Abstract
We show that the nuclear genes for the cytoplasmic and mitochondrial leucyl-tRNA synthetase (LeuRS) of Neurospora crassa are distinct in their encoded proteins, codon usage, mRNA levels, and regulation. The 4.2-kilobase-pair region representing the structural gene for cytoplasmic LeuRS and flanking regions has been sequenced. The positions of the 5' and 3' ends of mRNA and of a single 62-base-pair intron have been mapped. The methionine-initiated open reading frame encoded a protein of 1,123 amino acids and displayed a strong codon bias. Although cytoplasmic LeuRS shares with mitochondrial LeuRS some general features common to most aminoacyl-tRNA synthetases, there is little amino acid sequence similarity between them, mRNA levels for cytoplasmic LeuRS were much higher than those for mitochondrial LeuRS. This observation and the strong codon bias in the cytoplasmic LeuRS gene may contribute to a greater abundance of cytoplasmic LeuRS than mitochondrial LeuRS. The genes for cytoplasmic and mitochondrial LeuRS are regulated independently. The cytoplasmic LeuRS gene is regulated by the cross-pathway control system in N. crassa, which is analogous to general amino acid control in Saccharomyces cerevisiae. The cytoplasmic LeuRS mRNA levels are induced by amino acid starvation resulting from the addition of aminotriazole. Part of this increase is due to utilization of new transcription start sites. In contrast, the mitochondrial LeuRS gene is not induced by amino acid limitation. However, the mitochondrial LeuRS mRNA levels did increase dramatically upon inhibition of mitochondrial protein synthesis by chloramphenicol or ethidium bromide or in the temperature-sensitive strain leu-5 carrying a mutation in the mitochondrial LeuRS structural gene.
Full text
PDF




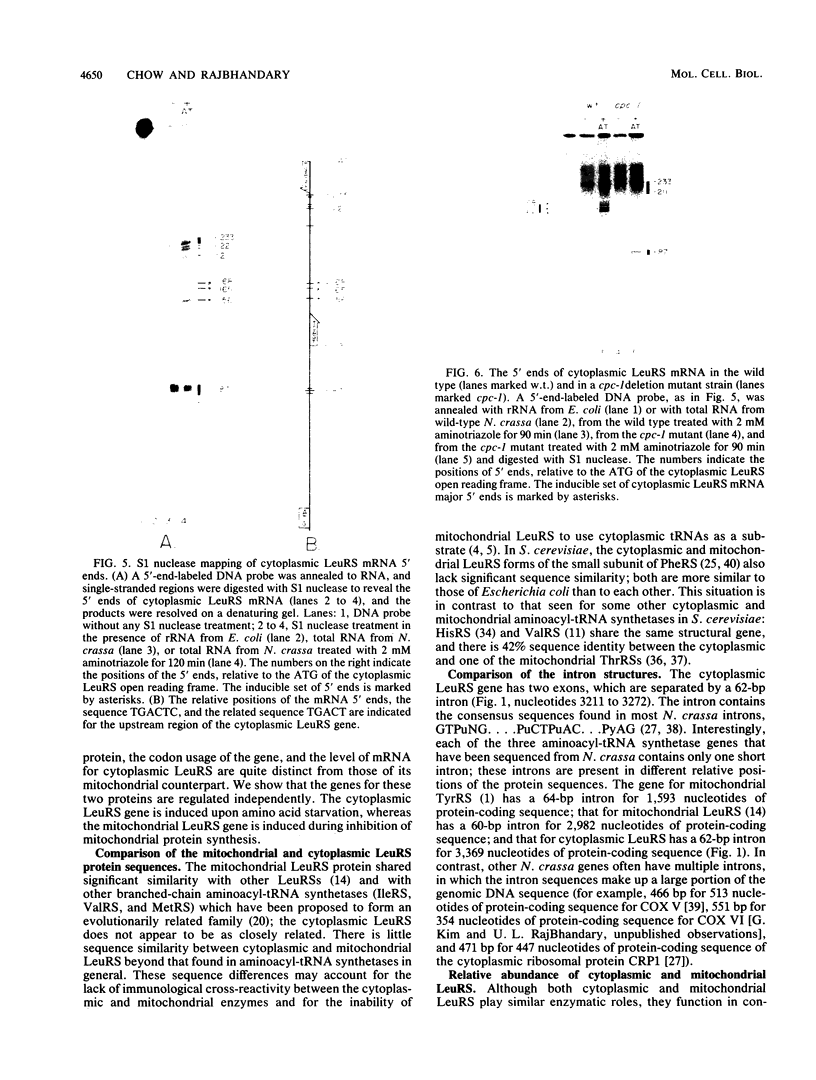


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Cooperation of mitochondrial and nuclear genes specifying the mitochondrial genetic apparatus in Neurospora crassa. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1371–1374. doi: 10.1073/pnas.69.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Induction of mitochondrial RNA polymerase in Neurospora crassa. Nat New Biol. 1972 Dec 13;240(102):195–197. doi: 10.1038/newbio240195a0. [DOI] [PubMed] [Google Scholar]
- Beauchamp P. M., Horn E. W., Gross S. R. Proposed involvement of an internal promoter in regulation and synthesis of mitochondrial and cytoplasmic leucyl-tRNA synthetases of Neurospora. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1172–1176. doi: 10.1073/pnas.74.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarous R., Chow C. M., RajBhandary U. L. Cytoplasmic leucyl-tRNA synthetase of Neurospora crassa is not specified by the leu-5 locus. Genetics. 1988 Aug;119(4):805–814. doi: 10.1093/genetics/119.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Beuchamp P. M., Gross S. R. Increased mitochondrial leucyl- and phenylalanyl-tRNA synthetase activity as a result of inhibition of mitochondrial protein synthesis. Nature. 1976 May 27;261(5558):338–340. doi: 10.1038/261338a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Yanofsky C. Nucleotide sequence of the Neurospora crassa trp-3 gene encoding tryptophan synthetase and comparison of the trp-3 polypeptide with its homologs in Saccharomyces cerevisiae and Escherichia coli. J Biol Chem. 1989 Mar 5;264(7):3840–3848. [PubMed] [Google Scholar]
- Chatton B., Walter P., Ebel J. P., Lacroute F., Fasiolo F. The yeast VAS1 gene encodes both mitochondrial and cytoplasmic valyl-tRNA synthetases. J Biol Chem. 1988 Jan 5;263(1):52–57. [PubMed] [Google Scholar]
- Cheung A. Y., Watson L., Söll D. Two control systems modulate the level of glutaminyl-tRNA synthetase in Escherichia coli. J Bacteriol. 1985 Jan;161(1):212–218. doi: 10.1128/jb.161.1.212-218.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Englisch U., Englisch S., Markmeyer P., Schischkoff J., Sternbach H., Kratzin H., Cramer F. Structure of the yeast isoleucyl-tRNA synthetase gene (ILS1). DNA-sequence, amino-acid sequence of proteolytic peptides of the enzyme and comparison of the structure to those of other known aminoacyl-tRNA synthetases. Biol Chem Hoppe Seyler. 1987 Aug;368(8):971–979. doi: 10.1515/bchm3.1987.368.2.971. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Gibson B. W., Walter P., Chatton B., Biemann K., Boulanger Y. Cytoplasmic methionyl-tRNA synthetase from Bakers' yeast. A monomer with a post-translationally modified N terminus. J Biol Chem. 1985 Dec 15;260(29):15571–15576. [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Baum J., Geever R., Huiet L., Patel V., Tyler B. Gene organization and regulation in the qa (quinic acid) gene cluster of Neurospora crassa. Microbiol Rev. 1985 Sep;49(3):338–358. doi: 10.1128/mr.49.3.338-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck J. D., Hatfield G. W. Valyl-tRNA synthetase gene of Escherichia coli K12. Primary structure and homology within a family of aminoacyl-TRNA synthetases. J Biol Chem. 1988 Jan 15;263(2):868–877. [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema A., Kastelein R. A., Vasser M., de Boer H. A. Codon replacement in the PGK1 gene of Saccharomyces cerevisiae: experimental approach to study the role of biased codon usage in gene expression. Mol Cell Biol. 1987 Aug;7(8):2914–2924. doi: 10.1128/mcb.7.8.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird J. H., Fincham J. R. The complete nucleotide sequence of the Neurospora crassa am (NADP-specific glutamate dehydrogenase) gene. Gene. 1983 Dec;26(2-3):253–260. doi: 10.1016/0378-1119(83)90195-6. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Myers A. M., Lee S., Tzagoloff A. Isolation and characterization of the yeast gene coding for the alpha subunit of mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1987 Mar 15;262(8):3690–3696. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreader C. A., Heckman J. E. Isolation and characterization of a Neurospora crassa ribosomal protein gene homologous to CYH2 of yeast. Nucleic Acids Res. 1987 Nov 11;15(21):9027–9042. doi: 10.1093/nar/15.21.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreader C. A., Langer C. S., Heckman J. E. A mitochondrial protein from Neurospora crassa detected both on ribosomes and in membrane fractions. Analysis of the gene, the message, and the protein. J Biol Chem. 1989 Jan 5;264(1):317–327. [PubMed] [Google Scholar]
- Kuiper M. T., Akins R. A., Holtrop M., de Vries H., Lambowitz A. M. Isolation and analysis of the Neurospora crassa Cyt-21 gene. A nuclear gene encoding a mitochondrial ribosomal protein. J Biol Chem. 1988 Feb 25;263(6):2840–2847. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meussdoerffer F., Fink G. R. Structure and expression of two aminoacyl-tRNA synthetase genes from Saccharomyces cerevisiae. J Biol Chem. 1983 May 25;258(10):6293–6299. [PubMed] [Google Scholar]
- Mirande M., Waller J. P. The yeast lysyl-tRNA synthetase gene. Evidence for general amino acid control of its expression and domain structure of the encoded protein. J Biol Chem. 1988 Dec 5;263(34):18443–18451. [PubMed] [Google Scholar]
- Natsoulis G., Hilger F., Fink G. R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986 Jul 18;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. K., Koerner T. J., Tzagoloff A. Characterization of a yeast nuclear gene (MST1) coding for the mitochondrial threonyl-tRNA1 synthetase. J Biol Chem. 1985 Dec 5;260(28):15362–15370. [PubMed] [Google Scholar]
- Pape L. K., Tzagoloff A. Cloning and characterization of the gene for the yeast cytoplasmic threonyl-tRNA synthetase. Nucleic Acids Res. 1985 Sep 11;13(17):6171–6183. doi: 10.1093/nar/13.17.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Berlin V., Hager K. M., Yanofsky C. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol Cell Biol. 1988 Jun;8(6):2411–2418. doi: 10.1128/mcb.8.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. S., Bertrand H., Metzenberg R. L., RajBhandary U. L. Cytochrome oxidase subunit V gene of Neurospora crassa: DNA sequences, chromosomal mapping, and evidence that the cya-4 locus specifies the structural gene for subunit V. Mol Cell Biol. 1989 Feb;9(2):566–577. doi: 10.1128/mcb.9.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanni A., Mirande M., Ebel J. P., Boulanger Y., Waller J. P., Fasiolo F. Structure and expression of the genes encoding the alpha and beta subunits of yeast phenylalanyl-tRNA synthetase. J Biol Chem. 1988 Oct 25;263(30):15407–15415. [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Luck D. J. A paracrystalline inclusion in Neurospora crassa. Induction by ethidium and acridine, isolation, and characterization. J Cell Biol. 1971 Oct;51(1):249–264. doi: 10.1083/jcb.51.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]






