Abstract
Atopic asthma is a chronic inflammatory disease of the lungs generally marked by excessive Th2 inflammation. The role of allergen-specific IgG in asthma is still controversial; however, a receptor of IgG–immune complexes (IgG-ICs), FcγRIII, has been shown to promote Th2 responses through an unknown mechanism. Herein, we demonstrate that allergen-specific IgG-ICs, formed upon reexposure to allergen, promoted Th2 responses in two different models of IC-mediated inflammation that were independent of a preformed T cell memory response. Development of Th2-type airway inflammation was shown to be both FcγRIII and TLR4 dependent, and T cells were necessary and sufficient for this process to occur, even in the absence of type 2 innate lymphoid cells. We sought to identify downstream targets of FcγRIII signaling that could contribute to this process and demonstrated that bone marrow–derived DCs, alveolar macrophages, and respiratory DCs significantly upregulated IL-33 when activated through FcγRIII and TLR4. Importantly, IC-induced Th2 inflammation was dependent on the ST2/IL-33 pathway. Our results suggest that allergen-specific IgG can enhance secondary responses by ligating FcγRIII on antigen-presenting cells to augment development of Th2-mediated responses in the lungs via an IL-33–dependent mechanism.
Introduction
The World Health Organization estimates that 300 million people worldwide suffer from asthma, a chronic inflammatory disease of the lungs marked by recurrent episodes of airway hyperresponsiveness (1). Asthma has heterogeneous phenotypes, but it is most commonly characterized by excessive Th2-driven inflammation and Th2 cytokines that mediate downstream events, including mast cell activation, eosinophilia, goblet cell hyperplasia, and airway remodeling (2). Development of Th2 inflammation relies on stimulation from antigen-presenting cells (APCs), primarily DCs, which can direct differentiation into specific T cell lineages (3). Th2 inflammation is characterized by the production of IL-4, IL-5, and IL-13, and it has been shown that IL-4 alone can induce potent Th2 differentiation in the absence of other cytokines (4). In mice, IL-4 is critical for B cell isotype switching to IgE and IgG1 (5). Several studies have supported a role for B cells in allergic lung diseases primarily via IgE and sensitization of mast cells (6). However, IgE-deficient mice are still able to develop systemic anaphylaxis reactions after OVA sensitization and i.v. antigen challenge, suggesting that other pathways may also mediate allergic reactions (7). One of the prime candidates put forth has been IgG antibodies (8). Some studies have suggested that antigen-specific IgG has a suppressive effect by acting through inhibitory Fcγ receptors (FcγRs) and competing with IgE (9, 10). Conversely, other studies have demonstrated a correlation between asthma susceptibility and increased IgG levels (11, 12). Thus, the role of IgG in the initiation and perpetuation of allergic lung disease is still poorly understood and controversial.
Four FcγRs have been identified in mice that provide a critical link between IgG and cellular effector mechanisms, including phagocytosis, release of inflammatory mediators, and antibody-dependent cell-mediated cytotoxicity (13). These FcγRs are divided into activating (FcγRI [also known as CD64], FcγRIII [also known as CD16], and FcγRIV [also known as CD16-2]) and inhibitory (FcγRIIb [also known as CD32b]) receptors (14). Each FcγR has varying affinities for the 4 subclasses of IgG: IgG1, IgG2a, IgG2b, and IgG3 (15). Of the activating receptors, FcγRI is known to bind to monomeric IgG with high affinity, while FcγRIII is efficiently engaged by IgG–immune complexes (IgG-ICs) (16, 17). Previous work in our laboratory investigated the contribution of activating FcγRs in conjunction with a TLR4 stimulus in regulating Th2-dependent inflammatory responses, and this study identified a key role for FcγRIII on DCs in the development of optimal Th2 airway inflammation (18).
In this study, we investigate the hypothesis that due to the presence of allergen-specific IgG in the airways of sensitized individuals, ICs would form upon secondary exposure to allergen, which, in turn, would promote Th2 mediated inflammation. Taken together with the fact that inhaled allergens can be contaminated with endotoxins, these 2 signals could augment Th2 inflammation in the lung during secondary responses (19). Intriguingly, this hypothesis is supported by clinical studies that have shown increased IgG levels in the bronchoalveolar lavage (BAL) of patients with asthma due to increased leakage from the blood as well as increased local IgG production (20, 21). Furthermore, other studies have identified allergen-specific ICs in the sera of allergic individuals (22). These studies suggest that allergen-specific IgG may contribute to the augmentation of allergic airway inflammation during secondary exposure to an allergen. To test this hypothesis, a model was developed to study the effect of allergen-specific IgG independent of a memory T cell response.
Our findings indicated that when antigen uptake was induced by ICs via FcγRIII signaling compared with simple soluble antigen uptake, the stimulated APCs produced a differential gene expression profile that included upregulation of IL33. IL-33, the most recently discovered member of the IL-1β family of cytokines, has been shown to act on Th2 cells, basophils, NK cells, iNKT cells, type 2 innate lymphoid cells (ILC2s), and mast cells to direct the development and expansion of Th2-mediated responses in vivo (23). We further demonstrated that this model of IC-mediated Th2 inflammation was T cell dependent and could occur in the absence of ILC2s. Our findings that previous sensitization and the presence of antigen-specific IgG dramatically change the response of APCs could help contribute to an evolving paradigm for the expansion and development of pathological Th2 inflammation.
Results
IC signaling through FcγRIII promotes Th2 responses.
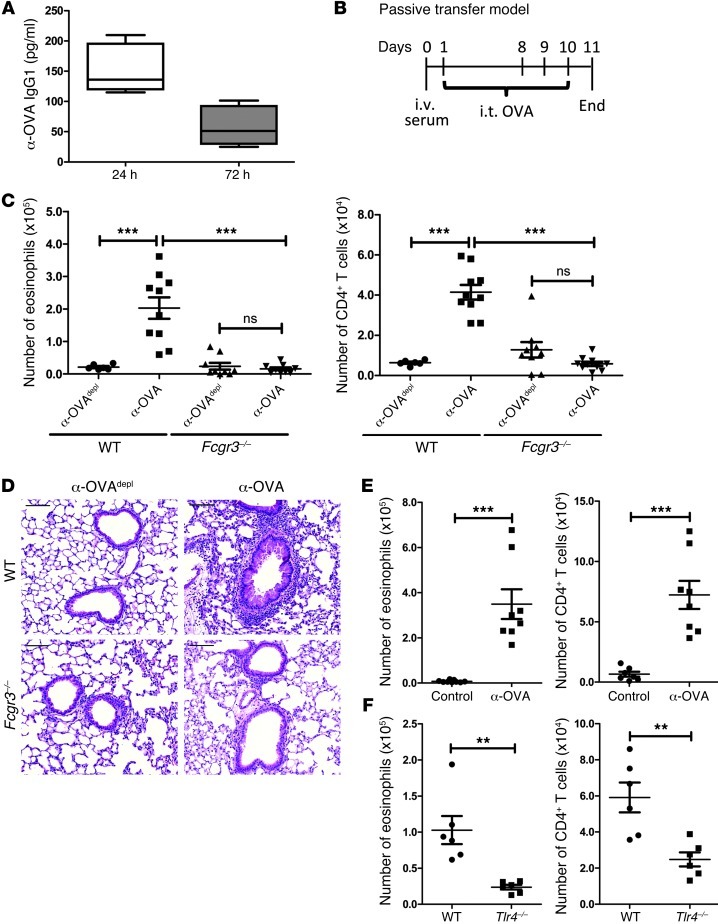
Our previous investigation demonstrated that when TLR4-stimulated DCs received an additional activating signal through FcγRIII, there was an augmentation in their ability to promote Th2 responses (18). Taken together with the clinical finding that patients with asthma or allergies had allergen-specific IgG in the airways, we hypothesized that secondary exposure to inhaled allergens may lead to IC formation. In the presence of a TLR4 signal, these allergen-specific ICs could then provide the signal through FcγRIII needed to heighten Th2 responses. To test this hypothesis, a model was developed to study the effect of allergen-specific IgG independent of a memory T cell response. Using ovalbumin (OVA) as the model antigen, it was first assessed whether antigen-specific IgG1 could be detected in the airways after injection of OVA-specific sera (α-OVA). OVA-specific IgG1 antibodies were detected in the BAL as soon as 24 hours after i.v. administration of α-OVA (Figure 1A). This finding suggested that antigen-specific IgG would be in a position in which it could encounter inhaled antigens and form ICs. Next, we developed a passive transfer model to test the effect of allergen-specific IgG in the absence of a secondary response. α-OVA or α-OVA that is IgG depleted (α-OVAdepl) was injected i.v. into mice followed by an OVA intratracheal (i.t.) sensitization the next day; 1 week later, animals were challenged with 3 OVA i.t. administrations (Figure 1B). After challenge, there was a striking increase in eosinophils and CD4+ T cells in the BAL of WT (C57BL/6) mice that had received α-OVA, with similar results obtained in an analysis of the lung cellular composition (Figure 1C and data not shown). Histological examination of the murine lungs showed that passive transfer of OVA-specific antibodies resulted in an influx of inflammatory cells that was not seen in the mice that had received α-OVAdepl (Figure 1D). To ensure that the effects being seen were not due to an artifact of the antibodies being generated in a rabbit, the experiment was also performed using OVA-specific mouse sera. Sera from unimmunized mice was used as a control. Only the WT mice that had received OVA-specific mouse sera developed inflammation, with an increase in eosinophils and CD4+ T cells in both the BAL and lungs (Figure 1E and data not shown). The data demonstrate increased inflammation in the presence of circulating antigen-specific IgG and point to a critical role for antigen-specific IgG in the development of airway inflammation upon challenge.
Figure 1. The presence of circulating antigen-specific IgG promotes Th2-mediated inflammation upon exposure to inhaled antigen.
(A) ELISA analysis of the BAL for α-OVA IgG1 after an i.v. injection of α-OVA into WT mice. At least 5 mice per time point were used. For the box-and-whisker plot, the median is shown as the line in the box, the box represents the 25th–75th percentile, and the whiskers represent the entire range of data from the minimum to maximum. (B) Time line of the passive transfer model. α-OVAdepl or α-OVA was administered i.v. to naive mice. On days 1, 8, 9, and 10, the mice were challenged i.t. with OVA. On day 11, the mice were sacrificed. Airway inflammation was assessed by determining (C) the number of eosinophils and CD4+ T cells in the BAL by flow cytometry and (D) by representative H&E-stained sections of lung tissue from treated WT and Fcgr3–/– mice. Scale bars: 100 μm. (E) Airway inflammation was assessed in WT mice that had received control or α-OVA mouse serum by determining the number of eosinophils and CD4+ T cells in the BAL. (F) Airway inflammation was assessed in WT and Tlr4–/– mice by determining the number of eosinophils and CD4+ T cells in the BAL. (C–F) The combined data represent at least 2 independent experiments, with a total of at least 6 mice analyzed per group. Symbols represent individual mice, horizontal bars indicate the mean, and error bars indicate SEM (**P < 0.01; ***P < 0.001).
Notably, the Fcgr3–/– mice that received an i.v. injection of α-OVA did not develop augmented airway inflammation. The cellular composition of the BAL indicated a significant decrease in the numbers of eosinophils and CD4+ T cells compared with those in WT mice treated with α-OVA (Figure 1C). Overall, the numbers of cells in the Fcgr3–/– mice were comparable in both treatment groups, and the histology from the Fcgr3–/– mice treated with α-OVA lacked inflammatory cell infiltrates (Figure 1D). As FcγRIII is efficiently activated by IgG1-ICs, the findings in the Fcgr3–/– mice confirm an important contribution for FcγRIII ligation in the development of Th2-type responses.
Our previous study found that signaling through FcγRIII diverted the TLR4 signal on DCs such that the resulting Th1 response was minimized and the Th2 response was augmented (18). To determine whether the in vivo passive transfer model was also TLR4 dependent, since endotoxin is present in the OVA, we repeated the model in the Tlr4–/– mice. Compared with the WT mice that had received α-OVA, the Tlr4–/– mice had decreased numbers of eosinophils and CD4+ T cells in the BAL (Figure 1F). Thus, the development of IC-mediated inflammation in the lungs is dependent on both FcγRIII and TLR4.
Since the previous model did not allow us to directly assess the role of ICs in allergic airway inflammation, it was important to address whether or not ICs alone could promote local Th2 responses in an FcγRIII-dependent manner. OVA-IC was formed by incubating OVA with α-OVA, while OVA was made by incubating OVA with α-OVAdepl as a control. WT or Fcgr3–/– mice were repeatedly challenged every other day with OVA or OVA-IC i.t. over a 2-week period in our local instillation model (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI63802DS1). With this model, there were increased numbers of eosinophils and CD4+ T cells in the BAL of WT mice challenged with OVA-IC (Supplemental Figure 1B). Repeated challenges with OVA were not enough to lead to the development of airway inflammation, highlighting the importance of ICs in this process. As seen in the passive transfer model, this process was dependent on FcγRIII, and neither the numbers of eosinophils or T cells, nor the histology exhibited increased inflammation in the Fcgr3–/– mice (Supplemental Figure 1, B and C). Collectively, the findings indicate that antigen-specific IgG and FcγRIII can contribute to the development of a potent Th2 inflammation during secondary responses.
IC-mediated Th2 inflammation is dependent on the presence of T cells but not ILC2s.
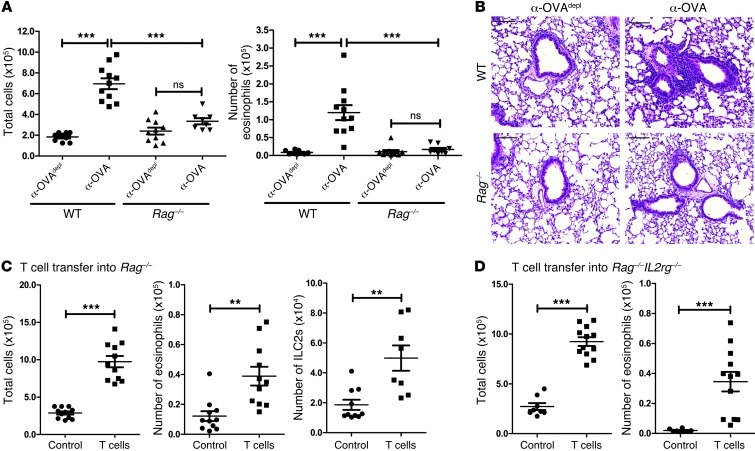
Recently, it has been found that many models of Th2-type inflammation do not require T cells for induction of the inflammatory response but are instead dependent on ILC2s that produce IL-5 and IL-13 after stimulation (24, 25). To determine whether IC-mediated inflammation was dependent on adaptive immunity, the passive transfer model was performed in Rag–/– mice. There was no significant increase in total cell numbers or eosinophil numbers in the BAL when comparing the Rag–/– mice that had received α-OVAdepl and those that had received α-OVA, and both of these values were significantly lower than those in the WT mice that had received α-OVA (Figure 2A). The decreased inflammatory response was also noted on histology, as the α-OVA–treated Rag–/– mice lacked inflammatory cell infiltrates around the airways and vasculature (Figure 2B). To determine whether T cells were specifically required for the induction of the Th2-type response in our model, WT nylon wool nonadherent T cells were adoptively transferred into naive Rag–/– mice 2 days prior to the injection of the anti-OVA sera. Transferring in T cells restored airway inflammation, with a significant increase in the number of total cells and eosinophils in the BAL (Figure 2C). Interestingly, there was also a significant increase in the number of ILC2s (gating strategy in Supplemental Figure 5A) found in the lungs of the mice that had received T cells, suggesting that Th2-mediated inflammation can affect the ILC2 response (Figure 2C).
Figure 2. IC-mediated Th2 inflammation is dependent on T cells but not ILC2s.
(A) WT or Rag–/– mice were treated as outlined in Figure 1B. Airway inflammation was assessed by determining the number of total cells and eosinophils in the BAL and (B) by representative H&E-stained sections of lung tissue from treated WT and Rag–/– mice. Scale bars: 100 μm. (C) Naive Rag–/– mice received vehicle control or nylon wool nonadherent T cells i.v. The mice were treated as outlined in Figure 1B, and airway inflammation was assessed by determining the total number of cells and eosinophils in the BAL as well as ILC2s (Lin–Sca-1+ICOS+ST2+, as described in Supplemental Figure 5) in the lung. (D) Naive Rag–/–Il2rg–/– mice received either a vehicle control or nylon wool nonadherent T cells i.v. The mice were treated as outlined in Figure 1B, and airway inflammation was assessed by determining the total number of cells and eosinophils in the BAL. The data are combined from 3 independent experiments, with a total of at least 8 mice analyzed per group. Symbols represent individual mice, horizontal bars indicate the mean, and error bars indicate SEM (**P < 0.01; ***P < 0.001).
To determine whether ILC2s were also necessary for the development of the inflammatory response in the passive transfer model, T cells were transferred into Rag–/–Il2rg–/– mice prior to administration of anti-OVA sera; these mice are deficient in adaptive immune cells and innate lymphoid cells, including ILC2s (26). One group of the Rag–/–Il2rg–/– mice received WT T cells, while the other group received only the vehicle control. As in the Rag–/– mice, reconstitution with T cells restored the inflammatory response in the ILC2-deficient Rag–/–Il2rg–/– mice (Figure 2D). It has been demonstrated that ILC2s can be found in the draining lymph node (dLN), but when we analyzed our nylon wool nonadherent T cell preparation, 0.002% of cells were Lin–Sca-1+ICOS+ST2+ (Supplemental Figure 5B). Furthermore, we did not see a repopulation of ILC2s in the Rag–/–Il2rg–/– mice that had received nylon wool nonadherent T cells (Supplemental Figure 5C). Thus, while T cells were necessary for the induction of IC-mediated Th2-type inflammation, the response could occur in the absence of ILC2s.
IC signaling through FcγRIII on APCs significantly upregulates IL-33 expression.
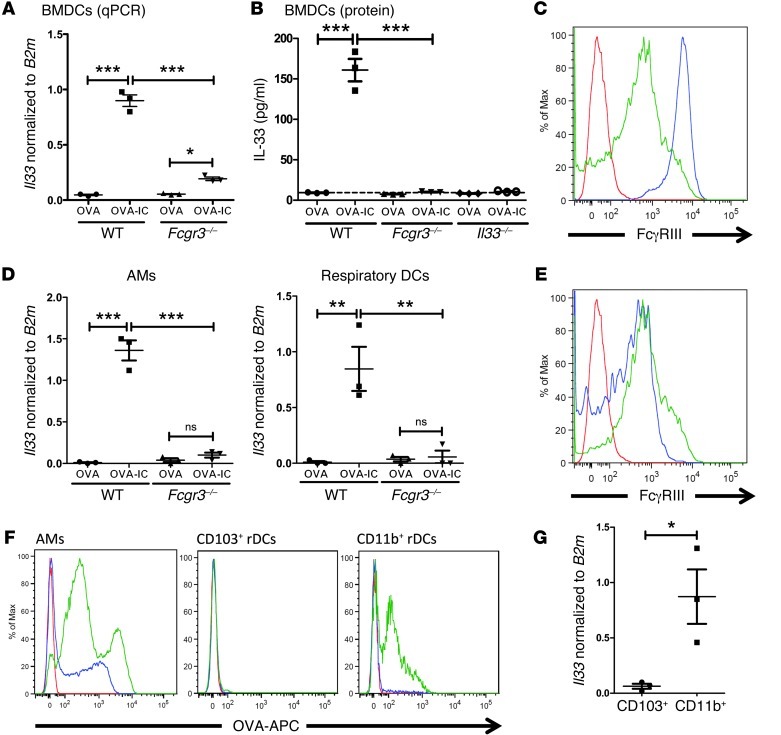
We next sought to identify downstream targets of FcγRIII signaling that could contribute to this process. We focused on DCs because it has been well established that DCs play a critical role in regulating Th cell differentiation that can affect development of airway inflammation (3). To address this question, we performed a microarray analysis on WT and Fcgr3–/– bone marrow–derived DCs (BMDCs) treated overnight with OVA or OVA-IC. The data from the microarray (not shown), which were confirmed by quantitative PCR (qPCR), highlighted Il33 as the most significantly upregulated gene upon OVA-IC treatment in WT BMDCs (Figure 3A). While there was a modest increase in Fcgr3–/– BMDCs treated with OVA-IC, it was significantly lower than that in treated WT BMDCs (Figure 3A). Further, IL-33 protein was produced by WT BMDCs treated with OVA-IC, but Fcgr3–/– and Il33–/– BMDCs failed to produce detectable levels of IL-33 protein (Figure 3B). Finally, treatment of Tlr4–/– BMDCs with OVA-IC did not lead to increased IL-33 expression (Supplemental Figure 2A). Thus, DCs can produce IL-33 after stimulation of FcγRIII in the presence of TLR4 signals, thereby suggesting that IL-33 may contribute to IC-mediated Th2 inflammation.
Figure 3. IC signaling through FcγRIII leads to a significant upregulation of IL-33 in WT APCs.
(A) BMDCs generated from WT or Fcgr3–/– mice were treated with OVA or OVA-IC overnight, and Il33 mRNA expression normalized to B2m mRNA expression was assessed by qPCR. (B) Multiplex array for IL-33 protein from OVA- or OVA-IC–treated WT, Fcgr3–/–, and Il33–/– BMDCs. (C) FcγRIII expression was assessed on naive WT AMs and rDCs, as gated in Supplemental Figure 4 (red, unstained; blue, AMs; green, rDCs). (D) Il33 mRNA expression in AMs and rDCs sorted from WT and Fcgr3–/– mice 3 hours after i.t. challenge with OVA or OVA-IC. (E) FcγRIII expression was assessed on rDC subpopulations (CD103+ and CD11b+ rDCs) from naive WT lungs (red, unstained; blue, CD103+ rDCs; green, CD11b+ rDCs). (F) WT mice were challenged i.t. with OVA-APC or OVA-APC IC, and OVA-APC expression on WT lung APCs was analyzed 3 hours after challenge (red, unchallenged; blue, OVA-APC; green; OVA-APC IC). (G) WT mice were challenged i.t. with OVA-IC, and rDC subpopulations were sorted 3 hours later. Il33 mRNA expression was determined by qPCR. The data in A, B, D, and G are from 3 independent experiments. Symbols represent independent experiments, horizontal bars indicate the mean, and error bars indicate SEM (*P < 0.05; **P < 0.01; ***P < 0.001). C, E, and F show representative flow plots, with at least 6 mice analyzed per group.
We next sought to determine whether respiratory APCs could be induced to upregulate IL-33 as seen with BMDCs. Alveolar macrophages (AMs; CD11c+SSCint/hiMHC II+) and respiratory DCs (rDCs; CD11c+SSCloMHC II–/+) were identified using the gating strategy outlined in Kim et al. (27) and shown in Supplemental Figure 4. To assess the effect of FcγRIII signaling by ICs on IL-33 upregulation, WT or Fcgr3–/– mice were challenged i.t. with OVA or OVA-IC. Three hours after instillation, AMs and rDCs were sorted from the treated mice since both populations expressed FcγRIII (Figure 3C). OVA-IC i.t. instillation led to an increase in Il33 mRNA expression in both AMs and rDCs, which was not seen in the Fcgr3–/– AMs and rDCs, suggesting that upregulation of IL-33 was, in fact, through IC activation of FcγRIII (Figure 3D). Similar results were found with mice injected with α-OVA or α-OVAdepl 24 hours prior to an i.t. instillation of OVA. Six hours after the OVA instillation, only the mice that had received α-OVA had a significant increase in IL-33 expression in both AMs and rDCs, with minimal expression of IL-33 in mice that received α-OVAdepl (Supplemental Figure 2B). Thus, these results demonstrate that the Th2 inflammation-inducing stimuli, FcγRIII plus TLR signaling, can induce IL-33 expression in pulmonary APCs.
CD103–CD11bhi lung DCs preferentially take up ICs and upregulate IL-33.
Augmentation of T cell responses has been shown to require lung DC migration to the dLNs for antigen presentation (3). Two migratory DC populations have been identified in the lungs: CD103+CD11blo (CD103+; CD11c+SSCloMHC II–/+CD103+CD11b–) and CD103–CD11bhi (CD11b+; CD11c+SSCloMHC II–/+CD103–CD11b+) (28). Both CD103+ and CD11b+ rDCs were shown to express FcγRIII (Figure 3E). To identify which DC subset was taking up ICs, we challenged mice i.t. with OVA-allophycocyanin (OVA-APC) or OVA-APC immune complex (OVA-APC IC) and harvested the lungs 3 hours later. Unlike in other studies that use a higher concentration of OVA and begin analysis at a later time point after airway instillation (29, 30), we did not note a significant uptake of OVA-APC in either rDC subset (Figure 3F). However, the CD11b+ population demonstrated a higher ability to take up OVA-APC IC compared with the CD103+ population, which took up a small amount of OVA-APC IC (Figure 3F). After sorting 3 hours after i.t. instillation with OVA-IC, the CD11b+ population was identified as preferentially upregulating IL-33 expression (Figure 3G).
Within the CD11c+CD11b+ rDC population, two populations of cells can be differentiated in the lungs following an inflammatory stimuli: a monocyte-derived DC (Ly6C+) population that is recruited to the lungs and a resident rDC (Ly6C–) population (28, 31). Using these markers, studies show that these two DC populations have different migratory properties, and only the CD11b+Ly6C– rDCs express CCR7 and migrate to the dLNs (32, 33). Using OVA-APC, it was determined that both CD11b+Ly6C+ (CD11c+SSCloMHC II–/+CD103–CD11b+Ly6C+) and CD11b+Ly6C– (CD11c+SSCloMHC II–/+CD103–CD11b+Ly6C–) DCs in the lungs took up OVA-IC APC (Supplemental Figure 3A). The results from these experiments indicate that early on ICs are primarily taken up by the CD11b+ rDC subset, including both Ly6C+ and Ly6C–, and may act in both the lungs and dLNs.
IC-mediated Th2 inflammation is dependent on the IL-33/ST2 pathway.
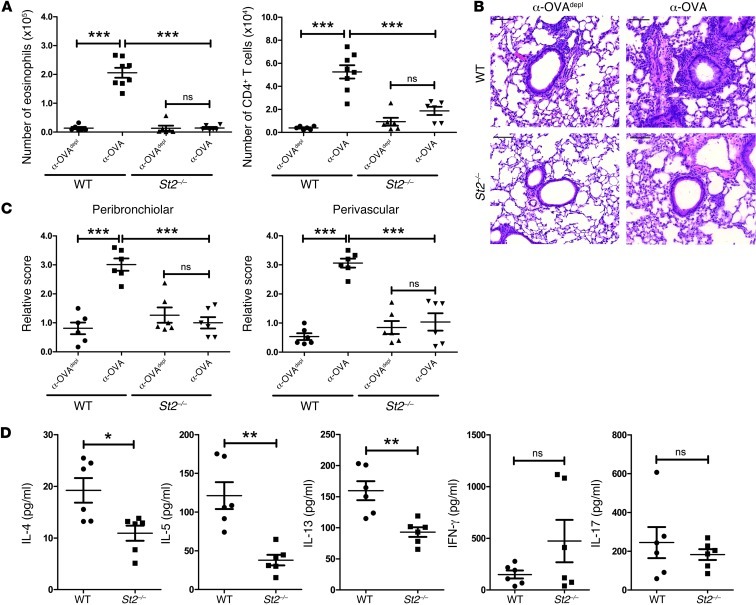
Previous studies have highlighted that IL-33 signaling induces Th2 cytokine production both in vitro and in vivo (34). Thus, upregulation of IL-33 in pulmonary APCs following activation by ICs could contribute to the pathogenesis of Th2 inflammation in the lungs. To investigate whether the IL-33 pathway was important in this process, the passive transfer model, outlined in Figure 1B, was carried out in mice deficient for the IL-33 receptor (St2–/– mice). The WT mice used in this experiment were either C57BL/6 or St2+/+ mice, and there was no significant difference between them. We confirmed that the St2–/– mice had equivalent numbers and percentages of AMs and rDCs compared to WT mice and that there was no defect in upregulating IL-33 expression in the presence of OVA-IC; this finding was further validated in the St2–/– BMDCs (data not shown). The St2–/– mice that received α-OVA had a significant decrease in eosinophils and CD4+ T cells compared with the WT mice and had less inflammation, as measured by histology (Figure 4, A–C). Furthermore, cytokine analysis of restimulated lung T cells demonstrated a significant decrease in IL-4, IL-5, and IL-13 in the α-OVA–treated St2–/– mice compared with that in the WT mice (Figure 4D). There was no significant difference in IFN-γ or IL-17 levels. The loss of eosinophilia and decreased histological inflammation was further confirmed using the local instillation model outlined in Supplemental Figure 1A (data not shown).
Figure 4. IC-mediated Th2 inflammation is ST2 dependent.
(A) WT (C57BL/6 or St2+/+ mice) or St2–/– mice were treated as outlined in Figure 1B. Airway inflammation was assessed by determining the number of eosinophils and CD4+ T cells in the BAL and (B) by H&E-stained sections of lung tissue from treated WT and St2–/– mice. Scale bars: 100 μm. (C) Perivascular and peribronchial inflammation were scored as described in Methods. (D) Lung cells from α-OVA–treated WT and St2–/– mice were restimulated in vitro with 2C11, and the amount of cytokines in culture supernatants was determined by Multiplex bead array. The data are combined from 2 independent experiments, with a total of at least 6 mice analyzed per group. Symbols represent individual mice, horizontal bars indicate the mean, and error bars indicate SEM (*P < 0.05; **P < 0.01; ***P < 0.001).
To ascertain whether IL-33 from DCs alone was sufficient to generate a Th2 response, WT and Il33–/– BMDCs were treated with OVA-IC overnight before instilling them into naive recipients. A week after sensitization, the mice were challenged i.t. with OVA on 3 consecutive days. The mice that received Il33–/– BMDCs had reduced numbers of eosinophils and CD4+ T cells in the BAL compared with the mice that received WT BMDCs, as well as decreased inflammation as shown by histology (Figure 5). Collectively, these findings indicate that the ST2/IL-33 pathway plays a critical role in the development of IC-mediated Th2 airway inflammation and that when DCs are directly stimulated with OVA-IC, IL-33 production from DCs is sufficient to lead to development of a Th2 response.
Figure 5. IL-33 from IC-stimulated DCs is sufficient for the development of a Th2 response.
(A) WT and Il33–/– BMDCs were generated and treated overnight with OVA-IC before being instilled i.t. into naive mice. On days 7, 8, and 9, the mice were challenged i.t. with OVA, before being sacrificed on day 11. Airway inflammation was assessed by determining the number of eosinophils and CD4+ T cells in the BAL and (B) by representative H&E-stained sections of lung tissue from mice that had received WT or Il33–/– BMDCs. Scale bars: 100 μm. The data are combined from 2 independent experiments, with a total of 8 mice analyzed per group. Symbols represent individual mice, horizontal bars indicate the mean, and error bars indicate SEM (*P < 0.05; ***P < 0.001).
T cells are sufficient to facilitate development of IC-mediated Th2 inflammation.
Since IC-mediated Th2 inflammation is T cell dependent (Figure 2), we tested whether WT T cells could restore an inflammatory response in the St2–/– mice. OVA-specific TCR transgenic T cells from the OTII mouse strain were transferred into WT or St2–/– mice. Both groups that received OTII cells had augmented numbers of eosinophils and CD4+ T cells in the BAL compared with the St2–/– control mice, as well as increased inflammation as noted by histology (Figure 6, A and B). These data demonstrate that adoptive transfer of WT antigen-specific cells can restore allergic airway inflammation in St2–/– mice and are sufficient for IC-mediated Th2 inflammation.
Figure 6. T cells are sufficient for the restoration of IC-mediated Th2 inflammation in St2–/– mice.
(A) Vehicle control or nylon wool nonadherent OTII cells were adoptively transferred into naive WT or St2–/– mice. The mice were treated as outlined in Figure 1B. Airway inflammation was assessed by determining the number of eosinophils and CD4+ T cells in the BAL and (B) by representative H&E-stained sections of lung tissue from treated mice. Scale bars: 100 μm. The data are combined from at least 2 independent experiments, with a total of at least 6 mice analyzed per group. Symbols represent individual mice, horizontal bars indicate the mean, and error bars indicate SEM (**P < 0.01).
Discussion
The focus of this study was to clarify the role of allergen-specific IgG during secondary responses in allergic airway inflammation. The results demonstrated that IC-mediated Th2 inflammation was dependent on FcγRIII and TLR4, and we found a novel downstream target of FcγRIII signaling on APCs, IL-33. Interestingly, we determined that this response required T cells and found that ILC2 expansion occurred only in the presence of T cells. Our study strengthens the link between the adaptive and innate immune response, and it demonstrates a role for allergen-specific IgG in potentiating the expansion of secondary T cell–mediated allergic inflammation. We propose a model in which allergen-specific IgG forms ICs upon secondary exposure that can then activate FcγRIII on APCs and upregulate IL-33. Once the IL-33 is released, it can act on neighboring resident lung cells, including T cells and ILC2s, to drive development of allergic airway inflammation. Additionally, IL-33 from migratory CD11b+ Ly6C– rDCs could enhance the expansion of Th2 cells in the dLNs. Thus, allergen-specific IgG may contribute to the heightened allergic response through multiple mechanisms.
This model could be particularly relevant when considering protein allergens, which are simpler than complex allergens (e.g., house dust mite, pollen, fungi, mold, and others). It is thought that complex allergens may injure the lung epithelium and promote primary sensitization through a variety of components that contribute to their allergenicity: cysteine proteases, serine proteases, MD2 mimic, pattern-associated molecular patterns, chitinase, and others (35–37). However, many allergens are simple protein allergens lacking enzymatic activity, including animal proteins, seed proteins, flour, latex, OVA, and others (38). With protein allergens, generation of allergen-specific IgG and subsequent IC formation during secondary responses may be necessary for continued development of allergic airway inflammation.
Our model tests the hypothesis that, during secondary Th2 responses, ICs form that positively augment the overall inflammatory response. To test this directly, we developed models in which the role of ICs could be tested in the absence of previously activated/memory T cell and B cell responses. The presence of allergen-specific IgG in the airways and IC formation during antigen administration recreates the physiological events seen in allergic asthma; however, the direct downstream mechanisms of IC stimulation may have global effects that function in the absence of memory T cell response. First, our findings demonstrate de novo Th2 responses after IC formation in the absence of preexisting memory T or B cells. Thus, even in the sensitization phase of the immune response, the expression of IL-33 from APCs may augment the expansion of newly differentiated Th2 cells. Second, sensitization to protein allergens is likely to be more complicated in humans than in mouse models. In mouse models, there are discrete sensitization phases and challenge phases by design. Use of adjuvants or other methodologies can induce Th2-type immunity specifically. How and why some humans are “sensitized” to allergens, while other exposed individuals do not becomes sensitized, remains one of the unanswered mysteries of asthma and allergy research. Individuals can be exposed to allergens for years prior to developing allergic responses, and they can develop IgG responses prior to developing allergic airway inflammation (38). Whether the presence of allergen-specific IgG in the airways plays a part in the development of allergy will require more investigations in the future.
Notably, our results demonstrated that IC-mediated Th2 inflammation was dependent on TLR4. Inhaled allergens are often contaminated with endotoxins, and our results suggest that allergen-specific IgG-ICs are needed to play a “costimulatory” role during secondary responses to modify the response of innate lymphoid cells and augment Th2 responses in the lungs (39). Our findings are supported by biochemical studies that have found a physical interaction between TLR4 and FcγRIII on macrophages following treatment with IgG-ICs (40). Thus, individuals who are not sensitized to an allergen and lack circulating allergen-specific IgG would fail to mount a Th2 response to inhaled allergens, because they would be lacking a signal through FcγRIII.
The downstream effects of FcγRIII signaling on DCs that contribute to the development of Th2 inflammation had not been previously identified. In our study, antigen uptake mediated by ICs via FcγRIII signaling on DCs both in vitro and in vivo led to a significant upregulation of IL-33. Due to its pro-Th2 effects, there has been a great deal of interest in understanding how IL-33 contributes to atopic asthma. Studies in murine asthma models and asthmatic patients have identified elevated levels of IL-33 protein in both the sera and tissues (41, 42). In humans, increased levels of IL-33 can be detected in the airway smooth muscle cells and lung epithelia of individuals with asthma (41, 43). Furthermore, several groups have used mouse models of asthma to demonstrate that in the absence of IL-33 there is reduced airway hyperresponsiveness, and overexpression of IL-33 in transgenic mice leads to increased airway inflammation, goblet cell hyperplasia, and eosinophilia (44, 45). A role for IL-33 in the pathogenesis of asthma in humans is further supported by 2 recent meta-analyses of North American and European genome-wide–associated studies in which asthma-susceptibility loci were identified in the regions for IL1RL1 (also known as ST2) and IL33 (46, 47). The importance and relevance of these susceptibility genes will rely on understanding their effects in individuals with the disease. In particular, our findings point to a potentially critical role for APCs in secondary responses to allergens. IL-33 may be the initiating signal that escalates the ongoing Th2-associated inflammatory response that occurs during antigen reexposure.
Although a correlation has been found between IL-33 and Th2 responses, it is not yet clear how IL-33 affects the development of Th2-type inflammation. How IL-33 enhances development of a Th2 response, including effects on DC activation, Th2 differentiation, Th2 expansion, and ILC2 activation, is an active area of study (24, 48, 49). Interestingly, while several studies have proposed that IL-33 works primarily on ILC2s, our findings suggest that while T cells are required for IC-induced inflammation, ILC2s are dispensable (44, 50). There is a small population of Lin–Sca-1+ICOS+ST2+ cells in the nylon wool nonadherent T cell preparations, but we did not note a significant increase in ILC2s in our Rag–/–Il2rg–/– experiments. The results suggest that IC-mediated Th2-type inflammation is not ILC2 dependent; however, it is still possible that when present ILC2s are contributing to the inflammatory response. Indeed, the increase in the number of ILC2s when T cells were transferred into Rag–/– mice suggests crosstalk between both the innate and adaptive arms of the immune system. Thus, it remains to be determined how these cells may interact with each other to promote allergic airway inflammation in asthmatics.
While it has been shown that there are multiple ways IL-33 can affect the environmental milieu, the release of IL-33 from cells is still a matter of controversy. Initial studies were unable to detect IL-33, unless the cells were induced to die by necrosis, suggesting that IL-33 was an intracellular protein that acted as an alarmin (51). Recently though, it has been shown that murine fibroblasts can release IL-33 during biomechanical overload (52). Our data demonstrating a reduction in airway inflammation in St2–/– mice suggest that IC-activated AMs and rDCs release IL-33 to act on other cells; these IL-33–responsive cells may then increase production of Th2 cytokines, thereby heightening the Th2 response. This hypothesis is supported by our finding that transferring WT antigen-specific T cells into St2–/– mice restored IC-mediated Th2 inflammation.
Since there are various rDC subsets in the lung, it was important to identify which ones were taking up ICs and increasing IL-33 expression. In our studies, we did not see either rDC subset taking up OVA; however, it has been shown in other studies using a higher concentration of OVA and looking at a later time point that OVA can be taken up by both CD103+ and CD11b+ rDCs (29, 30). Thus, it is possible that the rDCs continue to take up antigen past the early time point that was analyzed in this study. Our results do demonstrate that the CD11b+ rDCs have a higher ability to take up ICs and upregulate IL-33 in the lungs soon after challenge. It has been demonstrated that CD11b+ rDCs are primarily involved in CD4+ T cell proliferation (53). In addition, studies that depleted rDCs at the time of allergen challenge saw an abrogation in allergic characteristics, but this defect was restored with an i.t. administration of CD11b+ BMDCs (54). It was further shown that both the Ly6C+ and Ly6C– subsets of CD11b+ rDCs took up ICs. It has been suggested that CD11b+Ly6C– cells are resident rDCs and CD11b+Ly6C+ cells are monocyte-derived DCs recruited to the lungs (28, 31, 55, 56). Previous studies have shown that only CD11b+Ly6C– rDCs can express CCR7 and migrate to the dLN (32, 33). These findings are of particular interest considering that IC-mediated inflammation is T cell dependent. Thus, IC-activated CD11b+Ly6C– rDCs could deliver IL-33 to the dLN, in which it would be available for enhancing Th2 function. These data suggest that DCs may be playing a role in both the lungs and the dLN to promote IC-mediated Th2 inflammation.
In addition, our study may be relevant in the context of an effective immune response against intestinal helminth infections. Control and expulsion of a helminth infection is predominantly associated with a Th2-type immune response, marked by increased numbers of eosinophils as well as enhanced IgE and IgG responses (57, 58). μMT mice are more susceptible to helminth infections, suggesting a critical role for the humoral response in this setting (59, 60). Furthermore, several studies have shown that passive transfer of helminth-specific IgG aids in the rapid expulsion of a helminth infection (61, 62). More recently, it has been shown that IL-33 is a potent inducer of adaptive immune responses against helminths (63). Our findings support a role for IL-33 in the chronic stage of a helminth infection, whereby circulating helminth-specific ICs activate APCs to upregulate IL-33 and help drive the Th2 response.
In addition to asthma, IL-33 has been implicated in a variety of human diseases, including cardiovascular disease, central nervous system disease, rheumatoid arthritis, inflammatory bowel disease, and others (23). Interestingly, IgG-ICs have been shown to contribute to the pathogenesis of rheumatoid arthritis and inflammatory bowel disease, which are both associated with elevated levels of IL-33 in the sera and tissues (64–68). While our findings contribute to the understanding of how IL-33 affects Th2-mediated diseases, it is not clear how IL-33 may function in these Th17-mediated diseases. IL-33 has been shown to cause a wide range of effects, including neutrophil migration, mast cell activation, osteoclast and osteoblast function, wound healing, and others (69–71). Thus, the ability of IL-33 to affect disease pathogenesis through a multitude of mechanisms has made it an intriguing area of investigation in which a great deal still remains unknown.
Methods
Mice.
Female 6- to 8-week-old C57BL/6 mice (WT) were purchased from Harlan Laboratories. Fcgr3–/–, Tlr4–/–, Rag1–/–, and OTII mice were purchased from The Jackson Laboratory. St2–/– mice were generously provided by Andrew McKenzie (Medical Research Laboratory, University of Cambridge, Cambridge, United Kingdom) and generated as previously described (72). Il33–/– mice were provided to P.J. Bryce by Dirk Smith (Amgen). Rag–/–Il2rg–/– mice were purchased from Taconic. Animals were housed in a specific pathogen–free facility maintained by the University of Chicago Animal Resources Center. The studies detailed herein conform to the principles set forth by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Production and stimulation of BMDCs.
BMDCs were generated as previously described (73). Briefly, bone marrow was flushed from the femurs of mice. The cells were cultured in 10% complete DMEM in bacteriological Petri dishes at a concentration of 2 × 105 cells/ml. The cultures were supplemented with 20 ng/ml GM-CSF (Shenandoah Biotechnology) and replenished on day 3. On day 6, the suspension cells were harvested. For stimulation, 5 × 105 BMDCs were cultured in 24-well plates with OVA or OVA-IC at a concentration of 100 μg/ml.
Reagents.
Grade V chicken egg OVA (Sigma-Aldrich) and rabbit anti-chicken egg OVA IgG (080M4812, Sigma-Aldrich) were used in murine experiments. OVA-APC (Invitrogen) was used for antigen uptake experiments. OVA-ICs were made by mixing a 4:1 excess of anti-OVA/OVA at 37°C for 30 minutes. To control for nonspecific effects from the antisera, IgG was depleted overnight with protein G-agarose beads (Thermo Scientific). OVA-specific mouse serum was made by immunizing mice twice with 100 μg OVA and 4 mg alum; the mice were bled a week after the last injection.
Induction and assessment of airway inflammation.
Mice were sensitized and challenged as described in Figure 1B (passive transfer) and Supplemental Figure 1A (local instillation). For Figure 5, mice were sensitized by instilling 1 × 106 treated BMDCs i.t. into naive mice. On days 7, 8, and 9, the mice were challenged with 50 μg OVA i.t., and they were sacrificed on day 11. BAL was performed by delivering 0.8 ml sterile PBS into the airway and gently aspirating the fluid. The lavage was repeated 3 times to recover a total volume of approximately 3 ml. The cells were stained with trypan blue to determine viability, and total nucleated cell counts were obtained using a hemocytometer (Bright-Line).
IgG1 ELISA.
Nunc-Immuno MaxiSorp surface plates (NUNC Brand Products) were coated with 100 μg/ml OVA in carbonate buffer. Antigen-specific IgG1 was detected with a secondary isotype-specific biotinylated antibody (A85-1, BD Biosciences).
Isolation of lung cells and dLN cells.
Lungs were made into single cell suspensions as previously described (18). Briefly, the lungs were perfused with 25 U/ml heparin (Sigma-Aldrich) and minced with scissors. Dissociation of the tissue was achieved by agitating the tissue for 30 minutes at 37°C in 10 ml of 5% complete DMEM with 150 U/ml collagenase I (Invitrogen). The digest was passed through a nytex filter, and red blood cells were lysed with ammonium chloride-potassium lysing buffer. dLNs were digested for 30 minutes at 37°C in 10 ml of 5% complete DMEM with 100 U/ml collagenase I (Invitrogen). The digest was then passed through a nytex filter, after which the single cell suspension was stained for flow cytometry.
Nylon wool nonadherent T cell enrichment.
Inguinal, brachial, cervical, and mesenteric lymph nodes were isolated from mice and made into single cell suspensions by disrupting the tissue and passing the digest through a nytex filter. Autoclaved nylon wool columns (Polysciences Inc.) were equilibrated by washing with 5% FCS in PBS and incubated for 30 minutes at 37°C. Cell suspensions were resuspended in warm DMEM at a concentration of 1.0 × 108 cells/ml, loaded onto the column, and incubated for an hour at 37°C. Nonadherent cells were eluted with 5% FCS in PBS. For the adoptive transfer experiment in Figure 2C, 1.0 × 107 cells were administered i.v. into naive mice. For the adoptive transfer experiment in Figure 6, 1.0 × 106 cells were administered i.v. into naive mice.
Histology.
The right middle lobe was removed from the mice after BAL and fixed by immersion into 10% formalin. Lobes were embedded in paraffin, sectioned sagittally, cut into 5-μm sections, and stained with H&E for analysis of cellular infiltrates. An inflammation score was assigned in a blinded fashion as described previously (18).
Flow cytometric analysis.
For FACs analysis, 5 × 105 cells were resuspended in 100 μl of FACs buffer (PBS containing 0.1% sodium azide and 1% BSA). They were then blocked with 20 μl of anti-CD16/32 (antibody supernatant was produced in house from 2.4G2 hybridoma obtained from ATCC) and stained with fluorescently conjugated antibodies specific for CCR3 (83101) and ST2 (245707) (both from R&D Systems); CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), CD11c (N418), CD19 (MB19-1), F4/80 (BM8), ICOS (C398.4A), and Ly6C (HK1.4) (all from eBiosciences); B220 (RA3-6B2), Gr1 (1A8), and CD103 (M290) (all from BD Pharmingen); or FcεRI (MAR-1), I-A/I-E (M5/114.15.2), NK1.1 (PK136), Sca-1 (E13-161.7), and Ter119 (TER-119) (all from Biolegend). Eosinophils in both the BAL and lung were identified as SSChiCCR3+Gr1–, while CD4+ T cells were gated as SSCloCD3+CD4+CD8–. For detection of AMs and rDCs, the cells were first gated on CD11c+ cells. AMs were separated based on high side scatter, as demonstrated in Supplemental Figure 4, and then the rDCs were further divided into CD103+ and CD11b+ subpopulations as described above. For detection of ILC2s, the cells were gated on Lin– (B220, CD3, CD4, CD8, CD11b, CD11c, CD19, F4/80, FcεRI, Gr1, NK1.1, and Ter119) cells. These cells were then gated for positive expression of Sca-1, ST2, and ICOS, as shown in Supplemental Figure 5. Flow cytometric analysis was performed on an LSRFortessa or LSRII (BD Biosciences), and the data were analyzed with FlowJo software (Tree Star Inc.). Flow sorting was done on a FACSAria (BD Biosciences) at the University of Chicago Flow Cytometry Core Facility.
Cytokine analysis.
BMDCs were stimulated overnight as described above, and the plates underwent a freeze-thaw cycle before supernatants were collected. Cells from the lungs were cultured with 1 μg/ml of 2C11 at 2 × 105 cells per well for 48 hours, after which supernatants were collected. Supernatants were analyzed by Multiplex bead array according to the manufacturer’s protocol (Millipore).
Microarray.
BMDC samples were run on an ABI Mouse Genome Survey Microarray (Applied Biosystems) by the University of Chicago Genomics Facility. All microarray gene expression data in this study are available from the Gene Expression Omnibus (accession no. GSE44388).
qPCR.
RNA from cells or tissues was isolated using an RNeasy Micro or Mini Kit (Qiagen). For cDNA synthesis, the manufacturer’s instructions were followed for the SuperScript III Reverse Transcriptase Kit (Invitrogen). cDNA samples were amplified with the Power SYBR Green PCR Master Mix (Applied Biosystems) and run on a ABI 7300 cycler (Bio-Rad). The qPCR cycling conditions included an initial denaturation of 95°C for 2 minutes, followed by 40 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. The concentration of the target gene was determined with the comparative CT (threshold cycle number) method and normalized to B2m. PCR primers were as follows: IL-33, forward 5′-GCTGCGTCTGTTGACACATT-3′, reverse 5′-CACCTGGTCTTGCTCTTGGT-3′; β2M, forward 5′-CATACGCCTGCAGAGTTAAGCA-3′, reverse 5′-GATCACATGTCCGATCCCAGTAG-3′.
Statistics.
All statistical analyses were performed with GraphPad Prism software, and a P value of less than 0.05 was considered significant. Experiments with 2 groups were analyzed using an unpaired Student’s 2-tailed t test. Experiments with more than 2 groups were analyzed with a 1-way ANOVA and post-hoc Tukey test. Error bars represent the SEM.
Study approval.
All animal procedures were approved by the University of Chicago Animal Resources Center.
Supplementary Material
Acknowledgments
We would like to thank H.S. Bandukwala for his contributions to the preliminary data for this study. For lab support, we would like to thank K.M. Blaine, B.R. Fixsen, and C.L. Hsu. For critical evaluation of the manuscript, we would like to thank S.M. Takahashi, J.M. Lunderburg, E.M. Luo, and E. Reynoso. We would also like to thank A. McKenzie and D. Smith for generously providing mice used in this study. This work was supported by a Ragins-Goldsmith Fellowship, University of Chicago (to M.Y. Tjota), and National Institutes of Health grants R21AI094408 (to A.I. Sperling), 5T32HL007237 (to J.W. Williams), 5T32HL007605 (to B.S. Clay and C.L. Hrusch), and 5R25GM066522 (to T. Byrd).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(5):2287–2297. doi:10.1172/JCI63802.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242(1):220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11(8):647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17(1):7–17. doi: 10.1016/S1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 5.Snapper CM, Finkelman FD, Paul WE. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 7.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370(6488):367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 8.Williams JW, Tjota MY, Sperling AI. The contribution of allergen-specific IgG to the development of th2-mediated airway inflammation. J Allergy (Cairo). 2012;2012:236075. doi: 10.1155/2012/236075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408–416. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11(4):446–449. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 11.Hales BJ, et al. IgE and IgG anti-house dust mite specificities in allergic disease. J Allergy Clin Immunol. 2006;118(2):361–367. doi: 10.1016/j.jaci.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Stern DA, et al. Exposure to a farming environment has allergen-specific protective effects on TH2-dependent isotype switching in response to common inhalants. J Allergy Clin Immunol. 2007;119(2):351–358. doi: 10.1016/j.jaci.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 15.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 16.Hazenbos WL, et al. Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16). J Immunol. 1998;161(6):3026–3032. [PubMed] [Google Scholar]
- 17.Harrison PT, Allen JM. High affinity IgG binding by FcgammaRI (CD64) is modulated by two distinct IgSF domains and the transmembrane domain of the receptor. Protein Eng. 1998;11(3):225–232. doi: 10.1093/protein/11.3.225. [DOI] [PubMed] [Google Scholar]
- 18.Bandukwala HS, et al. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204(8):1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz DA. Does inhalation of endotoxin cause asthma? Am J Respir Crit Care Med. 2001;163(2):305–306. doi: 10.1164/ajrccm.163.2.ed2000a. [DOI] [PubMed] [Google Scholar]
- 20.Out TA, van de Graaf EA, van den Berg NJ, Jansen HM. IgG subclasses in bronchoalveolar lavage fluid from patients with asthma. Scand J Immunol. 1991;33(6):719–727. doi: 10.1111/j.1365-3083.1991.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 21.Kitz R, Ahrens P, Zielen S. Immunoglobulin levels in bronchoalveolar lavage fluid of children with chronic chest disease. Pediatr Pulmonol. 2000;29(6):443–451. doi: 10.1002/(SICI)1099-0496(200006)29:6<443::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Stevens WJ, Bridts CH. IgG-containing and IgE-containing circulating immune complexes in patients with asthma and rhinitis. J Allergy Clin Immunol. 1984;73(2):276–282. doi: 10.1016/S0091-6749(84)80020-2. [DOI] [PubMed] [Google Scholar]
- 23.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 24.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4(1):e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 29.Nakano H, et al. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012;5(1):53–65. doi: 10.1038/mi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337(2):121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook D, Burgents J, Nakano H. Migratory properties of pulmonary dendritic cells are developmentally programmed. J Immunol. 2012;188:61–64. [Google Scholar]
- 33.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205(12):2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011;17(10):604–611. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Hassim Z, Maronese SE, Kumar RK. Injury to murine airway epithelial cells by pollen enzymes. Thorax. 1998;53(5):368–371. doi: 10.1136/thx.53.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dyken SJ, et al. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187(5):2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan-Yeung M, Malo JL. Occupational asthma. N Engl J Med. 1995;333(2):107–112. doi: 10.1056/NEJM199507133330207. [DOI] [PubMed] [Google Scholar]
- 39.Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr. 2004;16(6):659–666. doi: 10.1097/01.mop.0000145920.00101.e4. [DOI] [PubMed] [Google Scholar]
- 40.Rittirsch D, et al. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5(6):e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prefontaine D, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183(8):5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 42.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179(9):772–781. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prefontaine D, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 44.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci. 2010;107(43):18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhiguang X, et al. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett. 2010;131(2):159–165. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Moffatt MF, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torgerson DG, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HY, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129(1):216–227. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 — cytokine of dual function or novel alarmin? Trends Immunol. 2009;30(5):227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287(9):6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178(11):6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 54.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201(6):981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 56.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 57.Urban JF, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065X.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 58.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32(2):80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Q, et al. B cells have distinct roles in host protection against different nematode parasites. J Immunol. 2010;184(9):5213–5223. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30(3):421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finkelman FD, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 62.Harris NL, et al. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177(9):6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- 63.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180(4):2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 64.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 65.Matsuyama Y, et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J Rheumatol. 2009;37(1):18–25. doi: 10.3899/jrheum.090492. [DOI] [PubMed] [Google Scholar]
- 66.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kemler BJ, Alpert E. Inflammatory bowel disease associated circulating immune complexes. Gut. 1980;21(3):195–201. doi: 10.1136/gut.21.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobori A, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45(10):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 69.Xu D, et al. IL-33 exacerbates autoantibody-induced arthritis. J Immunol. 2010;184(5):2620–2626. doi: 10.4049/jimmunol.0902685. [DOI] [PubMed] [Google Scholar]
- 70.Verri WA, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69(9):1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 71.Saidi S, et al. IL-33 is expressed in human osteoblasts, but has no direct effect on bone remodeling. Cytokine. 2011;53(3):347–354. doi: 10.1016/j.cyto.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 72.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191(6):1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.