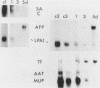
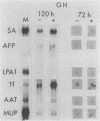
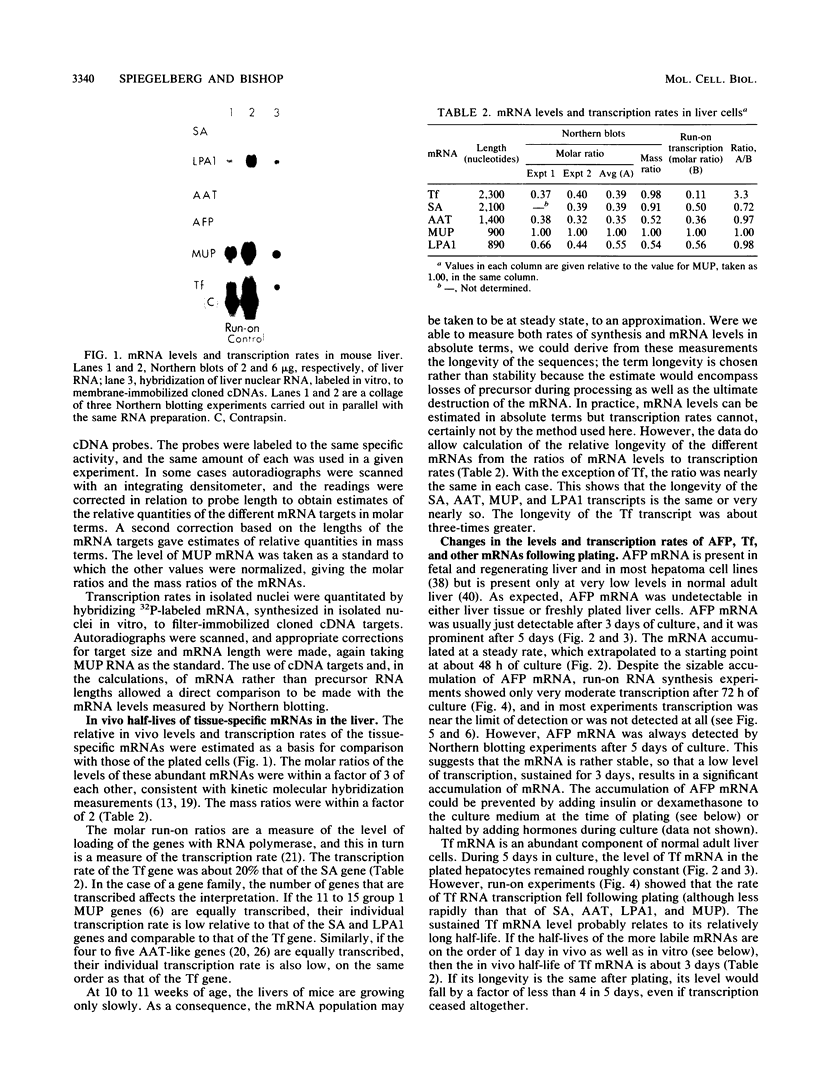
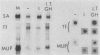Abstract
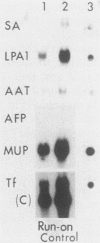
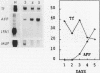
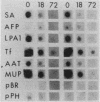
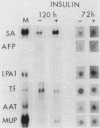
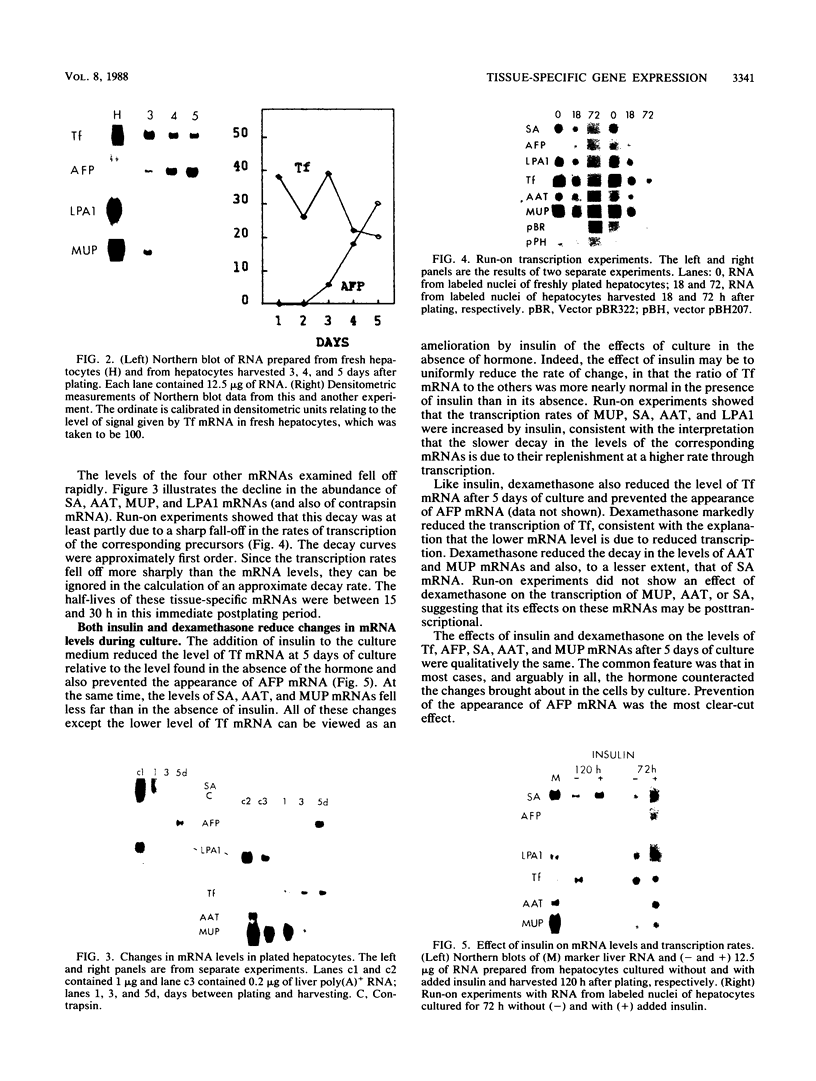
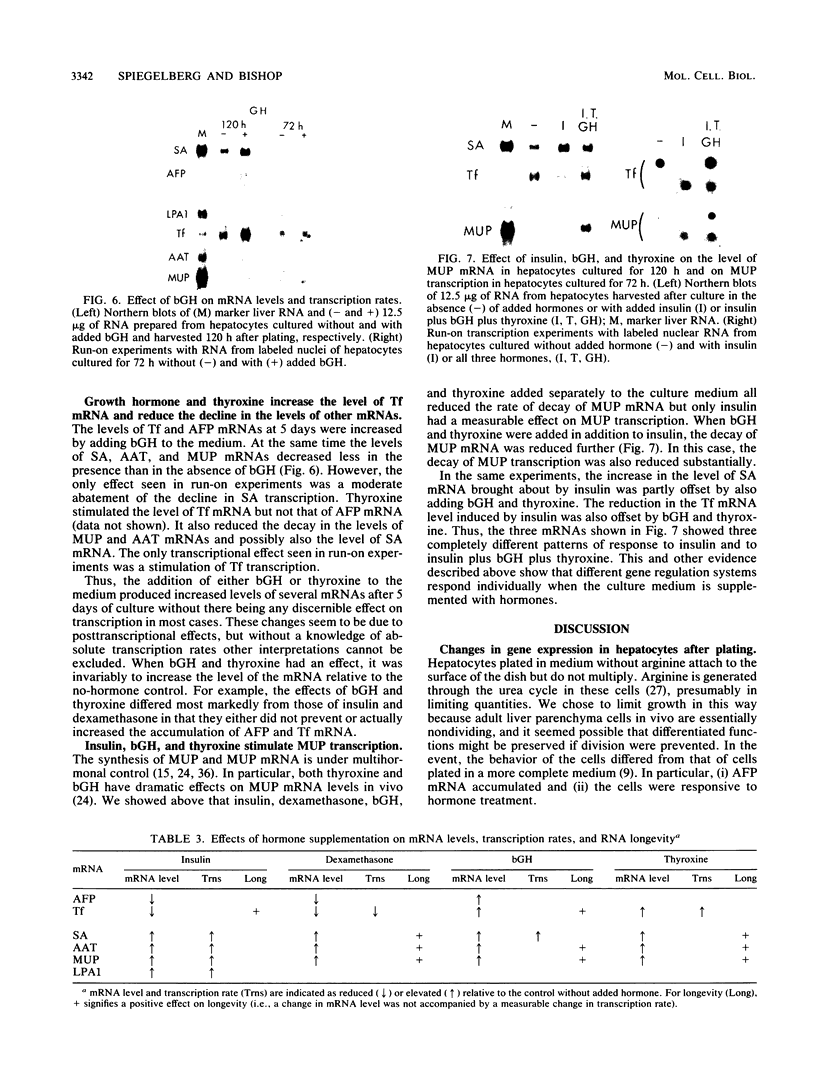
Culture conditions which maintain hepatocytes in their in vivo state are not known. This hampers the study of liver gene expression and of direct responses of liver genes to hormonal stimulation. We argued that hepatocytes that were unable to divide might retain in vivo characteristics. We therefore plated mouse (BALB/c) hepatocytes on plastic dishes in medium lacking arginine and measured the levels and transcription rates of six tissue-specific mRNAs over a period of days. Alpha-fetoprotein mRNA began to accumulate at about 48 h of culture, and transcription could sometimes be detected after 72 h. The levels and transcription rates of four mRNAs (albumin, alpha-1-antitrypsin, apolipoprotein A1, and major urinary protein [MUP]) fell sharply. The rate of transcription of transferrin mRNA fell less rapidly, and its level remained high, partly due to its longer half-life. The overall pattern of gene expression in the plated cells did not exactly parallel that of either fetal or regenerating liver. The hepatocytes remained responsive to hormonal stimulation. Insulin and dexamethasone each tended to counteract changes in mRNA levels, for example, preventing the accumulation of alpha-fetoprotein mRNA. The effects of insulin were primarily due to changes in transcription rates. Bovine growth hormone and thyroxine elevated the levels of most of the mRNAs. Many of the effects of these hormones, when added singly, could not be ascribed to changes in transcription. The level of MUP mRNA was strongly affected by added hormones. The mRNA level at 5 days was increased by added insulin, dexamethasone, growth hormone, and thyroxine. In the presence of these three hormones, the decay in the transcription rate of the MUP genes was reduced about 10-fold. We conclude that hepatocytes plated under these nongrowing conditions can provide insights into the hormonal responsiveness of tissue-specific genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth R. K., Gross K. W., Gremke L. C., Hastie N. D. Developmentally regulated mRNAs in mouse liver. Proc Natl Acad Sci U S A. 1982 Jan;79(2):500–504. doi: 10.1073/pnas.79.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Clark A. J., Clissold P. M., Hainey S., Francke U. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1982;1(5):615–620. doi: 10.1002/j.1460-2075.1982.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Davies J. A. Plasmid cloning vectors that can be nicked at a unique site. Mol Gen Genet. 1980;179(3):573–580. doi: 10.1007/BF00271747. [DOI] [PubMed] [Google Scholar]
- Borek C. Neoplastic transformation in vitro of a clone of adult liver epithelial cells into differentiated hepatoma-like cells under conditions of nutritional stress. Proc Natl Acad Sci U S A. 1972 Apr;69(4):956–959. doi: 10.1073/pnas.69.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Chave-Cox A., Ma X., Bishop J. O. Analysis of mouse major urinary protein genes: variation between the exonic sequences of group 1 genes and a comparison with an active gene out with group 1 both suggest that gene conversion has occurred between MUP genes. EMBO J. 1985 Dec 1;4(12):3167–3171. doi: 10.1002/j.1460-2075.1985.tb04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Weiss M., Darnell J. E., Jr Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985 Oct;5(10):2633–2641. doi: 10.1128/mcb.5.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Molecular cloning of cDNA sequences transcribed from mouse liver endoplasmic reticulum poly(A)mRNA. Gene. 1981 Nov;15(2-3):225–235. doi: 10.1016/0378-1119(81)90132-3. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Mason P. J., Bishop J. O. Comparison of poly(A)-mRNA prepared from membranes and free polyribosomes of mouse liver. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3697–3701. doi: 10.1073/pnas.78.6.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commer P., Schwartz C., Tracy S., Tamaoki T., Chiu J. F. Dexamethasone inhibits alpha-fetoprotein gene expression in developing mouse liver. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1294–1299. doi: 10.1016/0006-291x(79)92149-1. [DOI] [PubMed] [Google Scholar]
- Derman E. Isolation of a cDNA clone for mouse urinary proteins: age- and sex-related expression of mouse urinary protein genes is transcriptionally controlled. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5425–5429. doi: 10.1073/pnas.78.9.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Chung E. Y., Darnell J. E., Jr Gene expression during liver regeneration. J Mol Biol. 1984 Oct 15;179(1):37–53. doi: 10.1016/0022-2836(84)90305-x. [DOI] [PubMed] [Google Scholar]
- Gorin M. B., Cooper D. L., Eiferman F., van de Rijn P., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. I. A comparison of the primary amino acid sequences of mammalian alpha-fetoprotein and albumin. J Biol Chem. 1981 Feb 25;256(4):1954–1959. [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Shaw P. H., Barth R. K., Hastie N. D. A genetic locus closely linked to a protease inhibitor gene complex controls the level of multiple RNA transcripts. Mol Cell Biol. 1985 Aug;5(8):2114–2122. doi: 10.1128/mcb.5.8.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Jansson J. O., Downs T. R., Beamer W. G., Frohman L. A. Receptor-associated resistance to growth hormone-releasing factor in dwarf "little" mice. Science. 1986 Apr 25;232(4749):511–512. doi: 10.1126/science.3008329. [DOI] [PubMed] [Google Scholar]
- Knopf J. L., Gallagher J. F., Held W. A. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol. 1983 Dec;3(12):2232–2240. doi: 10.1128/mcb.3.12.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Krauter K. S., Citron B. A., Hsu M. T., Powell D., Darnell J. E., Jr Isolation and characterization of the alpha 1-antitrypsin gene of mice. DNA. 1986 Feb;5(1):29–36. doi: 10.1089/dna.1986.5.29. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Moran T., Boorstein R., Koch K. S. Procarcinogen activation and hormonal control of cell proliferation in differentiated primary adult rat liver cell cultures. Nature. 1977 May 5;267(5606):58–61. doi: 10.1038/267058a0. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Paul D. Studies on primary cultures of differentiated fetal liver cells. J Cell Biol. 1972 Mar;52(3):559–568. doi: 10.1083/jcb.52.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H., Moran T., Sell S., Skelly H., Ibsen K., Mueller M., Arias I. Growth state-dependent phenotypes of adult hepatocytes in primary monolayer culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1834–1838. doi: 10.1073/pnas.75.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. C., Barth R. K., Shaw P. H., Elliott R. W., Hastie N. D. Identification of a cDNA clone for mouse apoprotein A-1 (apo A-1) and its use in characterization of apo A-1 mRNA expression in liver and small intestine. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1511–1515. doi: 10.1073/pnas.80.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Powell D. J., Friedman J. M., Oulette A. J., Krauter K. S., Darnell J. E., Jr Transcriptional and post-transcriptional control of specific messenger RNAs in adult and embryonic liver. J Mol Biol. 1984 Oct 15;179(1):21–35. doi: 10.1016/0022-2836(84)90304-8. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B., Prasad M. S., Unakar N. J. Role of insulin in the regulation of the hepatic messenger RNA for alpha 2u-globulin in diabetic rats. J Biol Chem. 1980 Dec 10;255(23):11614–11618. [PubMed] [Google Scholar]
- Shaw P. H., Held W. A., Hastie N. D. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell. 1983 Mar;32(3):755–761. doi: 10.1016/0092-8674(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Isom H. C. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987 Oct;7(10):3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]