Abstract
Objective:
To describe the radiological and clinical features of adult non-puerperal mastitis and to determine the most accurate method of preventing unnecessary surgical procedures.
Methods:
Clinical and imaging findings were retrospectively reviewed in 51 females with non-puerperal mastitis, which was confirmed by biopsy/surgical pathology. All 51 patients had pre-operative MRI; 45 patients also had sonograms and 25 also had mammograms, pre-operatively.
Results:
Of the 51 cases with non-puerperal mastitis, 94.1% (48/51) were confirmed as having acute or chronic inflammation, and the other 3 had plasma cell mastitis; areola papillaris inflammation was found in 39.2% (20/51) of the cases. Overall, 6 of the 25 cases that were examined with mammography and 2 of the 45 cases that were examined with sonography appeared normal, but all 51 lesions were positively identified on MRI. Asymmetrical density (12/25) on mammograms and solitary or separated/contiguous, clustered, hypoechoic mass-like lesions (31/45) on ultrasound were the most common signs of non-puerperal mastitis. On enhanced MRI, 90.2% (46/51) of patients showed non-mass-like enhanced lesions. Multiple regional enhancements in the pattern of distribution (32/46) and separated or contiguous, clustered, rim-like enhancements in the pattern of internal enhancement (29/46) were the most common manifestations in non-mass-like enhanced lesions. Of the 51 patients, mastitis Type 1 and Type 2 in the time–signal intensity curve were detected in 47.1% and 51.0% of the patients, respectively. The breast imaging reporting and data system categories with the highest number of patients were Category 0 (9/25) on mammography, Category 4a on sonography (18/45) and Category 4a on MRI (29/51).
Conclusion:
The findings from mammography and ultrasound are non-specific; therefore, using MR can be helpful in the diagnosis, especially in the presence of non-mass-like enhancements that are multiple, regional, separated, or contiguous, clustered and rim-like.
Advances in knowledge:
Mastitis is often neglected because of the lack of typical clinical signs and symptoms. This study has assessed and described the clinical features and imaging findings of adult non-puerperal mastitis on mammograms, sonograms and MRI and found that MRI is more specific in the diagnosis of disease.
Non-puerperal mastitis, also known as non-lactating mastitis, encompasses all the causes of inflammatory changes in the female breast and mammilla not related to lactation. This inflammation of the breast is often neglected because of the lack of typical clinical signs and symptoms. The aetiology of this disease is still unknown, but it may or may not be accompanied by infection [1,2]. Non-puerperal mastitis includes a wide spectrum of different disorders, such as newborn mastitis, thelitis in young females, bacterial infections, non-bacterial infections that generally originate from deeper milk ducts (such as plasma cell mastitis and granulomatous mastitis), as well as inflammatory disorders of the breast skin [2–6]. The majority of patients with non-puerperal mastitis usually exhibit one or several breast masses without any other signs of inflammation. Because it can be very difficult to distinguish these symptoms from breast carcinoma, these patients can easily be misdiagnosed as having an inflammatory breast carcinoma or an invasive carcinoma [7–9].
Non-puerperal mastitis does not require surgery to be treated; therefore, a correct diagnosis will avoid unnecessary surgery. However, the appearance of non-puerperal mastitis on mammography and sonography is non-specific [5,10–13]. MR mammography is useful for the detection of non-puerperal mastitis, but the published literature on using MRI to detect non-puerperal mastitis is sparse [7,13,14]. The purpose of this study is to assess and describe the clinical features and image findings of adult non-puerperal mastitis on mammograms, sonograms and MRI as well as to discover the best method for accurately determining the disease.
Materials and methods
Patient population
We retrospectively reviewed the clinical and imaging records of 51 female patients who had biopsy/surgical pathology confirmed as non-puerperal mastitis collected between January 2007 and March 2011. All patients had pre-operative MRI. Of these patients, pre-operative sonography and mammography were performed on 45 and 25 patients, respectively. Patients were between 19 and 67 years old with a mean age of 37.5 years. Of these 51 patients, 47 were pre-menopausal and 4 were post-menopausal. 22 patients had a history of breast-feeding, but without a history of lactation mastitis. 44 patients had one or several breast lumps, including 3 with an operation history and 1 with a history of trauma. Five cases had only breast pain, and two cases had nipple discharge. The duration of the symptoms was between several days and 3 months. 31 cases were confirmed by core needle biopsy, and 20 were confirmed by surgical pathology.
Imaging techniques
Bilateral digital mammography was performed using the GE Senographe™ 2000D (GE Medical Systems, San Francisco, CA) or the Hologic® Selenia (Hologic Medical Systems, Bedford, MA). Bilateral breast images were obtained in the craniocaudal and mediolateral oblique views. Bilateral whole-breast ultrasound was performed before biopsy using a linear array broadband transducer [Aloka 5500 (Hitachi, Tokyo, Japan); IU 22 (Philips, Andover, MA); ACUSON (Siemens, Washington, DC)] with a centre frequency of 3.5–10 MHz. Ultrasound was performed by three ultrasound technologists with 3–15 years of experience in breast ultrasound. MRI was performed using a 1.5-T or 3.0-T twin speed scanner (Signa Twin speed Excite; GE Medical Systems). Patients were positioned prone in the dedicated breast coil. Routine sagittal and axial T1 weighted, high-resolution images were obtained before and after the administration of contrast material, with repetition time (TR)=480 s and echo time (TE)=10 s. The T2 weighted acquisition was performed using a fat-suppressed two-dimensional (2D) fast spin-echo sequence with TR=3200 ms, TE=85 ms, a 5-mm slice thickness and a 1-mm intersection gap. Dynamic imaging was performed utilising a T1 weighted, 2D fast spoiled gradient recalled echo sequence, with TR=200 ms, TE=5 ms, flip angle=80°, matrix size=256×160, number of excitations=1 and a field of view between 32 cm and 38 cm for bilateral axial view imaging. The contrast medium (Magnevist®; Schering, Berlin, Germany, 0.1 mmol kg−1) was administered as a bolus injection, followed by a 20-ml saline flush. The scans began after contrast injection at times of 0, 1, 2, 4 and 7 min, with a scanning time of 45 s. The time–signal intensity curves are categorised into one of the three types: a Type 1 (persistent enhancement) pattern was assigned if the signal intensity increased steadily throughout the dynamic period, a Type 2 (plateau) pattern was assigned if the peak signal intensity was reached soon after the injection of the contrast agents and followed by a signal intensity plateau in the remaining dynamic series and a Type 3 (washout) pattern was assigned if the peak signal intensity was reached in the early phase and was immediately followed by a loss of signal intensity soon after the injection of the contrast agent [15].
Imaging interpretation and data analysis
All the patients’ images (including mammography and MRI) were independently interpreted by two experienced radiologists with 5 or more years of radiological experience. Radiologists were blinded to the final pathological results, and the imaging features were described according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon [16,17]. Ultrasound findings were interpreted by two experienced ultrasound experts. The features of the lesion, including echo, shape, margin and vascularity, were recorded and classified according to the ultrasound BI-RADS description [18]. If the two readers differed in their assignment of a BI-RADS category, a consensus was reached through discussion. BI-RADS Categories 1–3 are considered benign, 4a is defined as a low-grade suspicious malignant lesion and 4b–5 are considered a malignant tumour; Category 0 is considered an incomplete diagnosis. To evaluate the blood vessels in non-puerperal mastitis on blood signal in colour Doppler ultrasound imaging (CDFI), we used a semi-quantitative analysis method. The vascularity was classified into three grades: grade I, in which the blood vessels are not present or not assessed; grade II, in which 1–3 punctate or rod-like blood vessels are present in the lesion or immediately adjacent to the lesion, and grade III, in which >3 punctate or rod-like blood vessels are present in the lesion or the surrounding tissue [19]. The size of the mastitis was measured using the longer diameter of the whole inflammatory lesion (this was performed because the majority of lesions are diffuse and multiple) and classified as <2.0, 2.0–5.0 or >5.0 cm. We then compared the image findings with the pathology results.
Results
The clinical features are shown in Table 1. Of the 51 cases of non-puerperal mastitis, 48 patients (94.1%, 48/51) were confirmed as having acute or chronic inflammation, and 3 patients (5.9%, 3/51) were diagnosed with plasma cell mastitis. 30 inflammatory lesions were located in the left breast, and 21 were located in the right breast. Red swelling occurred in 15 patients, and breast pain occurred in 5 patients. Of the cases, 13.7% (7/51, with an average 1.3 cm) were <2.0 cm, 51.0% (26/51, average 3.7 cm) were between 2.0 and 5.0 cm and 35.3% (18/51, average 6.3 cm) were measured to be >5.0 cm. There were 20 (39.2%) cases with areola papillaris inflammation (including 3 cases only located within the areola papillaris). 23 lesions were found in 1 quadrant (45.1%), including the upper outer (n=9), lower outer (n=8), upper inner (n=5) and lower inner (n=1). 19 were found in 2 quadrants (37.2%), and 6 were found in 3 quadrants (11.8%). Only three lesions were within the subareolar location (5.9%). In addition, 13 patients were found with an enlargement of the axillary lymph nodes, and 2 cases presented with a retracted nipple.
Table 1.
Clinical features of 51 patients with non-puerperal mastitis
| Features | n | Percentage (%) |
| Histology | ||
| Acute/chronic inflammation | 48 | 94.1 |
| Plasma cell mastitis | 3 | 5.9 |
| Red and swollen breast | 15 | 29.4 |
| Mastalgia | 5 | 9.8 |
| Lump in breast | ||
| Diameter <2.0 cm | 7 (average 1.3 cm) | 13.7 |
| Diameter 2.0–5.0 cm | 26 (average 3.7 cm) | 51.0 |
| Diameter >5.0 cm | 18 (average 6.3 cm) | 35.3 |
| Location | ||
| One quadrant | 23 | 45.1 |
| Two quadrants | 19 | 37.2 |
| Three quadrants | 6 | 11.8 |
| Subareolar | 3 | 5.9 |
| Axillary adenopathy | 13 | 25.5 |
| Retracted nipple | 2 | 3.9 |
Mammography was performed in 25 cases, including 23 with acute or chronic inflammation and 2 with plasma cell mastitis. The mammographic findings are listed in Table 2. In our study, 64% (16/25) of patients had Type III and IV mammary glands (>50% dense tissue). Mammography showed focal/diffuse asymmetrical density (n=12, 48%) (Figure 1), architectural disorder (n=4, 12%), ill-defined masses (n=2) (Figure 2), well-defined masses (n=1) and normal parenchymal pattern (n=6, 24%). Two cases involved the nipple itself. On mammography, none of the lesions showed calcification and spiculation, but one lesion showed skin thickening in the mammary areola. One case of plasma cell mastitis showed a normal parenchymal pattern on mammography, and the other case showed focal asymmetry density in our study.
Table 2.
Mammographic findings of 25 patients with non-puerperal mastitis
| Mammographic findings | n | Percentage (%) |
| Types of mammary gland | ||
| Type I (<25%) | 2 | 8 |
| Type II (≥25%, ≤50%) | 7 | 28 |
| Type III (>50%, <75%) | 8 | 32 |
| Type IV (≥75%) | 8 | 32 |
| Lesion description | ||
| Asymmetry density | 12 | 48 |
| Architectural disorder | 4 | 16 |
| Mass | 3 | 12 |
| Normal parenchymal pattern | 6 | 24 |
| Nipple involved | 2 | 8 |
Figure 1.
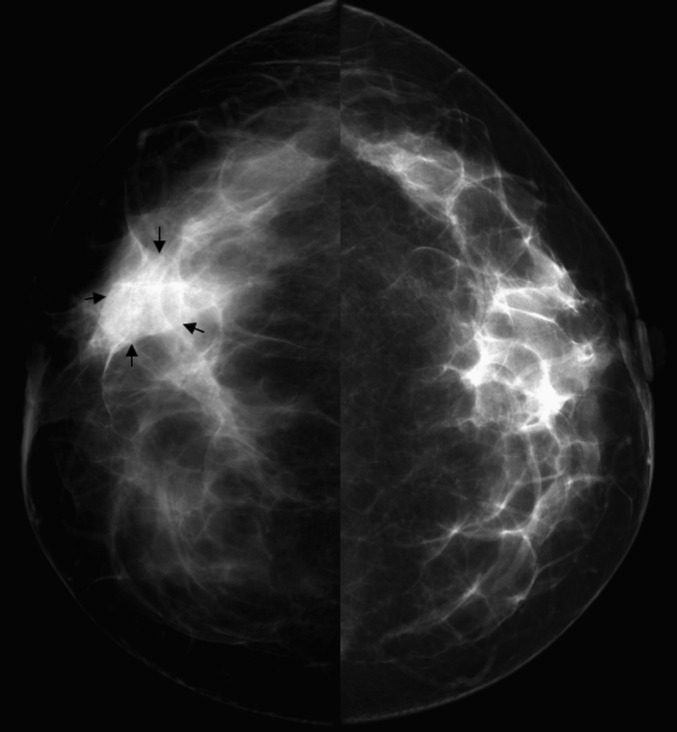
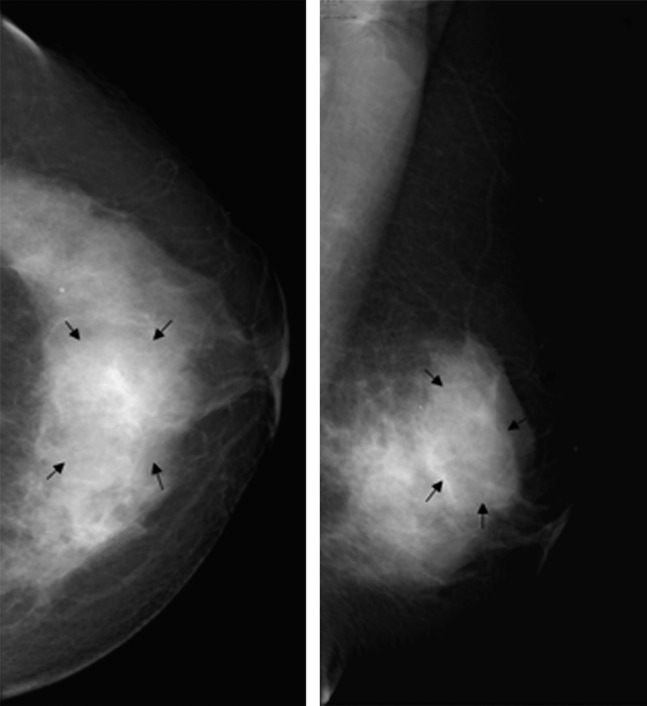
Female, 43 years old, chronic mastitis. Mammography showed a focal asymmetrical density in the right breast (arrows).
Figure 2.
Female, 34 years old, chronic mastitis. Mammography demonstrated an ill-defined, high-density mass in the left breast (arrows).
Of the 51 non-puerperal mastitis patients, 45 underwent sonography. The sonographic findings are shown in Table 3. 2 patients exhibited normal sonograms, while the majority of non-puerperal mastitis cases showed lesions that were irregular (83.7%, 36/43), heterogeneous (76.7%, 33/43), hypoechoic (83.7%, 36/43) and mass-like (68.9%, 31/45). Among the 31 sonograms showing mass-like lesions, the lesions were clustered but separate in 13 patients (Figure 3) and contiguous in 8 patients (Figure 4). 10 lesions appeared as a simple mass, while 26.7% (12/45) of the lesions appeared separately or contiguously lamellar and as a tubular hypoechoic structure. The signal from the blood in CDFI showed grade I, grade II and grade III blood flow in 12 (27.9%), 16 (37.2%) and 15 (34.9%) lesions, respectively. Three patients were found to exhibit skin thickening on the sonogram.
Table 3.
Sonographic findings of 45 patients with non-puerperal mastitis
| Sonographic findings | n | Percentage (%) |
| Lesion description | ||
| Mass-like | 31 | 68.9 |
| Non-mass-like | 12 | 26.7 |
| Normal | 2 | 4.4 |
| Lesion morphology | ||
| Regular | 7 | 16.3 |
| Irregular | 36 | 83.7 |
| Lesion echo | ||
| Hypoecho | 36 | 83.7 |
| Iso/hyperecho | 3 | 7.0 |
| Mixed echo | 4 | 9.0 |
| Lesion margin | ||
| Well defined | 19 | 44.2 |
| Poorly defined | 24 | 55.8 |
| Lesion homogeneity | ||
| Homogeneous | 10 | 23.3 |
| Heterogeneous | 33 | 76.7 |
| Lesion vascularity | ||
| Grade I | 12 | 27.9 |
| Grade II | 16 | 37.2 |
| Grade III | 15 | 34.9 |
| Skin thickening | 3 | 6.7 |
Figure 3.
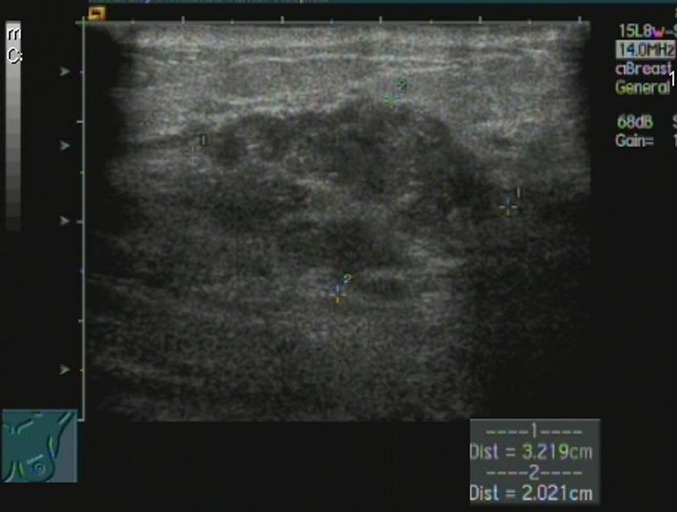
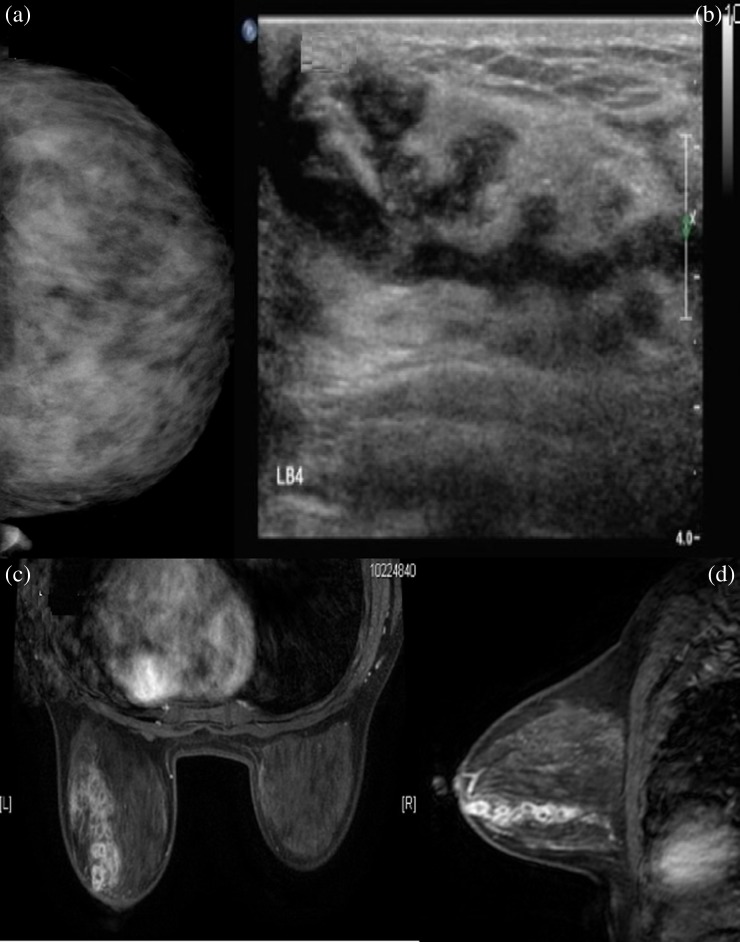
Female, 40 years old, acute/chronic mastitis. Sonogram showed a hypoechoic, mass-like lesion in the left breast, and the lesion was clustered but separate.
Figure 4.
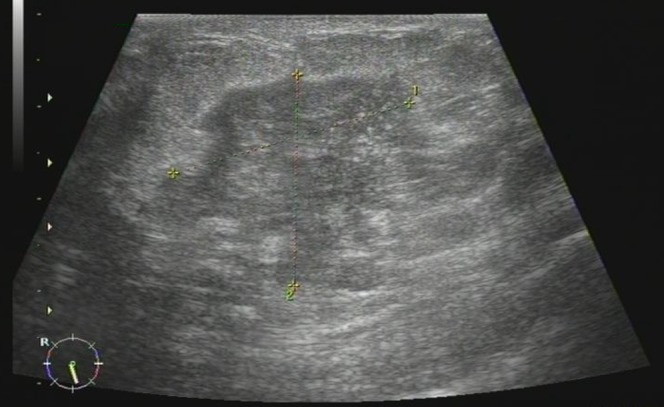
Female, 43 years old, chronic mastitis. Sonogram revealed a hypoechoic, mass-like lesion in the right breast, and the lesion showed a contiguous, clustered appearance.
Using enhanced MRI, all the inflammatory lesions were visible and the MRI findings are shown in Table 4. On T1 weighted images, the majority of the lesions were shown to be homogeneous (70.6%, 36/51). The lesions were found to have a low signal intensity (54.9%, 28/51) or equal signal intensity (45.1%, 23/51). All the lesions were found to have a high signal intensity in T2 weighted images, and 72.5% (37/51) of these signals were found to be heterogeneous. After enhancement, 90.2% (46/51) of patients showed non-mass-like enhanced lesions, with only 5 cases showing mass-like enhanced lesions. For the 46 non-mass-like enhanced lesions, 26.1% (12/46) were regional, 69.5% (32/46) were multiple regional, 2.2% (1/46) were segmental and 2.2% (1/46) were focal area distributed. Of the 12 regional and 32 multiple regional distributed lesions, 5 cases were complicated with segmental (n=2) and linear ductal (n=3) distribution patterns. With respect to internal enhancement, clustered rim-like enhancements were found in 29 patients (63.1%, 29/46), separate in 21 patients (Figure 5) and contiguous in 8 patients (Figure 6a). 10 patients (21.7%, 10/46) showed patchy enhancement (Figure 7), and 7 cases (15.2%, 7/46) showed heterogeneous enhancement. Of the 46 non-mass-like enhanced lesions, 30 cases (65.2%) were found directed to the nipple along the breast ducts (Figure 6d), and 4 had gross blood vessels behind the lesions (Figure 8). In the five mass-like lesions, two lesions were oval shaped, two were irregular shaped and one had a lobulated shape. Of the five lesions, four had an ill-defined margin. After enhancement, one lesion showed a heterogeneous enhancement while another exhibited rim-like enhancement. One of the lesions had a homogeneous enhancement (Figure 9). In comparison with the histological results, clustered rim-like enhancements were related to multiple small abscesses, which had formed among the inflammatory lesions. In all 51 patients, the percentages of the time–signal intensity curve in Type 1 (Figure 6b), Type 2 and Type 3 were 47.1%, 51.0% and 1.9%, respectively. Skin thickening was found in 17 patients, of whom 11 had skin thickening involving the skin of the areola. Five patients had a different degree of nipple retraction. Another five patients had axillary adenopathy, two of which were involved in the chest wall (Figure 10).
Table 4.
MRI findings of 51 patients with non-puerperal mastitis
| MRI findings | n | Percentage (%) |
| T1 weighted imaging | ||
| Low signal intensity | 28 | 54.9 |
| Iso-signal intensity | 23 | 45.1 |
| T2 weighted imaging | ||
| Homogeneous high signal | 14 | 27.5 |
| Heterogeneous high signal | 37 | 72.5 |
| Enhanced pattern | ||
| Non-mass-like | 46 | 90.2 |
| Mass-like | 5 | 9.8 |
| Time–signal intensity curve | ||
| Type 1 (persistent enhancement) | 24 | 47.1 |
| Type 2 (plateau) | 26 | 51.0 |
| Type 3 (washout) | 1 | 1.9 |
| Axillary adenopathy | 5 | 9.8 |
| Skin thickening | 17 | 33.3 |
Figure 5.
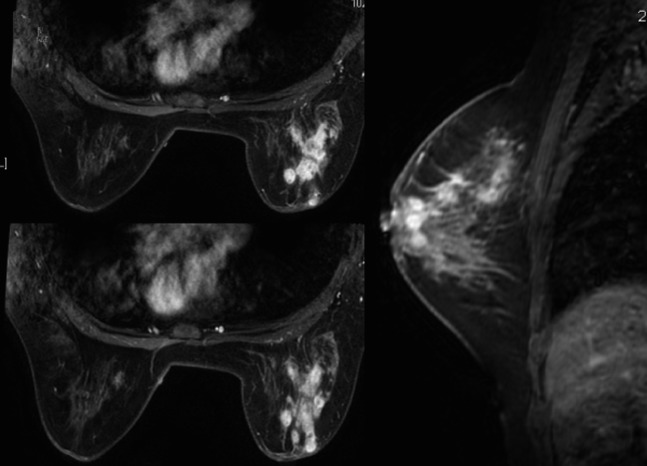
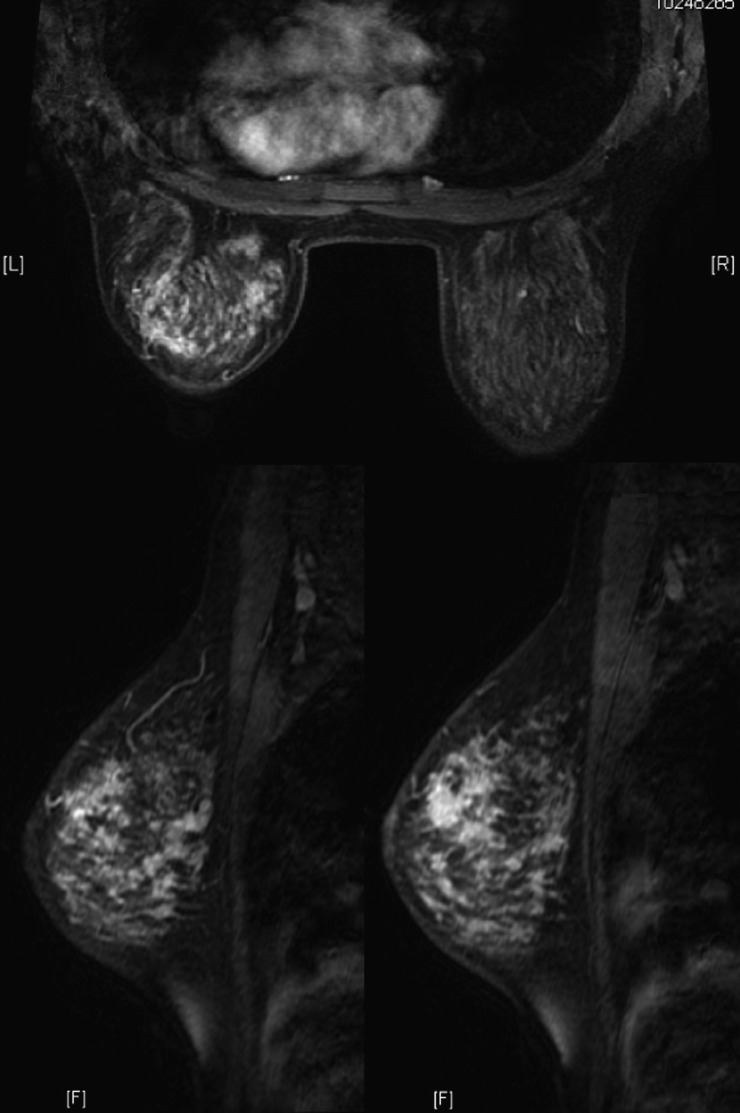
Female, 39 years old, chronic mastitis. Enhanced MR images showed multiple, separated, clustered, rim-like enhancements in the right breast.
Figure 6.
Female, 47 years old, acute/chronic mastitis. Enhanced MR image showed multiple, contiguous, clustered, rim-like enhancements in the left breast (a); Type 1 time–signal intensity curves were obtained (b, c) and the sagittal MR image showed a non-mass-like enhanced lesion directed to the nipple along the breast ducts (d).
Figure 7.
Female, 24 years old, acute mastitis. Enhanced MR images showed patchy enhancements.
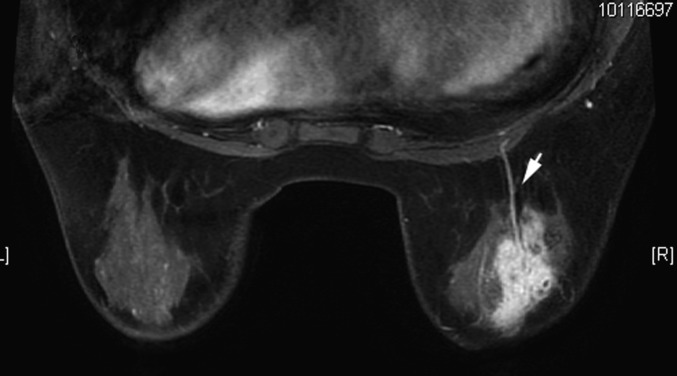
Figure 8.
Female, 39 years old, chronic mastitis. A gross blood vessel was found behind the heterogeneous enhanced lesion on enhanced MRI (arrow).
Figure 9.
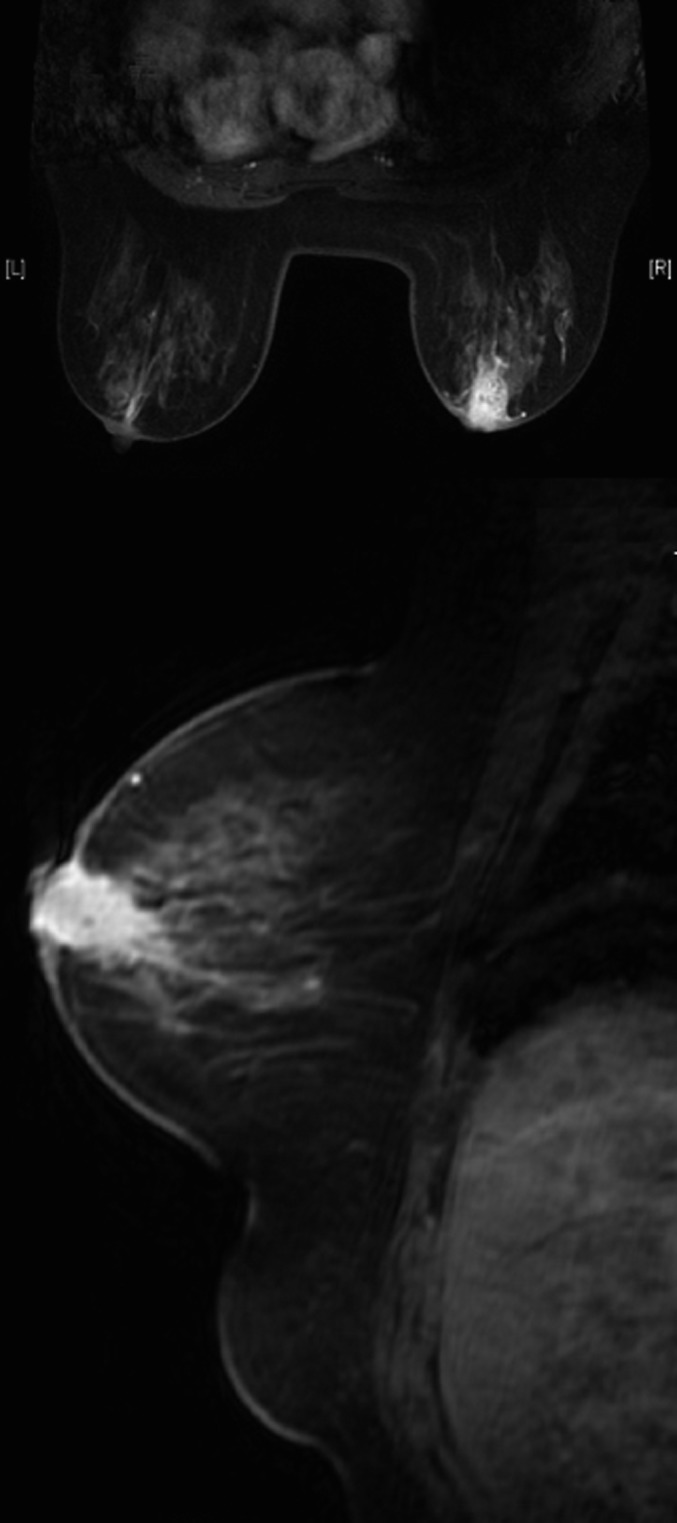
Female, 66 years old, chronic mastitis. MR images showed a subareolar, irregular shaped, homogeneous, enhanced mass in the right breast.
Figure 10.
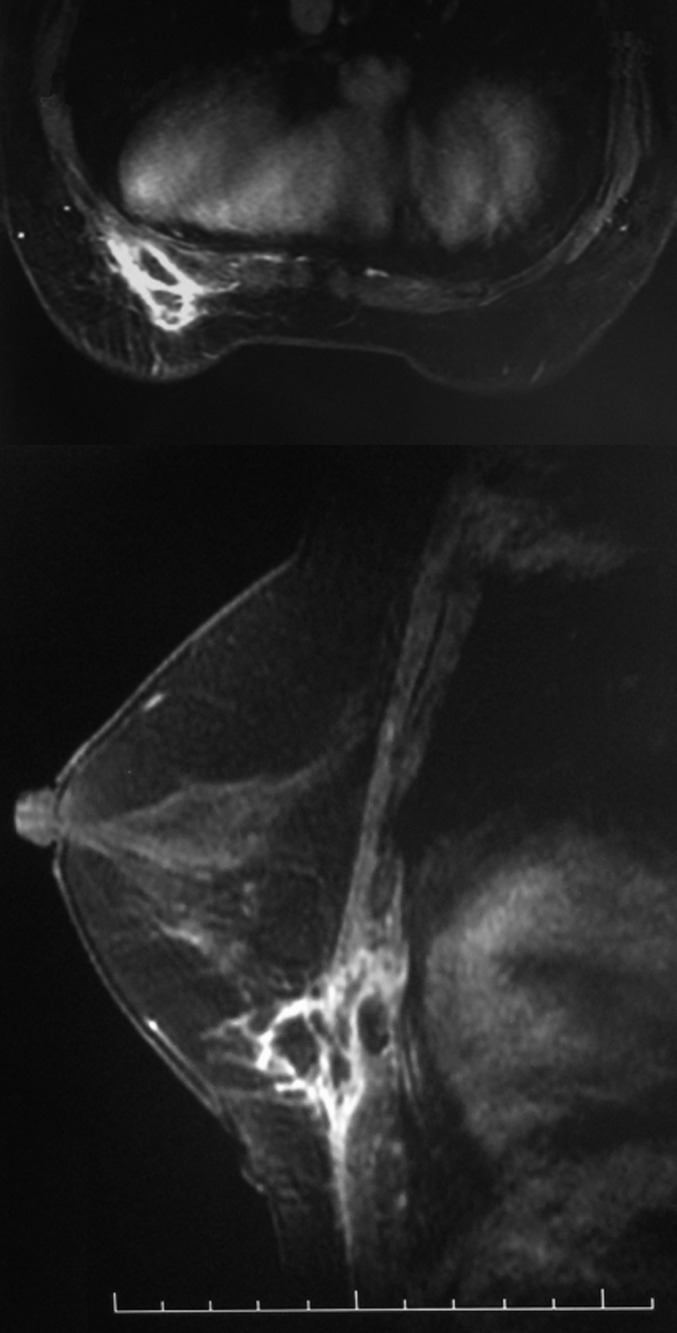
Female, 54 years old, chronic mastitis. Enhanced MR images showed that the chest wall was involved in the inflammatory lesion.
In our study, 6 of the 25 cases imaged by mammography and 2 of the 45 cases imaged by sonography were not positively detected; however, all 51 lesions were successfully identified using enhanced MRI. Table 5 shows the BI-RADS category assigned through mammography, ultrasound and MRI at diagnosis. The BI-RADS categories with the highest number of patients were Category 0 on mammography (36%, 9/25), Category 4a on sonography (40%, 18/45) and Category 4a on MRI (56.9%, 29/51). All the lesions were detected using enhanced MRI (Figure 11), and with a BI-RADS category no less than 4a; however, six of the cases (two with BI-RADS Category 0 and four with Category 3) imaged under mammography and two of the cases (each with BI-RADS Category 3 or lower) imaged using sonography appeared normal. Together, patients were categorised with BI-RADS Category ≥4b on mammography because these cases involved the nipple (n=2), skin thickening of the mammary areola (n=1) or a finding of an irregular mass (n=1). Of the 12 cases with a BI-RADS category ≥4b on sonography, 9 patients showed irregular mass-like lesions with an ill-defined margin and 3 exhibited skin thickening (including 2 which involved skin thickening of the mammary areola). On MRI, 22 patients were found to be in BI-RADS Category ≥4b. The MR images showed skin thickening (n=17), nipple retraction (n=5), axillary adenopathy (n=5) and chest wall involvement (n=2). If lesions labelled higher than BI-RADS Category 4b are considered to be malignant lesions, the rates of misdiagnosis using mammography, ultrasound and MRI would therefore be 16.0% (4/25), 26.7% (12/45) and 43.1% (22/51), respectively.
Table 5.
The Breast Imaging Reporting and Data System (BI-RADS) category of non-puerperal mastitis among the imaging techniques
| BI-RADS | Mammographya |
Ultrasound |
MRI |
Total (n) | |||||||||||||||
| 0 | 1–3 | 4a | 4b | 4c | 5 | 0 | 1–3 | 4a | 4b | 4c | 5 | 0 | 1–3 | 4a | 4b | 4c | 5 | ||
| Case (n) | 9 | 6 | 6 | 4 | 0 | 0 | 3 | 12 | 18 | 5 | 4 | 3 | 0 | 0 | 29 | 10 | 9 | 3 | 51 |
| Rate of misdiagnosisb (%) | 16.0 | 26.7 | 43.1 | ||||||||||||||||
Only 25 patients underwent a mammographic examination.
If the lesions with BI-RADS Category ≥4b are considered as malignant lesions, then this is the misdiagnosis rate.
Figure 11.
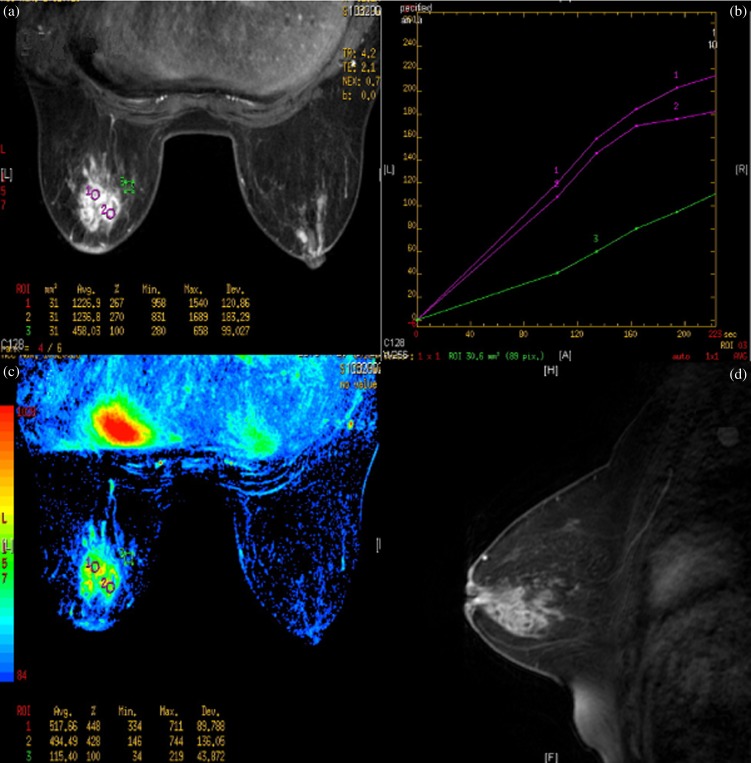
Female, 42 years old, acute/chronic mastitis. Mammogram showed diffuse asymmetrical density but with a Type IV mammary gland (≥75% dense tissue) (a); mammary sonogram revealed a multiple, contiguous, tubular hypoechoic lesion (b); enhanced MR images showed multiple, separated, clustered, rim-like enhancements in the left breast (c, d).
Discussion
The incidence of non-puerperal mastitis, which is clinically reported as currently increasing but <10%, is lower than that of puerperal mastitis [1,2]. Non-puerperal mastitis is not a single specific histopathological entity but includes a wide spectrum of different disorders [2–6]. The most frequently used classification subtypes are subareolar abscess of the breast, mastitis complicating fibrocystic disease, inflammation of a cyst and plasma cell mastitis [10,20]. Non-puerperal mastitis usually affects females under 40 years old [5,10,11,13], which was reflected in our study (the mean age of our patients was 37.5 years). Females with non-puerperal mastitis typically present with a breast mass that may be associated with pain, skin thickening or axillary adenopathy. Many females in our study were initially thought to have carcinoma because they presented with a unilateral mass and regional adenopathy and did not have a history or clinical findings suggestive of inflammation. Our study results showed that the majority of lesions were >2.0 cm and were located in one or several quadrants. This may be due to inflammatory lesions, which are spread along the breast ducts and the diffuse distributions. In our series, 39.2% involved areola papillaris inflammation; this number is slightly lower than other studies [10]. This may be due to our hospital specialising in the treatment of cancer, as patients tend to choose more general hospitals, unless they have highly suspicious, malignant disease. Antibiotic treatment is the primary clinical option for non-puerperal mastitis; therefore, a correct diagnosis of non-puerperal mastitis should not require surgery.
The mammographic and ultrasound findings of non-puerperal mastitis have been relatively well described. Mammographic findings are considered non-specific, and these data are in agreement with our findings. Although Lequin et al [10] first revealed non-circumscript lesions (24/41), other authors have described other findings such as multiple small masses, an irregular ill-defined mass or a focal asymmetric density to be more common [5,11–13,21]. Our study showed that the most common mammographic appearance was a focal/diffuse asymmetric density (12/25). Less common mammographic findings included architectural disorders (4/25), ill-defined masses (2/25) and well-defined masses (1/25). Lesions were mammographically undetectable in 6 of 25 females, possibly because of an overlying dense mammary gland pattern observed in most females. With mammography, two patients in our study showed nipple retraction, and one showed skin thickening in the mammary areola, which is more commonly observed in inflammatory breast carcinoma or invasive carcinoma [7–9]. The ultrasound manifestations of non-puerperal mastitis varied. In our study, the majority of the lesions were irregular, hypoechoic or mass-like lesions (31/45). Other ultrasound findings included lesions that were either separately or contiguously lamellar, tubular hypoechoic or non-mass-like (12/45). Two sonograms were found to be normal. These findings are similar to those previously described [5,10–13,21]. The blood signal of non-puerperal mastitis on CDFI was further studied in our series. Unfortunately, these findings were non-specific and similar to findings for carcinoma.
MRI features of non-puerperal mastitis described in the published literature are scarce [7,13,14]. These reports state that non-puerperal mastitis has a wide spectrum of appearances, such as a ring-like enhancement, intensively enhancing irregular mass and focal homogeneous enhancing masses, which are all consistent with our findings. Non-mass-like enhanced lesions were found in 90.2% (46/51) of our patients, and only 5 cases presented with mass-like enhanced lesions. The percentage of non-mass-like enhanced lesions in our series was slightly higher than that reported by Dursun et al (77%) [13] and Liu et al (81.5%, 22/27) [14]. This higher percentage may be due to different inflammatory stages correlating with histopathological findings. Multiple regional enhancements (32/46) and clustered rim-like enhancements (29/46) were the most common characteristics in our study. Multiple regional enhancements are more characteristic of benign breast diseases, although multicentric carcinomas, such as infiltrating ductal carcinoma or infiltrating lobular carcinoma, may also exhibit such appearances. However, these findings always present dominant lesions surrounded by multiple, small, enhancing foci [22,23], which is slightly different from our findings (multiple regional non-mass-like lesions). Clustered enhancements on MRI, such as clustered focal area, ductal or segmental enhancements, are observed more frequently in ductal carcinoma in situ (DCIS) [16,24]; but in our study, clustered rim-like enhancements were found in 29 patients (63.1%). Rim-like enhancement on MRI was reported to be more frequent in malignant breast diseases [16,24,25], especially for mass-like lesions, as this is related to a large area of tumour ischaemic necrosis due to the rapid growth of a malignant tumour. However, if treatment is delayed or if it is inadequate in mastitis, these rim-like enhancements can cause the formation of abscesses near mammary ducts because of the necrosis present in tissue cells. These abscesses can present solitary or multiple, separate or contiguous patterns. Multiple, small abscesses can also be connected to a larger abscess. Therefore, this explains why inflammatory lesions tend to distribute along the breast ducts and around the mammary areola. Our study did not contain many cases with mass-like, enhanced lesions showing ill-defined margins, heterogeneous and rim-like enhancements, which would have been similar to breast carcinoma. In addition, the time–signal intensity curve in non-puerperal mastitis was also studied, and our results showed that Type 1 and Type 2 curves were most prevalent (98.1%). Skin thickening, especially for the skin of the mammary areola, axillary adenopathy and chest wall invasion were also found in our series. However, these findings were non-specific because these findings are also found in carcinomas.
In our study, 6 of the 25 cases imaged using mammography and 2 of the 45 cases imaged using sonography were not positively detected; however, all the lesions were detected by enhanced MRI. In the three imaging modalities used in this study, the BI-RADS category with the highest number of patients was Category 0 on mammography, Category 4a on sonography and also Category 4a on MRI. If a BI-RADS rating greater than Category 4b is considered to be a malignant lesion, the rates of misdiagnosis on mammography, ultrasound and MRI would have been 16.0%, 26.7% and 43.1%, respectively. The reason why the rate of misdiagnosis in MRI was high in our study may be due to insufficient understanding of MRI appearances in non-puerperal mastitis, as well as some manifestations on MRI that could indicate other malignant tumours of the breast. Therefore, clinical presentations should always be considered when making diagnoses. Moreover, enhanced MRI should be used to diagnose mastitis if multiple regional and separated enhancements or contiguous clustered rim-like enhancements appear on MRI, and antibiotics or corticosteroids should be prescribed as treatment. If this treatment exhibits no effect, a biopsy should be performed.
Non-puerperal mastitis with signs of inflammation should be distinguished from inflammatory breast cancer. Clinically, inflammatory breast cancer is characterised by the rapid onset of swelling and enlargement of the breast, the characteristic “peau d’orange” (orange peel) skin appearance and quick progression to nodules and ulcerations. This type of breast cancer is usually poorly differentiated in histology from infiltrating ductal carcinoma, which often has distant metastases at the time of diagnosis [8,26]. These aforementioned manifestations in non-puerperal mastitis are slight or rare [8]. Inflammatory breast cancer usually affects females of approximately 50 years old [26,27], but non-puerperal mastitis is more common in females under 40 [5,10,11,13]. MRI is highly sensitive for depicting primary breast parenchymal lesions and global skin abnormalities that may help diagnose inflammatory breast cancer. Lesions that were either heterogeneous or inhomogeneous, consisting of a reticular or dendritic internal enhancement with a washout or plateau kinetic curve, were commonly noted [26–29]. This is different from our findings in non-puerperal mastitis. However, Rieber et al [7] revealed that breast MR cannot definitively distinguish inflammatory carcinoma from mastitis. Thus, further contrast studies between non-puerperal mastitis and inflammatory breast cancer are required. Because most non-puerperal mastitis cases occur without any signs of inflammation, it is clinically very difficult to distinguish these cases from other benign or malignant tumours on the basis of radiological features, especially from DCIS and invasive carcinoma. Therefore, clinical data and imaging data should be considered together, and a core needle biopsy should also be performed.
In conclusion, most non-puerperal mastitis cases clinically present as a palpable mass, with some cases exhibiting several signs of inflammation. The findings of mammography and ultrasound are not specified; therefore, the appearance of multiple regional enhancements, separated or contiguous clustered rim-like enhancements on enhanced MRI could be helpful in diagnosis. MRI is recommended because of the increased detail that MRI offers for clinically suspicious mastitis.
References
- 1.Bässler R. Mastitis. Classification, histopathology and clinical aspects. Pathologe 1997;18:27–36 [DOI] [PubMed] [Google Scholar]
- 2.Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009;15:367–80 doi: 10.1111/j.1524-4741.2009.00740.x [DOI] [PubMed] [Google Scholar]
- 3.Sabaté JM, Clotet M, Gómez A, De Las Heras P, Torrubia S, Salinas T. Radiologic evaluation of uncommon inflammatory and reactive breast disorders. Radiographics 2005;25:411–24 doi: 10.1148/rg.252045077 [DOI] [PubMed] [Google Scholar]
- 4.Stricker T, Navratil F, Forster I, Hürlimann R, Sennhauser FH. Nonpuerperal mastitis in adolescents. J Pediatr 2006;148:278–81 doi: 10.1016/j.jpeds.2005.08.074 [DOI] [PubMed] [Google Scholar]
- 5.Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol 2009;193:574–81 doi: 10.2214/AJR.08.1528 [DOI] [PubMed] [Google Scholar]
- 6.Seo HR, Na KY, Yim HE, Kim TH, Kang DK, Oh KK, et al. Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer 2012;15:111–18 doi: 10.4048/jbc.2012.15.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieber A, Tomczak RJ, Mergo PJ, Wenzel V, Zeitler H, Brambs HJ. MRI of the breast in the differential diagnosis of mastitis versus inflammatory carcinoma and follow-up. J Comput Assist Tomogr 1997;21:128–32 [DOI] [PubMed] [Google Scholar]
- 8.Robertson FM, Bondy M, Yang W, Yamauchi H, Wiggins S, Kamrudin S, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin 2010;60:351–75 doi: 10.3322/caac.20082 [DOI] [PubMed] [Google Scholar]
- 9.Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer 2012;15:119–23 doi: 10.4048/jbc.2012.15.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lequin MH, van Spengler J, van Pel R, van Eijck C, van Overhagen H. Mammographic and sonographic spectrum of non-puerperal mastitis. Eur J Radiol 1995;21:138–42 [DOI] [PubMed] [Google Scholar]
- 11.Han BK, Choe YH, Park JM, Moon WK, Ko YH, Yang JH, et al. Granulomatous mastitis: mammographic and sonographic appearances. AJR Am J Roentgenol 1999;173:317–20 [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz E, Lebe B, Usal C, Balci P. Mammographic and sonographic findings in the diagnosis of idiopathic granulomatous mastitis. Eur Radiol 2001;11:2236–40 doi: 10.1007/s003300100965 [DOI] [PubMed] [Google Scholar]
- 13.Dursun M, Yilmaz S, Yahyayev A, Salmaslioglu A, Yavuz E, Igci A, et al. Multimodality imaging features of idiopathic granulomatous mastitis: outcome of 12 years of experience. Radiol Med 2012;117:529–38 doi: 10.1007/s11547-011-0733-2 [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Peng W. Morphological manifestations of nonpuerperal mastitis on magnetic resonance imaging. J Magn Reson Imaging 2011;33:1369–74 doi: 10.1002/jmri.22464 [DOI] [PubMed] [Google Scholar]
- 15.Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 1999;211:101–10 [DOI] [PubMed] [Google Scholar]
- 16.American College of Radiology Breast Imaging Reporting and Data System atlas (BI-RADS® atlas). Reston, VA: American College of Radiology; 2003 [Google Scholar]
- 17.Tardivon AA, Athanasiou A, Thibault F, El Khoury C. Breast Imaging and Reporting Data System (BIRADS) magnetic resonance imaging illustrated cases. Eur J Radiol 2007;61:216–23 [DOI] [PubMed] [Google Scholar]
- 18.Levy L, Suissa M, Chiche JF, Teman G, Martin B. BIRADS ultrasonography. Eur J Radiol 2007;61:202–11 doi: 10.1016/j.ejrad.2006.08.035 [DOI] [PubMed] [Google Scholar]
- 19.Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol 1990;16:553–9 [DOI] [PubMed] [Google Scholar]
- 20.Hughes LE. Non-lactational inflammation and duct ectasia. Br Med Bull 1991;47:272–83 [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Oh KK, Kim EK, Kwack KS, Jung WH, Lee HK. Radiologic and clinical features of idiopathic granulomatous lobular mastitis mimicking advanced breast cancer. Yonsei Med J 2006;47:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstein SP, Orel SG, Heller R, Reynolds C, Czerniecki B, Solin LJ, et al. MR imaging of the breast in patients with invasive lobular carcinoma. AJR Am J Roentgenol 2001;176:399–406 [DOI] [PubMed] [Google Scholar]
- 23.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics 2009;29:165–76 doi: 10.1148/rg.291085100 [DOI] [PubMed] [Google Scholar]
- 24.Agrawal G, Su MY, Nalcioglu O, Feig SA, Chen JH. Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 2009;115:1363–80 doi: 10.1002/cncr.24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi M, Kawashima H, Matsui O, Zen Y, Suzuki M, Inokuchi M, et al. Two different types of ring-like enhancement on dynamic MR imaging in breast cancer: correlation with the histopathologic findings. J Magn Reson Imaging 2008;28:1435–43 doi: 10.1002/jmri.21622 [DOI] [PubMed] [Google Scholar]
- 26.Yang WT, Le-Petross HT, Macapinlac H, Carkaci S, Gonzalez-Angulo AM, Dawood S, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat 2008;109:417–26 doi: 10.1007/s10549-007-9671-z [DOI] [PubMed] [Google Scholar]
- 27.Carbognin G, Calciolari C, Girardi V, Camera L, Pollini G. Pozzi Mucelli R. Inflammatory breast cancer: MR imaging findings. Radiol Med 2010;115:70–82 doi: 10.1007/s11547-009-0475-6 [DOI] [PubMed] [Google Scholar]
- 28.Belli P, Costantini M, Romani M, Pastore G. Role of magnetic resonance imaging in inflammatory carcinoma of the breast. Rays 2002;27:299–305 [PubMed] [Google Scholar]
- 29.Chow CK. Imaging in inflammatory breast carcinoma. Breast Dis 2005;22:45–54 [DOI] [PubMed] [Google Scholar]