Table 2). The first component, which carried high loadings (absolute value >0.4) from all the pain intensity scores, explained 42% of the total variance. This component appears to represent residual pain after dose titration on oral morphine. Component 2, accounting for 18% of the total variance, received high loadings from the central side-effects of confusion/hallucinations, drowsiness, bad dreams and nausea. The final pattern matrix (Table 2) demonstrates the loadings of each variable onto each component after rotation.
Figure 1.
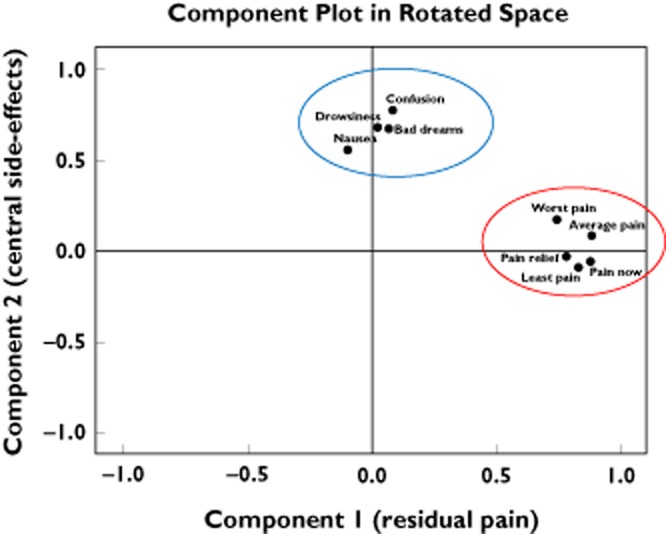
Principal components of morphine response. The co-ordinates of the variables along the axes (components) represent a measure of the strength of the relationship between the variable and the component. Worst pain, average pain, least pain, pain now and pain relief scores correlate highly with component 1 (x-axis); therefore, component 1 appears to represent residual pain on morphine. Confusion, drowsiness, nausea and bad dreams are correlated with component 2 (y-axis); therefore, component 2 appears to represent central side-effects. The variables that are highly correlated with component 1 (residual pain; red ring) have a low correlation with component 2. Likewise, variables that are highly correlated with component 2 (central side-effects; blue ring) have a low correlation with component 1
Table 2.
Pattern matrix showing the loading of each variable onto each component after rotation
| Variable | Component 1 | Component 2 |
|---|---|---|
| Eigenvalue (after rotation) | 3.61 | 2.13 |
| Percentage of variance explained | 41.83 | 17.8 |
| Average pain | 0.885 | 0.085 |
| Pain now | 0.882 | −0.061 |
| Least pain | 0.831 | −0.087 |
| Percentage pain relief | 0.781 | −0.027 |
| Worst pain | 0.744 | 0.173 |
| Confusion* | 0.083 | 0.774 |
| Drowsiness | 0.024 | 0.678 |
| Bad dreams | 0.070 | 0.671 |
| Nausea | −0.099 | 0.554 |
Confusion includes hallucinations. Only factor loadings with an absolute value of >0.4 were interpreted [36](p. 638).
Component 1 (residual pain) and component 2 (central side-effects) were only mildly correlated, with Spearman correlation coefficient rs = 0.27 (95% confidence interval 0.15–0.38).
A higher component 1 score (residual pain) was associated with a worse outcome in terms of pain control on morphine, as evidenced by higher average, least, worst and pain now scores and lower pain relief scores. Likewise, a higher component 2 score (central side-effect) score was associated with a higher individual symptom severity score. Confusion and hallucinations were associated with the highest loading of individual factors onto component 2 (central side-effects).
All genotypes were in Hardy–Weinberg equilibrium. Genotype frequency data are detailed in Table 3. The clinical and genetic factors associated with variability in residual pain and central side-effects on univariate analysis after titration on oral morphine are shown in Tables 1 and 3.
Table 3.
Genotype frequencies and univariate linear regression results
| Gene/SNP | Genotype [% (n)] | P value | |||
|---|---|---|---|---|---|
| AA | Aa | aa | Component 1 (residual pain) | Component 2 (central side-effects) | |
| OPRM1 | |||||
| rs6912029 | 0.91 (226) | 0.09 (23) | 0.0 (0) | 0.414 | 0.737 |
| rs1799971 | 0.74 (183) | 0.23 (58) | 0.03 (8) | 0.921 | 0.134 |
| rs589046 | 0.53 (128) | 0.41 (100) | 0.06 (15) | 0.338 | 0.299 |
| rs563649 | 0.81 (197) | 0.17 (41) | 0.02 (5) | 0.629 | 0.267 |
| rs9479757 | 0.01 (2) | 0.2 (49) | 0.79 (198) | 0.011 | 0.412 |
| rs2075572 | 0.34 (83) | 0.48 (118) | 0.19 (47) | 0.963 | 0.007 |
| rs533586 | 0.46 (112) | 0.43 (104) | 0.11 (27) | 0.47 | 0.02 |
| OPRK1 | |||||
| rs10504151 | 0.8 (196) | 0.18 (43) | 0.02 (5) | 0.628 | 0.123 |
| rs7836120 | 0.68 (167) | 0.28 (67) | 0.04 (10) | 0.299 | 0.977 |
| rs6473799 | 0.6 (146) | 0.36 (87) | 0.04 (11) | 0.39 | 0.179 |
| rs1365098 | 0.48 (116) | 0.42 (104) | 0.1 (24) | 0.209 | 0.499 |
| rs7016778 | 0.73 (178) | 0.26 (64) | 0.01 (2) | 0.254 | 0.429 |
| rs7824175 | 0.8 (196) | 0.18 (44) | 0.02 (4) | 0.012 | 0.487 |
| rs16918875 | 0.844 (206) | 0.15 (37) | 0.004 (1) | 0.319 | 0.307 |
| rs963549 | 0.67 (164) | 0.29 (71) | 0.04 (9) | 0.056 | 0.69 |
| OPRD1 | |||||
| rs1042114 | 0.72 (176) | 0.26 (63) | 0.02 (4) | 0.687 | 0.668 |
| rs533123 | 0.63 (152) | 0.32 (78) | 0.05 (13) | 0.573 | 0.798 |
| rs419335 | 0.5 (121) | 0.42 (101) | 0.08 (21) | 0.141 | 0.259 |
| rs2236857 | 0.58 (142) | 0.35 (86) | 0.06 (15) | 0.35 | 0.249 |
| rs2234918 | 0.33 (82) | 0.49 (118) | 0.18 (43) | 0.89 | 0.861 |
DNA was available for the following genes/SNPs: OPRM1 rs6912029, rs1799971, rs9479757 and rs9479757, n = 249; OPRM1 rs589046, rs563649 and rs533586, n = 243; OPRK1, n = 244; and OPRD1, n = 243. SNP, single nucleotide polymorphism.
Using multivariate linear regression, the following five factors were retained as independent predictors of residual pain on morphine: OPRK1 rs7824175, use of a β-blocker, taking an antiemetic, having a diagnosis of a haematological cancer and the total daily morphine dose (Table 4). Being on a β-blocker was found to be associated with higher residual pain, whereas being on an antiemetic was associated with lower residual pain. Daily morphine dose contributed only slightly to the model. The five-factor multivariate model accounted for 11.8% of variability in residual pain on morphine.
Table 4.
Clinical and genetic factors predictive of residual pain and central side-effects on morphine, stepwise linear regression (additive model)
| B (95% confidence interval) | β | P value | |
|---|---|---|---|
| Model 1: residual pain anovaF = 6.2, P = 2 × 10−5, R2 = 11.8 | |||
| Antiemetic | −0.126 (−0.203 to −0.049) | −0.203 | 0.001 |
| OPRK1 rs7824175 | −0.118 (−0.202 to −0.034) | −0.17 | 0.006 |
| β-Blocker | 0.207 (0.058–0.355) | 0.17 | 0.007 |
| Haematological cancer | 0.200 (0.027–0.374) | 0.14 | 0.023 |
| Morphine dose [mg (24 h)−1] | <0.001 | 0.12 | 0.045 |
| Model 2: central side-effects anovaF = 6.99, P = 0.009, R2 = 3 | |||
| OPRM1 rs2075572 | −0.04 (−0.07 to −0.01) | −0.174 | 0.009 |
Transformed components 1 and 2 were used as dependent variables in models 1 and 2, respectively. Abbreviations are as follows: B is the regression coefficient, which represents the change in the dependent variable associated with a unit change in the predictor variable; β is the standardized regression coefficient; and R2 is the proportion of the variability in the outcome variable that can be explained by its relationship with the predictor variables. Unadjusted α = 0.05; and Bonferroni-adjusted α = 0.005.
Only one SNP (OPRM1 rs2075572) was retained in the multivariate model as an independent predictor of developing central side-effects on morphine (Table 4). This model accounted for only 3% of variability in central side-effects of morphine.
Ten predictor variables were significant at the level of 10% (P < 0.1) on univariate analysis and were entered into the multivariate models; therefore, the Bonferroni corrected α was 0.005. When the Bonferroni adjustment for multiple testing was applied, taking an antiemetic medication remained associated with residual pain, with taking a β-blocker and OPRK1 rs7824175 being just outside significance. No factors were retained as predictors of central side-effects.
Discussion
The results from this study suggest that response to morphine is not a homogeneously defined outcome. The finding that residual pain and central side-effect scores loaded onto separate components and the lack of sizeable correlation between these two components indicates that there are two different dimensions of morphine response. This is supported by our regression analyses, which suggest that the genetic and clinical factors associated with these two morphine response outcomes may be markedly different. The independence of these components may be important for subsequent improvement in therapy.
Pain researchers may intuitively consider pain and side-effects to be different outcome phenotypes in terms of response to opioids. However, until now, this has never been rigorously explored. In this study, a statistical model of response to morphine for cancer pain was defined using principal components analysis. Instead of analysing each pain or side-effect score separately, or instead of choosing one single dimension of pain intensity to study, in this study all pain and side-effect scores were included in the PCA modelling. Principal components analysis has been used in validation studies of the Brief Pain Inventory, and in most of these studies the pain intensity scores loaded highly onto the same component [38, 39], consistent with our findings.
In this study, instead of concentrating on single SNPs, as has been done in other studies, we used multivariate regression to explore both the clinical and the genetic factors associated with variability in clinical outcome on morphine. The two independent principal components (residual pain and central side-effects) accounted for a significant proportion of variance in morphine response, 42 and 18%, respectively. However, the proportion of variability in the PCA-defined clinical phenotypes (components) explained by clinical and genetic factors in the regression models was much lower (12 and 3% for residual pain and central side-effects, respectively). Furthermore, many of the clinical and genetic predictors of residual pain and central side-effects did not remain significant after conservative correction for multiple testing. These association analyses can therefore be considered only exploratory in nature, and further scrutiny of these results is merely theoretical.
Morphine acts on opioid receptors; therefore, the genes coding for the opioid receptors were natural candidates for an exploratory genetic association study. Although morphine, like most other opioids, acts primarily through μ opioid receptors, polymorphisms in the δ and κ opioid receptors were also examined because: (i) morphine also binds, albeit with a much weaker affinity, to δ and κ opioid receptors [40, 41]; (ii) opioid receptors are thought to interact with each other and form heterodimers with altered function, potentially affecting opioid response [42–47]; and (iii) μ, δ and κ opioid receptors are all involved in nociception. Interindividual variation in response to morphine is the result of a complex interplay between variability in nociception as well as sensitivity to the drug itself.
In humans, there is much less data regarding the influence of polymorphisms in OPRD1 and OPRK1 than OPRM1 in pain sensitivity or opioid response. Previous studies have focused primarily on one polymorphism, OPRM1 rs1799971 (A118G) [7, 11, 13, 14, 48]. The clinical relevance of OPRM1 A118G, however, is difficult to interpret. Despite some positive associations, a number of studies demonstrate a negative or weak association between this SNP and opioid requirements [14, 49, 50]. There is also controversy about the functional relevance of this polymorphism [51–53]. In our study, OPRM1 rs1799971 (A118G) was not retained in the model of either residual pain or central side-effects. The genetic factor associated with residual pain was an SNP in OPRK1 (rs7824175). Association between this SNP and opioid response has not been published previously.
Pain is one of the most complex measurable traits [54], and it is reasonable to propose that the response to morphine for cancer pain must be equally complex. Response to morphine in terms of pain control and side-effects is likely to be determined by many factors, including the underlying pain sensitivity of the patient, along with the nature and extent of the painful process, concomitant medications, genetic and other clinical and environmental factors. The wide range of clinical, drug and laboratory data collected from each patient in this study allowed investigation of other non-opioid factors that may influence response to morphine. In this study, genetic and clinical factors, such as concomitant medications and tumour diagnosis, were retained as independent predictors of morphine response. Taking an antiemetic medication was associated with less residual pain on morphine, even after correction for multiple testing. This is clinically logical, because some antiemetics are known to have analgesic properties [55]. Furthermore, being on an antiemetic may also facilitate improved pain control because it may allow adequate dose escalation through avoidance of dose-limiting nausea. Being on a β-blocker was associated with higher residual pain scores on morphine. Although this factor resided just outside statistical significance after correction for multiple testing, there are documented molecular mechanisms that may support the biological plausibility of this finding. There have been previous reports of interaction between the adrenergic and opioidergic systems in terms of morphine response, tolerance and dependence [56, 57]. Animal data suggest specific molecular interactions and even dimerization between β-adrenergic and κ opioid receptors [58–60]; therefore, it is conceivable that drugs which act on adrenergic receptors may alter morphine response in association with genetic variation in OPRK1, as suggested by our model. Only a very low proportion of variability in central side-effects was explained by the multivariate regression analysis. Central side-effects were predicted by a single SNP, OPRM1 rs2075572, a finding which was nonsignificant after correction for multiple testing. Haplotypes containing OPRM1 rs2075572 have been found to be associated with variability in experimental pain sensitivity [61], but this SNP has not previously been associated with response to opioids. Central side-effects as a clinical outcome may be confounded by phenotypic variability (i.e. variability in onset/severity of symptoms), which is not captured by the study data. It is also likely that there are many other clinical factors that play a role in morphine response variability. For example, there is documented association between blood pressure, psychological distress and sleep and pain sensitivity and response to opioids [21, 62, 63].These factors were not measured as part of this study. A prospective follow-up study in which data are collected before and after patients were started on morphine would add further information, particularly about factors such as pain intensity at initiation of morphine therapy, the development of morphine tolerance and side-effects, the fact that cancer pain is likely to change in location, character and severity with time and the context-dependent nature of pain perception. Furthermore, although the number of SNPs included for analysis in this study exceeds other studies in this area, only a limited number of SNPs was genotyped from each gene. Likewise, there are undoubtedly other genes that influence morphine response, including COMT, MDR-1 and UDR2B7. There have been some studies exploring the association of single SNPs or two-SNP interactions in these genes on pain perception and analgesia [8, 11, 64, 65], but these have not yet been included in large multivariate studies.
The findings presented in this paper are based on data-driven analyses rather than a priori hypotheses. One of the limitations of using any data reduction or multivariate cluster analysis is that the components or clusters are derived from statistical patterns and structures, rather than necessarily being underpinned by the same genetic architecture or biological mechanisms. To date, however, our understanding of the biological and genetic mechanisms that underlie the analgesic and toxic side-effects of opioids is sparse; therefore, the use of such statistical techniques to extract trends and patterns from multiple measures of morphine response may identify phenotype subtypes or endophenotypes, which may in turn assist in understanding the heterogeneity in this complex trait. The use of endophenotypes and phenotype subtypes has proved useful in areas such as psychiatry and respiratory disease [66, 67]. Before application in clinical practice, however, such phenotype subtypes need to be replicated and, in terms of genetics, should be shown to have common genetic risk factors, for example, in twin studies [66].
A further limitation of this study is that the sample size is relatively small compared with other genetic association studies in different fields. Genetic variability is likely to be associated with small or modest effect sizes in complex traits such as pain and analgesic response [68]. Therefore, although the sample size was adequate to carry out PCA, a larger sample size is probably required to identify the smaller genetic effect sizes [69]. In addition, these findings have not yet been replicated. Thus, the regression modelling must be considered preliminary and hypothesis generating. However, in terms of correcting for multiple testing, the use of PCA component scores in the genetic association study does reduce the multiplicity of analyses that would be necessary if each score was to be examined separately, whilst retaining as much clinical information as possible. Additionally, although the findings presented here require testing in larger, prospective studies, biologically sound hypotheses may be proposed to explain the associations presented in the modelling.
Conclusion
Residual pain and side-effects on morphine appear to be distinct dimensions of response to morphine. This hypothesis is supported by the fact that residual pain and central side-effect scores load onto separate components in PCA, are only weakly correlated and may be associated with different genetic and clinical predictors.
The data presented are exploratory and serve to present a method of defining clinical outcomes and approaching genetic analyses in studies of response to morphine. Future work requires replication and confirmation of the PCA modelling. It is likely that in order to carry out a study of the size required to take into account multiple testing and with the power to identify small genetic effect sizes, collaborations among research groups is needed. We hope that this paper might generate interest in carrying out such large studies, in which these factors may be considered in the study design.
Acknowledgments
This study was supported by money from the Asmarley Trust fund and the Royal Marsden Palliative Care Research fund.
Competing Interests
There are no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1
Primer sequences used for genotyping
References
- 1.Riley J, Ross JR, Rutter D, Shah S, Gwilliam B, Wells AU, Welsh K. A retrospective study of the association between haematological and biochemical parameters and morphine intolerance in patients with cancer pain. Palliat Med. 2004;18:19–24. doi: 10.1191/0269216304pm856oa. [DOI] [PubMed] [Google Scholar]
- 2.Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, Mercadante S, Pasternak G, Ventafridda V. Strategies to manage the adverse effects of oral morphine: an evidence-based report. [Review] [172 refs] J Clin Oncol. 2001;19:2542–2554. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 3.Riley J, Ross JR, Rutter D, Wells AU, Goller K, Du BR, Welsh K. No pain relief from morphine? Individual variation in sensitivity to morphine and the need to switch to an alternative opioid in cancer patients. Support Care Cancer. 2006;14:56–64. doi: 10.1007/s00520-005-0843-2. [DOI] [PubMed] [Google Scholar]
- 4.Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25:2133–2150. doi: 10.1185/03007990903120158. [DOI] [PubMed] [Google Scholar]
- 5.Kasai S, Hayashida M, Sora I, Ikeda K. Candidate gene polymorphisms predicting individual sensitivity to opioids. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:269–281. doi: 10.1007/s00210-007-0205-3. [DOI] [PubMed] [Google Scholar]
- 6.Turk DC, Dworkin RH, Burke LB, Gershon R, Rothman M, Scott J, Allen RR, Atkinson JH, Chandler J, Cleeland C, Cowan P, Dimitrova R, Dionne R, Farrar JT, Haythornthwaite JA, Hertz S, Jadad AR, Jensen MP, Kellstein D, Kerns RD, Manning DC, Martin S, Max MB, McDermott MP, McGrath P, Moulin DE, Nurmikko T, Quessy S, Raja S, Rappaport BA, Rauschkolb C, Robinson JP, Royal MA, Simon L, Stauffer JW, Stucki G, Tollett J, von Stein T, Wallace MS, Wernicke J, White RE, Williams AC, Witter J, Wyrwich KW. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125:208–215. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Klepstad P, Rakvag TT, Kaasa S, Holthe M, Dale O, Borchgrevink PC, Baar C, Vikan T, Krokan HE, Skorpen F. The 118 A > G polymorphism in the human micro-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48:1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 8.Rakvag TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, Krokan HE, Skorpen F. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain. 2005;116:73–78. doi: 10.1016/j.pain.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Galvan A, Skorpen F, Klepstad P, Knudsen AK, Fladvad T, Falvella FS, Pigni A, Brunelli C, Caraceni A, Kaasa S, Dragani TA. Multiple Loci modulate opioid therapy response for cancer pain. Clin Cancer Res. 2011;17:4581–4587. doi: 10.1158/1078-0432.CCR-10-3028. [DOI] [PubMed] [Google Scholar]
- 10.Romberg RR, Olofsen E, Bijl H, Taschner PE, Teppema LJ, Sarton EY, van Kleef SW, Dahan A. Polymorphism of mu-opioid receptor gene (OPRM1:c.118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 12.Landau R, Kern C, Columb MO, Smiley RM, Blouin JL. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14. doi: 10.1016/j.pain.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, Lee TH, Concejero A, Hsu CJ. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 14.Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Gibby CC, Shete S, Rakvag T, Bhat SV, Skorpen F, Bruera E, Kaasa S, Klepstad P. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130:25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klepstad P, Fladvad T, Skorpen F, Bjordal K, Caraceni A, Dale O, Davies A, Kloke M, Lundstrom S, Maltoni M, Radbruch L, Sabatowski R, Sigurdardottir V, Strasser F, Fayers PM, Kaasa S. Influence from genetic variability on opioid use for cancer pain: a European genetic association study of 2294 cancer pain patients. Pain. 2011;152:1139–1145. doi: 10.1016/j.pain.2011.01.040. May. [DOI] [PubMed] [Google Scholar]
- 17.Ross JR, Riley J, Taegetmeyer AB, Sato H, Gretton S, du Bois RM, Welsh KI. Genetic variation and response to morphine in cancer patients: catechol-o-methyltransferase and multidrug resistance-1 gene polymorphisms are associated with central side effects. Cancer. 2008;112:1390–1403. doi: 10.1002/cncr.23292. March 15. [DOI] [PubMed] [Google Scholar]
- 18.Laugsand EA, Fladvad T, Skorpen F, Maltoni M, Kaasa S, Fayers P, Klepstad P. Clinical and genetic factors associated with nausea and vomiting in cancer patients receiving opioids. Eur J Cancer. 2011;47:1682–1691. doi: 10.1016/j.ejca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Hjermstad MJ, Gibbins J, Haugen DF, Caraceni A, Loge JH, Kaasa S. Pain assessment tools in palliative care: an urgent need for consensus. Palliat Med. 2008;22:895–903. doi: 10.1177/0269216308095701. [DOI] [PubMed] [Google Scholar]
- 20.Hjermstad MJ, Fainsinger R, Kaasa S. Assessment and classification of cancer pain. Curr Opin Support Palliat Care. 2009;3:24–30. doi: 10.1097/SPC.0b013e3283260644. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen AK, Brunelli C, Kaasa S, Apolone G, Corli O, Montanari M, Fainsinger R, Aass N, Fayers P, Caraceni A, Klepstad P. Which variables are associated with pain intensity and treatment response in advanced cancer patients? – Implications for a future classification system for cancer pain. Eur J Pain. 2011;15:320–327. doi: 10.1016/j.ejpain.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF, Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 23.Neddermeyer TJ, Fluhr K, Lotsch J. Principle components analysis of pain thresholds to thermal, electrical, and mechanical stimuli suggests a predominant common source of variance. Pain. 2008;138:286–291. doi: 10.1016/j.pain.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Ger LP, Ho ST, Sun WZ, Wang MS, Cleeland CS. Validation of the Brief Pain Inventory in a Taiwanese population. J Pain Symptom Manage. 1999;18:316–322. doi: 10.1016/s0885-3924(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 25.Fan G, Hadi S, Chow E. Symptom clusters in patients with advanced-stage cancer referred for palliative radiation therapy in an outpatient setting. Support Cancer Ther. 2007;4:157–162. doi: 10.3816/SCT.2007.n.010. [DOI] [PubMed] [Google Scholar]
- 26.Skerman HM, Yates PM, Battistutta D. Multivariate methods to identify cancer-related symptom clusters. Res Nurs Health. 2009;32:345–360. doi: 10.1002/nur.20323. [DOI] [PubMed] [Google Scholar]
- 27.Chen E, Nguyen J, Khan L, Zhang L, Cramarossa G, Tsao M, Danjoux C, Barnes E, Sahgal A, Holden L, Jon F, Chow E. Symptom clusters in patients with advanced cancer: a reanalysis comparing different statistical methods. J Pain Symptom Manage. 2012;44:23–32. doi: 10.1016/j.jpainsymman.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 29.Rakvag TT, Ross JR, Sato H, Skorpen F, Kaasa S, Klepstad P. Genetic variation in the catechol-O-methyltransferase (COMT) gene and morphine requirements in cancer patients with pain. Mol Pain. 2008;4:64–75. doi: 10.1186/1744-8069-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercadante S, Ferrera P, Villari P, Casuccio A, Intravaia G, Mangione S. Frequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unit. J Pain Symptom Manage. 2009;37:632–641. doi: 10.1016/j.jpainsymman.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Salazar LA, Hirata MH, Cavalli SA, Machado MO, Hirata RD. Optimized procedure for DNA isolation from fresh and cryopreserved clotted human blood useful in clinical molecular testing. Clin Chem. 1998;44(8 Pt 1):1748–1750. [PubMed] [Google Scholar]
- 32.Bunce M, O'Neill CM, Barnardo M, Krausa P, Browning MJ, Morris PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilising sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 33.Welsh K, Bunce M. Molecular typing for the MHC with PCR-SSP. Rev Immunogenet. 1999;1:157–176. [PubMed] [Google Scholar]
- 34.Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20:141–151. [Google Scholar]
- 35.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th edn. Upper Saddle River, NJ: Pearson Education Inc; 2012. p. 620. 649. [Google Scholar]
- 36.Field A. Discovering Statistics Using SPSS. 2nd edn. London: SAGE Publishers LtD; 2005. p. 638.p. 642. [Google Scholar]
- 37.Mundform DJ, Perrett JJ, Schaffer J, Piccone A, Roozeboom M. Bonferroni adjustments in tests for regression coefficients. Multiple Linear Regression Viewpoints. 2006;32:1–6. [Google Scholar]
- 38.Klepstad P, Loge JH, Borchgrevink PC, Mendoza TR, Cleeland CS, Kaasa S. The Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manage. 2002;24:517–525. doi: 10.1016/s0885-3924(02)00526-2. [DOI] [PubMed] [Google Scholar]
- 39.Caraceni A, Mendoza TR, Mencaglia E, Baratella C, Edwards K, Forjaz MJ, Martini C, Serlin RC, De Conno F, Cleeland CS. A validation study of an Italian version of the brief pain inventory (Breve Questionario per la Valutazione del Dolore) Pain. 1996;65:87–92. doi: 10.1016/0304-3959(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 40.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 41.Satoh M, Minami M. Molecular pharmacology of the opioid receptors. Pharmacol Ther. 1995;68:343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 42.Khotib J, Narita M, Suzuki M, Yajima Y, Suzuki T. Functional interaction among opioid receptor types: up-regulation of mu- and delta-opioid receptor functions after repeated stimulation of kappa-opioid receptors. Neuropharmacology. 2004;46:531–540. doi: 10.1016/j.neuropharm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Rozenfeld R, bul-Husn NS, Gomez I, Devi LA. An emerging role for the delta opioid receptor in the regulation of mu opioid receptor function. ScientificWorldJournal. 2007;7:64–73. doi: 10.1100/tsw.2007.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park PS, Palczewski K. Diversifying the repertoire of G protein-coupled receptors through oligomerization. Proc Natl Acad Sci U S A. 2005;102:8793–8794. doi: 10.1073/pnas.0504016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lotsch J, Skarke C, Grosch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12:3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Janicki PK, Schuler G, Francis D, Bohr A, Gordin V, Jarzembowski T, Ruiz-Velasco V, Mets B. A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain. Anesth Analg. 2006;103:1011–1017. doi: 10.1213/01.ane.0000231634.20341.88. [DOI] [PubMed] [Google Scholar]
- 50.Coulbault L, Beaussier M, Verstuyft C, Weickmans H, Dubert L, Tregouet D, Desco C, Parc Y, Lienhart A, Jaillon P, Becquemont L. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79:316–324. doi: 10.1016/j.clpt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroslak T, LaForge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 53.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 54.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Patt RB, Proper G, Reddy S. The neuroleptics as adjuvant analgesics. J Pain Symptom Manage. 1994;9:446–453. doi: 10.1016/0885-3924(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, He J, Chen YM, Wang JH, Ma YY. Morphine and propranolol co-administration impair consolidation of Y-maze spatial recognition memory. Brain Res. 2008;1230:150–157. doi: 10.1016/j.brainres.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 57.Cecchi M, Capriles N, Watson SJ, Akil H. Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- 58.Yu XC, Wang HX, Zhang WM, Wong TM. Cross-talk between cardiac kappa-opioid and beta-adrenergic receptors in developing hypertensive rats. J Mol Cell Cardiol. 1999;31:597–605. doi: 10.1006/jmcc.1998.0896. [DOI] [PubMed] [Google Scholar]
- 59.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan J, Yu XC, Fung ML, Wong TM. Attenuated ‘cross talk’ between kappa-opioid receptors and beta-adrenoceptors in the heart of chronically hypoxic rats. Pflugers Arch. 2002;444:126–132. doi: 10.1007/s00424-002-0814-0. [DOI] [PubMed] [Google Scholar]
- 61.Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, Tchivileva IE, Belfer I, Mishra B, Kiselycznyk C, Wallace MR, Staud R, Spiridonov NA, Max MB, Goldman D, Fillingim RB, Maixner W, Diatchenko L. Expansion of the human {micro}-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18:1037–1051. doi: 10.1093/hmg/ddn439. March 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG. Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science. 1979;205:1299–1301. doi: 10.1126/science.472749. [DOI] [PubMed] [Google Scholar]
- 63.McCubbin JA, Helfer SG, Switzer FS, III, Galloway C, Griffith WV. Opioid analgesia in persons at risk for hypertension. Psychosom Med. 2006;68:116–120. doi: 10.1097/01.psy.0000195742.24850.79. [DOI] [PubMed] [Google Scholar]
- 64.Holthe M, Klepstad P, Zahlsen K, Borchgrevink PC, Hagen L, Dale O, Kaasa S, Krokan HE, Skorpen F. Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy. Eur J Clin Pharmacol. 2002;58:353–356. doi: 10.1007/s00228-002-0490-1. [DOI] [PubMed] [Google Scholar]
- 65.Holthe M, Rakvag TN, Klepstad P, Idle JR, Kaasa S, Krokan HE, Skorpen F. Sequence variations in the UDP-glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J. 2003;3:17–26. doi: 10.1038/sj.tpj.6500139. [DOI] [PubMed] [Google Scholar]
- 66.Walters JT, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- 67.Roy K, Smith J, Kolsum U, Borrill Z, Vestbo J, Singh D. COPD phenotype description using principal components analysis. Respir Res. 2009;10:41–48. doi: 10.1186/1465-9921-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 69.Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet. 2005;6:109–118. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


