Abstract
Spontaneous antigen-specific T cell responses can be generated in hosts harboring a variety of solid malignancies, but are subverted by immune evasion mechanisms active within the tumor microenvironment. In contrast to solid tumors, the mechanisms that regulate T cell activation versus tolerance to hematological malignancies have been underexplored. A murine acute myeloid leukemia (AML) model was used to investigate antigen-specific T cell responses against AML cells inoculated i.v. versus s.c. Robust antigen-specific T cell responses were generated against AML cells after s.c., but not i.v., inoculation. In fact, i.v. AML cell inoculation prevented functional T cell activation in response to subsequent s.c. AML cell challenge. T cell dysfunction was antigen specific and did not depend on Tregs or myeloid-derived suppressor cells (MDSCs). Antigen-specific TCR-Tg CD8+ T cells proliferated, but failed to accumulate, and expressed low levels of effector cytokines in hosts after i.v. AML induction, consistent with abortive T cell activation and peripheral tolerance. Administration of agonistic anti-CD40 Ab to activate host APCs enhanced accumulation of functional T cells and prolonged survival. Our results suggest that antigen-specific T cell tolerance is a potent immune evasion mechanism in hosts with AML that can be reversed in vivo after CD40 engagement.
Introduction
Although it is widely accepted that cancer cells can express antigens that are recognizable to host T cells (1), spontaneous immune-mediated elimination of established malignancies is rare. This is believed to be due in large part to immune evasion pathways active within the tumor microenvironment that subvert the generation or execution of an effective antitumor immune response (2). Analysis of the major immune evasion pathways has predominantly focused on solid tumor models, either preclinically or in clinical specimens. Such investigations have been profitable, as strategies to overcome these immune-inhibitory pathways are meeting with early clinical success. For example, immune checkpoint blockade is rapidly emerging as an effective strategy to enhance antitumor immunity in patients with melanoma and several other solid malignancies. In particular, phase II and III studies of anti–CTLA-4 and anti–PD-L1 Abs have demonstrated impressive objective tumor response rates (3, 4), and administration of the anti–CTLA-4 Ab ipilimumab (Yervoy; Bristol-Myers Squibb) has been shown to prolong survival in patients with advanced melanoma (3). In addition, early-phase clinical trials are underway to test strategies to deplete CD4+CD25+FoxP3+ Tregs (5); to block the enzymatic activity of indoleamine-2,3-dioxygenase (IDO); and to reverse tumor-induced T cell anergy through T cell homeostatic proliferation, OX40 ligation, and LAG-3 blockade (6, 7). Each of these interventions has been supported by preclinical studies in solid tumor models (8–11), often induced through s.c. tumor cell inoculation.
In contrast to the translational research progress being made uncoupling immune inhibitory mechanisms in the setting of solid tumors, the negative regulatory mechanisms orchestrated by hematologic malignancies, such as acute myeloid leukemia (AML), have been underexplored. However, several groups have investigated T cell tolerance in systemic hematological cancer models. The first observation of T cell tolerance to a systemic hematological malignancy was demonstrated in the transplantable A20 lymphoma model. TCR-Tg CD4+ T cells specific for a model tumor antigen were rendered “anergic” in tumor-bearing mice (12). The CD4+ T cell tolerance was regulated by host APCs (13) and could not be prevented with CTLA-4 blockade and vaccination (14). Furthermore, in a model of CD8+ T cell tolerance in hosts harboring Friend murine leukemia virus–transformed leukemia (FBL), which expresses an immunogenic peptide derived from the retroviral Gag protein, it was observed that Gag-specific CD8+ T cells were tolerized in FBL-bearing hosts in which the Gag antigen was also conditionally expressed in the liver. This antigen-specific CD8+ T cell tolerant state could not be prevented by administration of agonistic anti-CD40 Ab or LPS, but was reversible after in vivo administration of IL-15 (15).
Because hematological malignancies differ greatly in their growth rate and pattern and stromal milieu compared with tumors that progress locally as a solid mass, it seemed likely that their interactions with the host immune system might be distinct. Recent observations from solid tumor models have suggested that local inflammation generated by tumor cell death can result in the elaboration of “danger signals” that activate host innate immune cells (16, 17), including CD8α+ DCs (18). Activated DCs can consequently cross-present tumor-derived antigens and initiate CD8+ T cell activation, resulting in a spontaneous antitumor T cell response. However, in the case of disseminated leukemia, it is conceivable that this immunogenic cell death might not occur to a similar degree. Therefore, the nature of the major immune evasion mechanisms active in hosts with leukemia also might be distinct. Understanding these mechanisms should point toward the most logical immunotherapeutic strategies for patients with hematologic malignancies.
With these notions in mind, we used a transplantable model of AML in which leukemia cells were introduced i.v. or s.c. into mice in order to analyze both spontaneous immune responses and mechanisms of immune escape. After i.v. inoculation, AML cells infiltrated the liver and, to a lesser extent, the bone marrow and peripheral blood of recipient mice (19, 20). Interestingly, it was observed that i.v. inoculation of AML cells prevented the generation of an antigen-specific T cell response induced by s.c. inoculation in the same mouse, indicating a rapid induction of peripheral tolerance. This tolerance appeared to be due to the intrinsic dysfunction and deletion of antitumor T cells, and was reversed by administration of an agonistic anti-CD40 Ab that has been previously demonstrated to overcome peripheral T cell tolerance in several preclinical solid tumor models (21–23). Our findings suggest that dominant peripheral tolerance is a major mechanism of immune escape with hematogenous dissemination of leukemia and that anti-CD40 mAb may have a therapeutic benefit that could be translated clinically.
Results
Diminished survival in C57BL/6 mice after i.v. versus s.c. challenge with C1498 AML.
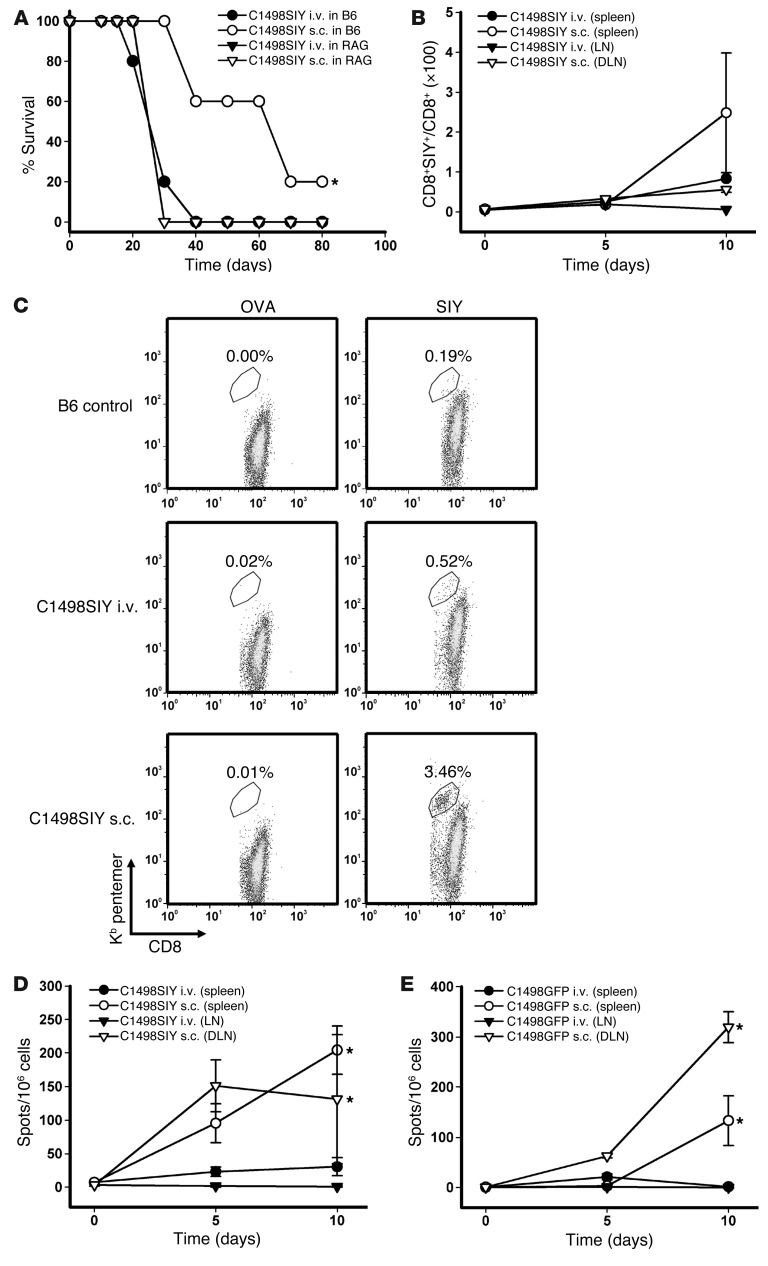
To begin to investigate the role of adaptive immunity in the control of AML progression, we challenged cohorts of C57BL/6 and T cell/B cell–deficient Rag2–/– hosts i.v. or s.c. with 106 C1498.SIY cells (engineered by retroviral transduction using the pLEGFP plasmid expressing cDNA for the SIYRYYGL model peptide antigen; see Methods), and survival was assessed. Whereas no difference in survival was seen after inoculation of C1498.SIY cells i.v. versus s.c. in Rag2–/– hosts, C57BL/6 mice challenged with s.c. C1498.SIY cells demonstrated significantly prolonged survival compared with i.v. inoculation, and approximately 20% of mice survived long-term (Figure 1A). These results suggested that a partial adaptive immune response was generated when C1498 cells were implanted s.c., but not i.v. Furthermore, the similar survival we observed in C1498.SIY cell–challenged Rag2–/– mice (unable to mount an adaptive immune response against C1498.SIY cells), regardless of inoculation route, argued that the “antigen” load to which mice were exposed was similar when comparing s.c. and i.v. routes of inoculation.
Figure 1. Decreased survival and antigen-specific T cell responses after i.v. versus s.c. C1498.SIY cell challenge.
(A) Rag2–/– or C57BL/6 (B6) mice received 106 C1498.SIY cells i.v. or s.c., and survival was assessed. *P = 0.009, s.c. versus i.v. Data are representative of 2 independent experiments with 5 mice/group. (B–D) C57BL/6 mice received C1498.SIY cells i.v. or s.c. (B) Spleen and LN cells (DLNs from s.c.-challenged mice and pooled axillary and inguinal LNs from i.v.-challenged mice) were analyzed for SIY-reactive CD8+ T cells after SIY/Kb pentamer staining. P = 0.09, s.c. versus i.v., day 10. 2 independent experiments were performed with 3–5 mice/group per time point. (C) Representative FACS plots of SIY and OVA pentamer staining. Gated areas represent percent pentamer-reactive CD8+ T cells among the entire CD8+ T cell population. (D) IFN-γ ELISPOT of spleen and LN cells. *P < 0.001, s.c. versus i.v., days 5 and 10. (E) Mice received 106 C1498.GFP cells i.v. or s.c. On days 5 and 10, IFN-γ ELISPOT was performed after restimulation with media or irradiated C1498.GFP cells. *P < 0.05, s.c. versus i.v., day 10. Data are representative of 2 experiments with 3 mice/group.
Minimal functional antigen-specific T cell responses are generated in mice harboring C1498.SIY cells i.v.
To test directly whether antigen-specific T cell responses were occurring in C57BL/6 mice after i.v. versus s.c. C1498 cell inoculation, spleens and LNs were harvested from groups of C57BL/6 mice at various time points after either i.v. or s.c. C1498.SIY cell inoculation, and the number and function of SIY-reactive CD8+ T cells were analyzed using SIY/Kb pentamers and IFN-γ ELISPOT. SIY pentamer–reactive CD8+ T cells were more numerous in the spleens of C57BL/6 mice challenged with C1498.SIY cells s.c. versus i.v. on day 10 after C1498.SIY cell challenge (Figure 1, B and C). Furthermore, when the function of SIY-specific T cells was analyzed with IFN-γ ELISPOT, significantly higher numbers of IFN-γ spot-forming cells were observed in mice 5 and 10 days after s.c. C1498.SIY cell challenge (Figure 1D). In contrast, in C57BL/6 mice challenged with C1498.SIY cells i.v., only minimal functional responses were detected at all time points analyzed. A similar, although slightly delayed, kinetic pattern of functional activation of endogenous C1498–specific T cells was seen in mice challenged with control C1498.GFP cells (Figure 1E), which indicates that the impaired priming or activation of tumor antigen-specific T cells in hosts harboring leukemia cells systemically was not limited to T cells specific for the model SIY antigen.
Generation of antigen-specific T cell dysfunction after i.v. C1498.SIY cell inoculation.
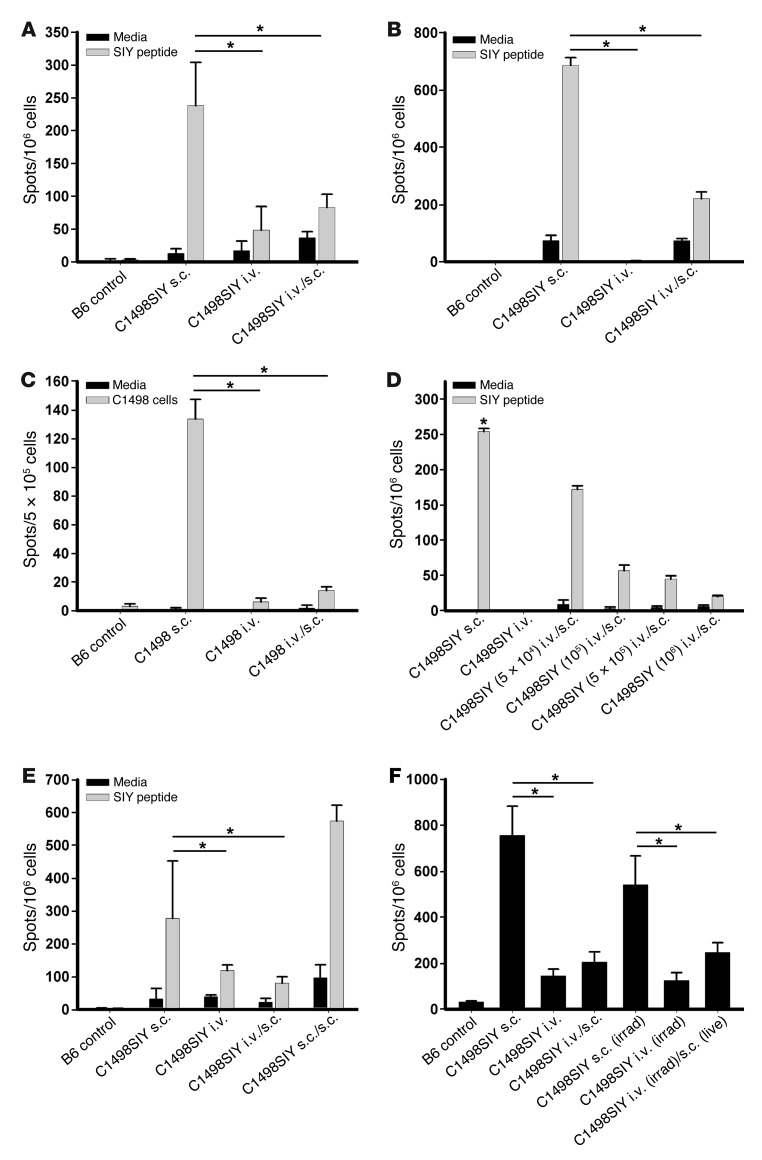
Given the equivalent antigen load after i.v. versus s.c. inoculation of an identical number of C1498.SIY cells, it was conceivable that the i.v.-disseminated leukemia cells not only failed to prime a specific T cell response, but might have actively induced peripheral tolerance. To determine whether this was the case, mice received i.v. C1498.SIY cell inoculation on day –6, followed by s.c. C1498.SIY cell challenge on day 0 (a dual-challenge approach referred to herein as i.v./s.c.). In fact, strikingly diminished functional SIY-specific T cell responses were observed in the spleens and tumor-draining LNs (DLNs) of mice subjected to i.v./s.c. administration (Figure 2, A and B). Similar findings were observed in parallel experiments in which control C1498 cells were used (Figure 2C), which suggests that T cell dysfunction induced by i.v. C1498 cells was not dependent upon their expression of the SIY antigen. Thus, hematogenous dissemination of AML cells actively promoted the induction of T cell dysfunction in C57BL/6 mice.
Figure 2. Inoculation of C1498.SIY cells i.v. generates a T cell–dysfunctional state.
(A and B) C1498.SIY cells were inoculated into C57BL/6 mice i.v., s.c., or i.v./s.c. Spleen (A) and LN (B) cells were restimulated 6 days later with media or SIY peptide, and IFN-γ ELISPOT was performed. *P < 0.05. Data are representative of 3 independent experiments with 3 mice/group. (C) 6 days after inoculation of control C1498 cells i.v., s.c., or i.v./s.c., LN cells were restimulated with media or irradiated C1498 cells in an IFN-γ ELISPOT assay. *P < 0.05. (D) Indicated numbers of i.v. C1498.SIY cells were introduced on day –6, followed by 106 s.c. C1498.SIY cells on day 0. On day 6, IFN-γ ELISPOT was performed. *P < 0.05 versus all other groups. (E) Mice received C1498.SIY cells as in A. A fourth group received s.c. C1498.SIY cells in one flank on day –6, and the opposite flank on day 0 (s.c./s.c.). IFN-γ ELISPOT was performed on day 6. *P < 0.05. (F) Live or irradiated C1498.SIY cells were inoculated i.v., s.c., or i.v./s.c., and IFN-γ ELISPOT was performed. *P < 0.05. (C–F) Data are representative of 2 experiments with 3 mice/group.
To ensure that the T cell tolerance to i.v.-disseminated leukemia was not an artifact of an individual cell line, parallel experiments were performed using murine FBL cells that naturally express the retroviral Gag protein. C57BL/6 mice received i.v., s.c., or i.v./s.c. inoculation of FBL cells as above, and Gag-specific CD8+ T cell responses were analyzed by IFN-γ ELISPOT after ex vivo restimulation with Gag peptide. Strikingly diminished functional Gag-specific endogenous CD8+ T cell responses were again observed in mice that received i.v./s.c. inoculation (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI63980DS1). These results argue that induction of peripheral T cell tolerance is a common mechanism of immune evasion in hosts with disseminated AML.
To determine whether the ability of i.v. C1498 cell inoculation to induce peripheral tolerance was dose dependent, a range of cell numbers was introduced i.v. Indeed, increasing numbers of i.v. C1498.SIY cells led to progressively diminished functional SIY-specific T cell responses after subsequent s.c. inoculation with 106 C1498.SIY cells 6 days later (Figure 2D). To determine whether the induction of peripheral tolerance was unique to the i.v. setting, groups of C57BL/6 mice were inoculated i.v. or s.c. with C1498.SIY cells on the left flank on day –6; on day 0, both groups received a second inoculation of s.c. C1498.SIY cells on the right flank. Whereas the i.v./s.c. recipients failed to generate a functional SIY-specific T cell response, in sharp contrast, enhanced SIY-specific T cell responses were seen in spleens of s.c./s.c. recipients (Figure 2E). This suggests that the initial s.c. C1498.SIY cell inoculation on day –6 actually promoted antigen-specific T cell priming, similar to what might be expected with a tumor cell–based vaccine. To determine whether a functional antigen-specific T cell response after s.c. C1498.SIY cell inoculation could be inhibited by subsequent i.v. C1498.SIY cell inoculation, groups of C57BL/6 mice were challenged with s.c. C1498.SIY cells on day –6, and some received subsequent i.v. C1498.SIY cell inoculation on day 0. Functional SIY-specific T cell responses were analyzed in the spleens of these mice 6 days later, which demonstrated that antigen-specific T cell responses in mice receiving s.c./i.v. C1498.SIY cell inoculation were similar to those in mice receiving s.c. C1498.SIY cell inoculation alone (Supplemental Figure 2). This result suggested that once antigen-specific CD8+ T cells were functionally primed after s.c. C1498.SIY cell challenge, they were no longer sensitive to tolerization with a subsequent i.v. C1498.SIY cell challenge.
It was important to exclude the possibility that global immune suppression as a result of advanced tumor burden was responsible for the defective antigen-specific T cell responses seen in mice after i.v. C1498.SIY cell inoculation. To address this, C57BL/6 mice were challenged with live or irradiated (150 Gy) C1498.SIY cells i.v. on day –6, followed by s.c. C1498.SIY cell challenge on day 0. This dose of radiation was found to be nearly 100% lethal to C1498.SIY cells, as assessed by a trypan blue exclusion assay (data not shown). Diminished SIY-specific T cell responses against s.c. C1498.SIY tumors were observed whether live or irradiated C1498.SIY cells were previously introduced (Figure 2F), which argues that systemic immune suppression from a rapidly growing tumor was not the cause of peripheral tolerance induced after i.v. C1498.SIY cell inoculation.
T cell dysfunction in mice bearing i.v. C1498.SIY cells occurs in an antigen-specific manner.
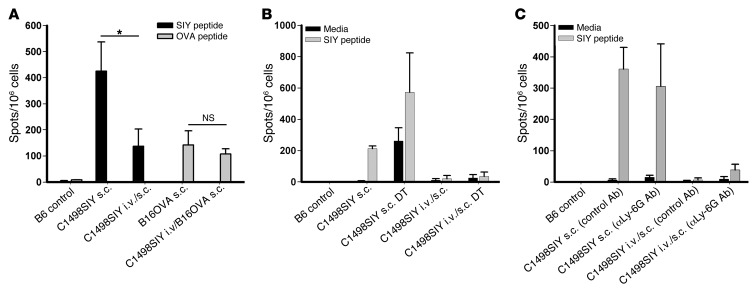
To determine whether the T cell dysfunction induced by i.v. C1498.SIY cells was specific to the antigens expressed on the tumor cells, 2 experiments were performed. First, groups of C57BL/6 mice were challenged i.v. with either C1498.GFP or C1498.SIY cells on day –6. On day 0, these mice received s.c. C1498.SIY cells, and 6 days later, spleen cells from these mice were restimulated ex vivo with the SIY peptide in an IFN-γ ELISPOT assay. Surprisingly, i.v. inoculation of either C1498.GFP or C1498.SIY cells led to a severely blunted SIY-specific T cell response against a subsequent s.c. C1498.SIY cell inoculation (data not shown). We speculated that a state of “shared tolerance” to unknown antigens on C1498 cells might explain this result. Thus, we next used a different cancer cell line expressing a different model antigen to determine whether T cell tolerance in i.v.-challenged mice was antigen specific. Groups of C57BL/6 mice were challenged with i.v. C1498.SIY cells on day –6 and received a subsequent s.c. challenge on day 0 with C1498.SIY cells or B16.OVA cells (B16 melanoma cells engineered to express the full-length chicken OVA protein). On day 6, spleen cells were restimulated ex vivo with either the SIY peptide or a Kb-restricted OVA-derived peptide (SIINFEKL) in an IFN-γ ELISPOT assay. SIY-specific CD8+ T cell responses were reduced as before, whereas OVA-specific T cell responses remained intact (Figure 3A). This result suggests that T cell dysfunction in mice inoculated with i.v. C1498.SIY cells occurred in an antigen-specific manner.
Figure 3. T cell dysfunction in mice after i.v. C1498.SIY cell inoculation is antigen specific, and is not regulated by Tregs or MDSCs.
(A) C57BL/6 mice received 106 C1498.SIY or B16.OVA cells s.c. only. Additional cohorts of mice received 106 C1498.SIY cells i.v. on day –6, followed by either C1498.SIY or B16.OVA cells s.c. on day 0. On day 6, spleen cells were restimulated with SIY or OVA peptide in an IFN-γ ELISPOT assay. *P < 0.05. (B) FoxP3-DTR mice received C1498.SIY cells s.c. or i.v./s.c. and were treated with diphtheria toxin (DT; 1 μg in 0.1 ml per mouse) or PBS as follows: s.c. C1498.SIY cell–challenged mice, days –2, –1, 0, 2, and 5; i.v./s.c. C1498.SIY cell–challenged mice, days –8, –7, –4, –1, 2, and 5. On day 6, an IFN-γ ELISPOT assay was performed. (C) C57BL/6 mice received s.c. or i.v./s.c. C1498.SIY cells and received either the anti–Ly-6G Ab 1A8 or isotype control Ab (300 μg i.p. on days 0 and 3 for s.c. challenge, and on days –6, –3, 0, and 3 for i.v./s.c. challenge). On day 6, spleen cells were restimulated with media or SIY peptide in an IFN-γ ELISPOT assay. (A–C) Data are representative of 2 experiments with 3 mice/group.
T cell dysfunction in mice harboring i.v. C1498.SIY cells is not reversed after depletion of Tregs or MDSCs.
As Tregs and MDSCs have been shown to suppress antitumor T cell responses in murine cancer models (24, 25), we sought to clarify whether they were regulating T cell dysfunction in mice harboring i.v. C1498.SIY cells. To address this possibility, Tregs and MDSCs were depleted from FoxP3-DTR mice or via administration of an anti–Ly-6G Ab to C57BL/6 mice, respectively, in host mice that received dual i.v./s.c. C1498.SIY cell inoculation. Depletion of FoxP3+ Tregs upon administration of diphtheria toxin to FoxP3-DTR mice did not restore functional SIY-specific T cell responses in C1498.SIY i.v./s.c. dual-challenged mice (Figure 3B), which suggests that Tregs were dispensable for the induction of T cell tolerance in this setting. Furthermore, although the anti–Ly-6G mAb effectively depleted splenic and DLN CD11b+Gr-1+ cells (data not shown), its administration did not reverse the T cell dysfunction induced in i.v./s.c. C1498.SIY cell dual-challenged mice (Figure 3C). Identical results were obtained when an anti–Gr-1 Ab was administered in vivo to deplete MDSCs (data not shown). Thus, despite meaningful depletion of these potentially suppressive cell populations, functional SIY-specific T cell responses were not restored, which argues that neither Tregs nor MDSCs were required for the early induction of tolerance in mice with i.v. C1498.SIY cells.
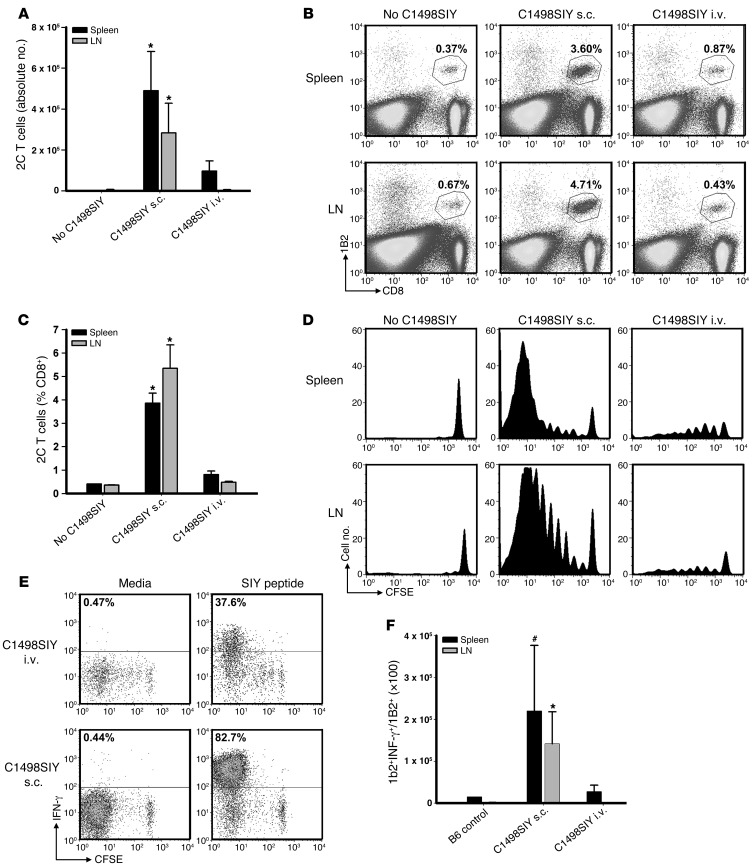
Antigen-specific T cells proliferate, but fail to accumulate, in hosts bearing i.v. C1498.SIY cells.
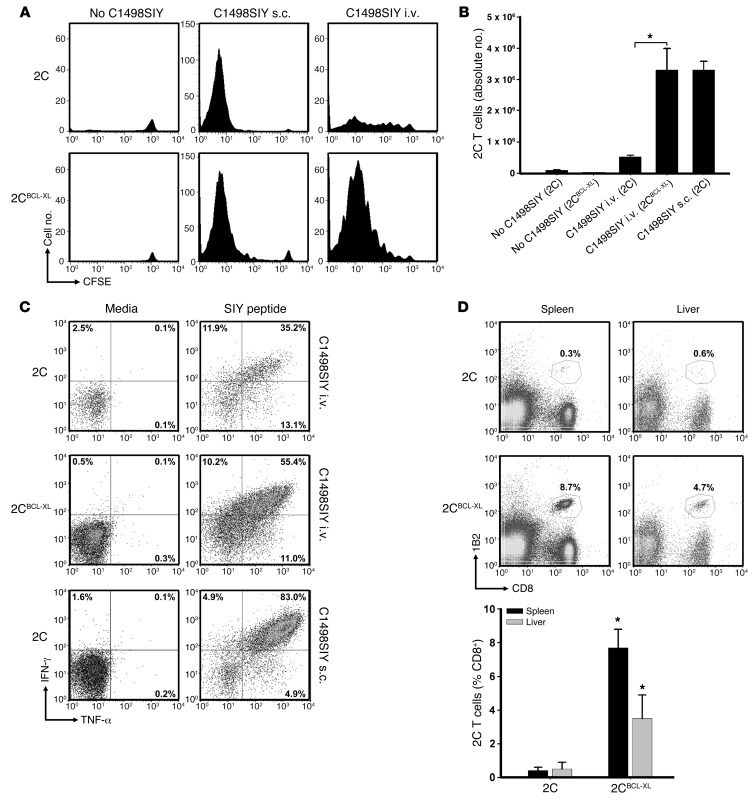
To further clarify the mechanism underlying the induction of T cell dysfunction in mice inoculated with i.v. C1498.SIY cells, we employed an adoptive transfer model using SIY antigen–specific TCR-Tg CD8+ T cells (referred to herein as 2C T cells). Purified 2C T cells (4 × 106) were CFSE labeled and adoptively transferred into C57BL/6 mice; 1 day later, mice received 106 C1498.SIY cells i.v. or s.c., or no tumor as a control. At 6 days after C1498.SIY cell inoculation, the absolute numbers, percentages, and CFSE dilution profiles of 2C T cells present in spleens and LNs were analyzed (Figure 4, A–D, and Supplemental Figure 3A). 2C T cells both proliferated and accumulated in spleens and LNs of mice receiving s.c. C1498.SIY cell challenge. In contrast, whereas 2C T cells from mice harboring i.v. C1498.SIY cells were induced to proliferate, they did not accumulate, and were recovered in significantly lower numbers than those in mice after s.c. C1498.SIY cell challenge (Figure 4A). Functional analysis of 2C T cells from tumor-bearing mice revealed decreased production of IFN-γ by 2C T cells from mice with i.v. C1498.SIY (Figure 4E). This difference was further accentuated by comparison of absolute numbers of IFN-γ–producing 2C T cells from mice challenged with i.v. versus s.c. C1498.SIY cells (Figure 4F). Collectively, these data confirmed the results of experiments examining the endogenous response to i.v. C1498.SIY cell challenge, and suggest that deletion and/or anergy of antigen-specific T cells may occur in hosts inoculated with i.v. C1498.SIY.
Figure 4. SIY-specific 2C T cells undergo abortive peripheral tolerance in mice with i.v. C1498.SIY.
CFSE-labeled 2C T cells (4 × 106) were adoptively transferred into C57BL/6 mice, followed 1 day later by inoculation with i.v. or s.c. C1498.SIY cells. (A) On day 7, 2C T cells were enumerated. *P < 0.05, i.v. versus s.c. Data are representative of 4 experiments with 3–5 mice/group. (B) Representative FACS plots from mice in A. Gated areas represent percent 2C T cells among the entire CD8+ T cell population. (C) Mean percent 2C T cells from mice in A. *P < 0.05, i.v. versus s.c. (D) CFSE dilution of 2C T cells from mice in A. (E) Mice received 2C T cells and C1498.SIY challenge as in A. On day 7, spleen and LN cells were restimulated with media or SIY peptide. Production of IFN-γ by 2C T cells was analyzed. Numbers represent percent IFN-γ+ 2C T cells. (F) Numbers of IFN-γ–producing 2C T cells after i.v. or s.c. C1498.SIY cell challenge. #P = 0.10, *P < 0.05, i.v. versus s.c. (E and F) Data are representative of 3 experiments with 3 mice/group.
Tg expression of the antiapoptotic BCL-XL protein in 2C T cells restores their ability to accumulate in hosts harboring i.v. C1498.SIY cells.
The above data demonstrating the failure of 2C T cells to accumulate in hosts harboring disseminated C1498.SIY cells raised the possibility that they were being specifically targeted for deletion. To test this possibility, we interbred 2C mice with Bcl-XL Tg mice, in which BCL-XL expression is directed within the T cell compartment (referred to herein as 2CBCL-XL mice). CFSE-labeled 2C or 2CBCL-XL T cells were adoptively transferred into groups of C57BL/6 mice, which were inoculated the following day with i.v. or s.c. C1498.SIY cells or remained tumor free. On day 6, the absolute numbers and extent of CFSE dilution of 2C versus 2CBCL-XL T cells were analyzed. As shown in Figure 5A and Supplemental Figure 3B, the ability of 2C T cells to both proliferate and accumulate in mice inoculated with C1498.SIY cells i.v. was restored upon Tg expression of Bcl-XL (i.e., in 2CBCL-XL T cells). Both 2C and 2CBCL-XL T cells failed to proliferate in leukemia-free hosts and were recovered in similar numbers (Figure 5, A and B), arguing against an intrinsic advantage of 2CBCL-XL T cells to survive and proliferate after adoptive transfer into hosts in which their cognate antigen was not present. Furthermore, 2CBCL-XL T cells were recovered in significantly higher numbers from mice with i.v. C1498.SIY cells compared with control 2C T cells (Figure 5B), and 2CBCL-XL T cells produced higher levels of IFN-γ and TNF-α than did control 2C T cells upon ex vivo restimulation with SIY peptide, although not to the level of control 2C T cells isolated from hosts with s.c. C1498.SIY cell challenge (Figure 5C). When spleens and livers (a primary location of C1498 cell progression) of mice were analyzed 3–4 weeks after 2C versus 2CBCL-XL adoptive transfer and i.v. C1498.SIY challenge, 6- and 20-fold increases in the percentage of 2CBCL-XL versus 2C T cells were observed in the livers and spleens, respectively (Figure 5D). In fact, almost no 2C T cells could be identified in the spleens and livers of mice after i.v. C1498.SIY cell inoculation at this later time point. Together, these data argue that T cell deletion represents a potent mechanism of tolerance induced in hosts with AML.
Figure 5. Tg expression of Bcl-XL in 2C T cells rescues them from deletion in hosts with i.v. C1498.SIY cells.
(A) CFSE-labeled 2C or 2CBCL-XL T cells were transferred into C57BL/6 mice. On day 1, mice received i.v. or s.c. C1498.SIY cells. On day 7, CFSE dilution of splenic 2C and 2CBCL-XL T cells was analyzed. Representative CFSE dilution profiles are shown. (B) Absolute numbers of 2C T cells in spleens of mice in A. *P < 0.05. (C) 2C or 2CBCL-XL T cells were transferred into mice and subsequently challenged with i.v. or s.c. C1498.SIY cells as in A. On day 7, spleen cells were restimulated with media or SIY peptide, and production of IFN-γ and TNF-α was analyzed. Numbers represent percent 2C T cells producing the indicated cytokines. (B and C) Data are representative of 2 experiments with 3 mice/group. (D) Percent 2C and 2CBCL-XL T cells in spleens and livers of mice 24 days after i.v. C1498.SIY cell challenge. Representative plots are shown. Gated areas represent percent 2C or 2CBCL-XL T cells among the entire CD8+ T cell population. Mean percent 2C and 2CBCL-XL T cells in groups of 3 mice is also shown. *P < 0.05, 2CBCL-XL versus 2C. Data are representative of 2 experiments.
Despite restored accumulation and enhanced early effector function of 2CBCL-XL T cells in mice harboring i.v. C1498.SIY cells, their adoptive transfer did not lead to improved control of leukemia cell progression or significantly enhanced survival compared with adoptive transfer of control 2C T cells (data not shown). In fact, when analyzed at this later time point after i.v. C1498.SIY cell inoculation, 2CBCL-XL T cells produced low levels of IFN-γ (data not shown), which suggests that additional negative regulatory mechanisms are involved in leukemia-specific T cell tolerance during the course of disease progression.
Endogenous antigen-specific T cell responses are restored, and mouse survival is prolonged, after administration of agonistic anti-CD40 Ab.
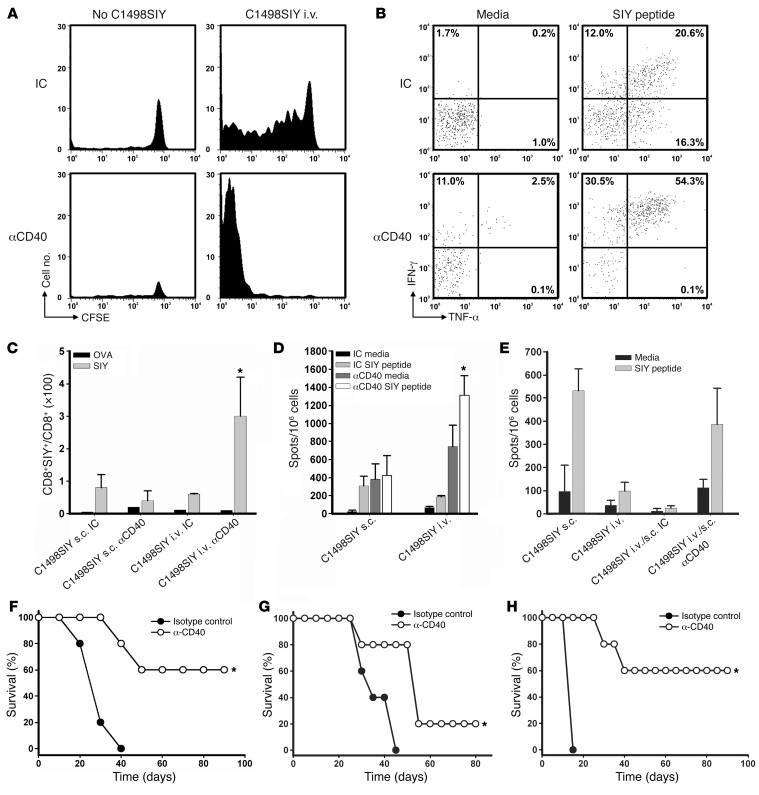
In other models of induction of peripheral tolerance, for example through the use of costimulatory ligand blockade (26), T cell deletion and anergy appear to operate in concert to induce and maintain the tolerant state. It seemed plausible that a similar process might be occurring with i.v. dissemination of tumor, if antigen cross-presentation was occurring by host DCs that were not activated or matured. Although CD11c+ cells from spleens and LNs of mice after i.v. versus s.c. C1498.SIY cell inoculation did not differ significantly in their expression of MHC class I or classical costimulatory molecules (data not shown), we nevertheless hypothesized that there might be a qualitative defect in the ability of DCs from i.v.-challenged mice to functionally prime leukemia antigen-specific T cells. As CD40 ligation has previously been shown to activate DCs in vivo (21, 22, 27), we investigated whether administration of an agonistic anti-CD40 Ab in mice inoculated with i.v. C1498.SIY cells would restore T cell activation and persistence, improving leukemia control and, hence, mouse survival. In the 2C T cell adoptive transfer system, anti-CD40 treatment of mice led to a markedly enhanced ability of 2C T cells to proliferate and accumulate in hosts harboring i.v. C1498.SIY cells (Figure 6A), which suggests that deletion of antigen-specific T cells was prevented. CD40 ligation also led to markedly enhanced production of IFN-γ and TNF-α by antigen-specific 2C T cells (Figure 6B).
Figure 6. Agonistic CD40 ligation prevents T cell deletion, priming large numbers of activated T cells, in mice harboring C1498.SIY cells i.v.
(A) CFSE dilution of 2C T cells 7 days after transfer into C57BL/6 mice challenged with i.v. C1498.SIY cells and treated with anti-CD40 or isotype control Ab (IC). (B) Splenocytes from mice in A were restimulated with media or SIY peptide, and IFN-γ and TNF-α production by 2C T cells was assessed. Numbers represent percent cytokine-producing 2C T cells. (C) C57BL/6 mice received i.v. or s.c. C1498.SIY cells and were treated with anti-CD40 or isotype control Ab. On day 6, percent SIY-reactive splenic CD8+ T cells was analyzed. A negative control OVA tetramer was also used. *P < 0.05 versus all other groups. (D) IFN-γ ELISPOT analysis of splenocytes from mice in C. *P < 0.05 versus control Ab. (E) C57BL/6 mice received C1498.SIY cells i.v. on day –6 and were treated with anti-CD40 or isotype control Ab on days –6 and –3. On day 0, these mice were challenged with C1498.SIY cells s.c. Control mice received C1498.SIY cells i.v. or s.c. on day 0 only. IFN-γ ELISPOT analysis was performed on day 6. (F) C57BL/6 mice received C1498.SIY cells i.v. On days 0, 2, and 4, anti-CD40 or isotype control Ab was administered, and survival was assessed. *P = 0.002 versus control Ab. (G) C57BL/6 mice received i.v. C1498.SIY cells on day 0. On days 8, 10, 12, 17, 22, and 27, anti-CD40 or isotype control Ab was administered, and survival was assessed. *P = 0.05 versus control Ab. (H) C57BL/6 mice received FBL cells i.v. on day 0. On days 5, 7, 9, 13, and 18, anti-CD40 or isotype control Ab was administered, and survival was assessed. *P < 0.05 versus control Ab. Data are representative of 2 independent experiments with 3 (A–E) or 5 (F–H) mice/group.
We then examined the effect of anti-CD40 mAb on the endogenous T cell response to i.v. C1498.SIY cells. Anti-CD40 mAb induced markedly higher frequencies and absolute numbers of endogenous SIY-specific CD8+ T cells in C57BL/6 mice with i.v. C1498.SIY cell challenge compared with those seen in isotype control Ab–treated mice (Figure 6C and data not shown). In contrast, anti-CD40 treatment had no significant effect on the frequency of SIY-reactive CD8+ T cells in mice after s.c. C1498.SIY cell challenge (Figure 6C). Similarly, functional SIY-specific T cell responses were strikingly enhanced in mice after i.v. C1498.SIY cell inoculation and anti-CD40 treatment. Again, anti-CD40 treatment did not significantly augment the already robust functional SIY-specific T cell responses that occurred naturally after s.c. C1498.SIY cell challenge (Figure 6D). Furthermore, anti-CD40 treatment prevented the T cell tolerance induced by i.v. C1498.SIY cell inoculation in i.v./s.c. C1498.SIY cell dual-challenged mice, as measured by functional SIY-specific T cell responses (Figure 6E).
In keeping with augmented SIY-specific T cell responses, significantly prolonged survival — and, in some cases, disease cure — was observed in mice after i.v. C1498.SIY cell inoculation and treatment with anti-CD40 versus isotype control Ab (Figure 6F), even when i.v. C1498.SIY cells were established 8 days prior to initiation of anti-CD40 treatment (Figure 6G). To determine whether anti-CD40 treatment could prolong survival in a second transplantable AML model, groups of C57BL/6 mice received i.v. challenge with FBL cells. Because of the aggressive nature of FBL (death within 2.5 weeks of i.v. challenge with 105 FBL cells), C57BL/6 mice were inoculated with i.v. FBL cells and treated with anti-CD40 or isotype control Ab 5 days later. Similar to what was observed in the C1498 model, anti-CD40 treatment of C57BL/6 mice harboring i.v. FBL cells led to an impressive prolongation of survival (Figure 6H). Collectively, these results argue that the T cell–tolerant state generated in mice with i.v. C1498.SIY cells is likely regulated by tolerogenic host APCs, in a way that can be prevented and, more importantly, reversed in vivo after treatment with an agonistic anti-CD40 Ab.
Discussion
The mechanisms that regulate T cell activation versus tolerance in the setting of hematological malignancies such as AML have not been well clarified. A more thorough understanding of these pathways is important in order to ultimately develop strategies to enhance leukemia-specific immunity in patients. Our results revealed a striking contrast between the nature of antigen-specific T cell responses generated against malignant cells introduced at a local s.c. site versus those inoculated systemically. In the former scenario, robust antigen-specific CD8+ T cell responses were induced, while in the latter, a profound state of antigen-specific T cell tolerance was generated. This peripheral tolerance appeared to result from a combination of T cell deletion and T cell–intrinsic dysfunction. An overarching implication of the present results is that leukemia cells may promote immune evasion indirectly through host APCs that cross-present leukemia-derived antigens in a context unfavorable for T cell activation.
The observation that T cell deletion played an important role in the promotion of immune dysfunction generated by AML cells has not been described in other experimental tumor models. Ohlen and colleagues used FBL in order to study the CD8+ T cell response to an immunodominant epitope (Gag) expressed by FBL cells, in a setting in which the Gag protein was also transgenically expressed in the liver and, to a lesser extent, the thymus (Alb:Gag mice) (28). In this model, tolerant Gag-specific CD8+ T cells failed to proliferate or produce IL-2 upon restimulation and demonstrated abnormal calcium flux and Ras/MAPK signaling, a picture most consistent with T cell anergy, which was later demonstrated to be reversible after IL-15 administration (15, 28). However, Tg expression of the target antigen in the liver likely skewed the peripheral tolerance mechanism toward anergy as the dominant outcome; the tolerance in our experiments resulted from antigen derived only from the leukemia cells. Staveley-O’Carroll et al. developed a model in which A20 lymphoma cells were engineered to express a model MHC class II–restricted antigen derived from the influenza virus (HA), and showed that naive HA-specific CD4+ TCR Tg T cells became anergic after their adoptive transfer into hosts along with systemic challenge with A20-HA cells (12). The induction of anergy in this model was not generated by the A20 cells themselves, but rather depended upon host APCs, as it did not occur in bone marrow chimeric mice in which hematopoietic cells were incapable of cross-presenting the HA antigen to CD4+ T cells (13). Similarly, in the current model, it is unlikely that C1498 cells were acting as suppressive APCs after their i.v. inoculation into mice, as they expressed similar levels of MHC class I molecules and failed to upregulate costimulatory molecules, such as B7-1, B7-2, or CD40 when analyzed directly ex vivo from hosts into which they had been inoculated i.v. or s.c. (data not shown). These observations support our findings suggesting a role for host APCs in the induction of tolerance in hosts with hematological malignancies. In contrast to prior models, T cell tolerance in hosts with systemic dissemination of C1498 leukemia involved a combined effect of deletion and T cell dysfunction to explain peripheral tolerance, which could be prevented with CD40 ligation on host APCs.
It is interesting to speculate that T cell deletion and anergy might represent a continuum of dysfunctional T cell activation. Whether a T cell becomes functionally activated, is anergized, or is deleted likely depends upon the affinity of the TCR for its antigen and the context in which the antigen is encountered. For example, Sherman et al. have demonstrated that TCR Tg CD8+ T cells were instructed to undergo an abortive proliferative response and to become tolerant upon transfer into mice in which the cognate antigen was cross-presented by quiescent APCs in a noninflamed LN environment (29, 30). By administering the antigenic peptide systemically into mice, it was determined that higher doses of antigen led to T cell anergy, while repeated low doses of antigen promoted T cell deletion (31). While T cell deletion appears to be a major mechanism of T cell tolerance in the C1498 model, it is likely that the small number of antigen-specific T cells that escaped deletion may had been rendered anergic. 2C T cells analyzed from mice with i.v. C1498.SIY produced significantly lower levels of IFN-γ and TNF-α compared with 2C T cells from mice with s.c. C1498.SIY, consistent with this notion.
Recent data from transplantable solid tumor models have indicated that innate signals, such as type I IFNs (18, 32), ATP (33), uric acid (34), tumor cell–derived DNA, and HMGB-1 (17), can be produced or released locally in the solid tumor microenvironment and lead to an adaptive T cell response against tumor antigens. However, because leukemia cells progressing in the circulation may not be capable of inducing the level of local inflammation necessary for productive T cell priming, it is possible that the APCs that cross-present leukemia-specific antigens do so in a context not favorable to T cell activation, and rather, they induce T cell tolerance.
An area of ongoing research in our laboratory is to define more precisely which APC populations mediate antigen-specific T cell tolerance to leukemia. It is conceivable that a specific APC subpopulation (35, 36), or that an immature activation state of any host APC, is responsible for promoting T cell tolerance. Future characterization of this mechanism may allow further refinements in strategies to prevent and/or reverse leukemia-induced tolerance.
Our results have 2 important implications for clinical translation of immunotherapeutic approaches in AML. First, the ideal scenario for promoting a leukemia-specific T cell response will likely be in the minimal residual disease setting, for example, after remission-induction therapy, so that the systemic delivery of leukemia-derived antigens that promote tolerance will be minimized and immune reconstitution of the host will have occurred. Second, our results suggest that agonistic CD40 Ab should be explored in patients with AML, a strategy that has become feasible given the availability of clinical-grade anti-CD40 Abs being explored for cancer immunotherapy (37, 38).
Methods
Mice and tumor cell lines.
C57BL/6 (H-2b) mice, aged 6–12 weeks, were purchased from either Jackson Laboratories or Taconic laboratories. Thy1.1+ congenic C57BL/6 mice were purchased from Jackson Laboratories and bred in our facility. 2C TCR-Tg mice on the C57BL/6 background (39) were bred in our animal facility. Bcl-XL Tg mice, in which BCL-XL expression is controlled by the LCK promoter, and is thus T cell specific, have been reported previously (40) and were a gift from A. Sperling (University of Chicago). 2CBCL-XL double-Tg mice were generated through cross-breeding in our animal facility. FoxP3-DTR animals (41) were obtained from A. Chervonsky (University of Chicago), with permission from A. Rudensky. Animals were maintained in a specific pathogen–free environment. The C1498 murine AML cell line (19) was purchased from ATCC. C1498 cells were cultured in complete DMEM supplemented with 10% fetal calf serum. C1498.GFP cells were engineered by retroviral transduction using the pLEGFP plasmid; C1498.SIY cells were engineered by retroviral transduction using the pLEGFP plasmid expressing cDNA for the SIY model peptide antigen in frame with eGFP. Cell surface expression of the SIY peptide is Kb restricted, and thus can be recognized by a small fraction of endogenous C57BL/6 CD8+ T cells and is also specifically recognized by the 2C TCR Tg CD8+ T cells. B16.OVA cells, expressing the full-length chicken OVA protein, were a gift from Y.-X. Fu (University of Chicago). The FBL cell line is an MHC class I+, MHC class II– AML cell line expressing the FMuLV gag peptide (CCLCLTVFL), which is presented in the context of Kb.
Tumor cell inoculation.
C1498, C1498.GFP, C1498.SIY, and B16.OVA cells were washed 3 times with PBS to remove FCS and resuspended in PBS at a concentration of 106–107 cells/ml. For i.v. challenge, a volume of 0.1 ml (105–106 tumor cells) was injected into the lateral tail vein of each mouse. For s.c. challenge, a volume of 0.1 ml (106 tumor cells) was injected under the skin of the right lower lateral abdominal wall. For experiments with FBL, 105 cells were inoculated i.v. or s.c.
IFN-γ ELISPOT.
ELISPOT was conducted with the BD Bioscience mouse IFN-γ ELISPOT kit according to the provided protocol. Briefly, ELISPOT plates were coated with anti-mouse IFN-γ Ab and stored overnight at 4°C. Plates were then washed and blocked with DMEM supplemented with 10% FCS for 2 hours at room temperature. Splenocytes or LN cells (DLNs for s.c. inoculation; pooled inguinal and axillary LNs for i.v. inoculation) from individual tumor-challenged mice were harvested at various time points and plated in triplicate at between 5 × 105 and 1 × 106 cells/well. Unless otherwise indicated, stimulation was performed with irradiated (150 Gy) C1498 cells (5 × 104 cells/well) or SIY peptide (80 nM). Stimulation with media alone or with PMA (50 ng/ml) and ionomycin (500 nM) served as negative and positive controls, respectively. Plates were stored at 37°C in an 8% CO2 incubator overnight, washed, and coated with detection Ab for 2 hours at room temperature. Plates were again washed and coated with avidin peroxidase for 1 hour at room temperature, then washed and developed by addition of AEC substrate. Developed plates were dried overnight, read using an ImmunoSpot Series 3 Analyzer, and analyzed with ImmunoSpot software.
Pentamer staining and FACS analysis.
The SIY and negative control OVA peptide pentamers were purchased from Proimmune. After cell surface staining with anti-CD4–PerCP-Cy5.5 and anti-B220–PerCP-Cy5.5 Abs (for exclusion of CD4+ T cells and B cells, respectively) as well as an anti-CD8–allophycocyanin Ab, pentamer staining was performed on spleen or LN cells from individual C1498-challenged mice according to the manufacturer’s protocol. 2C T cells were recognized using an anti-Thy1.2–PE Ab (in experiments using congenic Thy1.1 mice as tumor-bearing recipients) or with a 2C TCR-specific biotinylated 1B2 Ab, followed by secondary staining with streptavidin-PE or streptavidin-allophycocyanin. FACS analysis was performed on a FACScanto cytometer using BD FACSDiva software. Data analysis was performed using FlowJo software (Tree Star Inc.).
Adoptive transfer of 2C T cells into mice harboring C1498.SIY cells.
2C T cells were purified from 2C C57BL/6 mice by positive selection using CD8 microbeads (Miltenyi). Purified 2C T cells were resuspended at a concentration of 107 cells/ml and washed twice with cold PBS. Labeling with CFSE (5 μM) was performed for 6 minutes at room temperature. Subsequently, cells were washed with cold FCS, washed 3 times with complete DMEM, and resuspended in PBS at a concentration of 4 × 107 cells/ml. 4 × 106 CFSE-labeled 2C T cells (0.1 ml) were injected into C57BL/6 mice i.v. through the lateral tail vein. 24 hours later, mice received C1498.SIY cells i.v. or s.c. as above. A cohort of mice receiving 2C T cell transfer, but no C1498.SIY cell challenge, served as control. 6 days after C1498.SIY cell challenge, spleens and LNs of tumor-challenged mice were harvested and analyzed by flow cytometry for CFSE dilution after staining with either an anti-Thy1.2–allophycocyanin Ab or a combination of anti-CD8–allophycocyanin and anti-1B2–biotin Abs, followed by secondary labeling with streptavidin-PE. The number of events acquired during flow cytometry was kept constant between samples for data analysis purposes. Absolute numbers of 2C cells were calculated by multiplying the total number of live spleen or LN cells by percent CD8+ T cells present and, finally, by percent Thy1.2+ or 1B2+CD8+ cells present per sample.
Intracellular cytokine staining.
Spleens and LNs were harvested from C57BL/6 mice 6 days after 2C T cell transfer and C1498.SIY cell challenge as described above. 106 spleen or LN cells from individual mice were plated in flat-bottomed 96-well tissue culture plates and stimulated with medium alone, or in medium supplemented with SIY peptide (500 nM), or PMA and ionomycin at 37°C for 4 hours in the presence of brefeldin-A (1 μg/ml). Subsequently, cells were recollected, stained with anti-Thy1.2–allophycocyanin or anti-1B2–biotin Ab and streptavidin-PE or PerCP-Cy5.5 in combination with an anti-CD8–allophycocyanin Ab. After washing, wells were fixed (Cytofix; BD Bioscience) for 10 minutes at room temperature; permeabilized; stained with anti–IFN-γ–PE-Cy7 and/or anti–TNF-α–PE Abs; and analyzed for cytokine production by flow cytometry after gating on 2C T cells (Thy1.2+ or 1B2+CD8+).
In vivo administration of agonistic anti-CD40 Ab.
Groups of C57BL/6 mice were challenged with 106 C1498.SIY cells i.v. or s.c. on day 0, or remained tumor free. On days 0, 2, and 4, mice received i.p. injection of agonistic anti-CD40 Ab (FGK45; 100 μg) or isotype control Ab. On day 6, spleen and LN cells from tumor-challenged and naive mice treated with anti-CD40 or isotype control Ab were isolated, analyzed by flow cytometry after SIY or OVA pentamer staining (as above), and also restimulated using IFN-γ ELISPOT assay (as above).
Statistics.
A 2-tailed Student’s t test was used to analyze differences in numbers of IFN-γ spot-forming cells and SIY/Kb pentamer–reactive CD8+ cells in individual mice assigned to various treatment groups, and also to compare numbers of 2C T cells present in mice challenged with i.v. versus s.c. C1498.SIY cells. A P value of 0.05 or less between groups was considered statistically significant. The log-rank test was used to compare survival differences between groups of tumor-challenged mice. Data are presented as mean ± SD in all experiments performed.
Study approval.
Animals were used according to protocols approved by the IACUC of University of Chicago according to NIH guidelines for animal use.
Supplementary Material
Acknowledgments
This work was supported by K23 CA133196 and R01 CA16670 (to J. Kline).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(5):1999–2010. doi:10.1172/JCI63980.
References
- 1.Urban JL. Tumor antigens. Annu Rev Immunol. 1992;10:617–644. doi: 10.1146/annurev.iy.10.040192.003153. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rech AJ. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244(1):218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kline J. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14(10):3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 9.Uyttenhove C. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 10.Grosso JF. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmond WL. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39(8):2184–2194. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staveley-O’Carroll K. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotomayor EM. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98(4):1070–1077. doi: 10.1182/blood.V98.4.1070. [DOI] [PubMed] [Google Scholar]
- 14.Sotomayor EM. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96(20):11476–11481. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teague RM. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12(3):335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 16.Obeid M. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 17.Apetoh L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 18.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116(14):2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl L. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5(7):774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 22.Sotomayor EM. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5(7):780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 23.Staveley-O’Carroll K. In vivo ligation of CD40 enhances priming against the endogenous tumor antigen and promotes CD8+ T cell effector function in SV40 T antigen transgenic mice. J Immunol. 2003;171(2):697–707. doi: 10.4049/jimmunol.171.2.697. [DOI] [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wekerle T. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14(5):592–600. doi: 10.1016/S0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 27.French RR. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5(5):548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 28.Ohlen C. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195(11):1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez J. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194(6):707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez J. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002;196(3):323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redmond WL. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174(4):2046–2053. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 32.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghiringhelli F. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 34.Hu DE. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64(15):5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 35.Asano K, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34(1):85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Iyoda T. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonderheide RH. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 39.Spiotto MT. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17(6):737–747. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 40.Chao DT. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182(3):821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JM. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.