Abstract
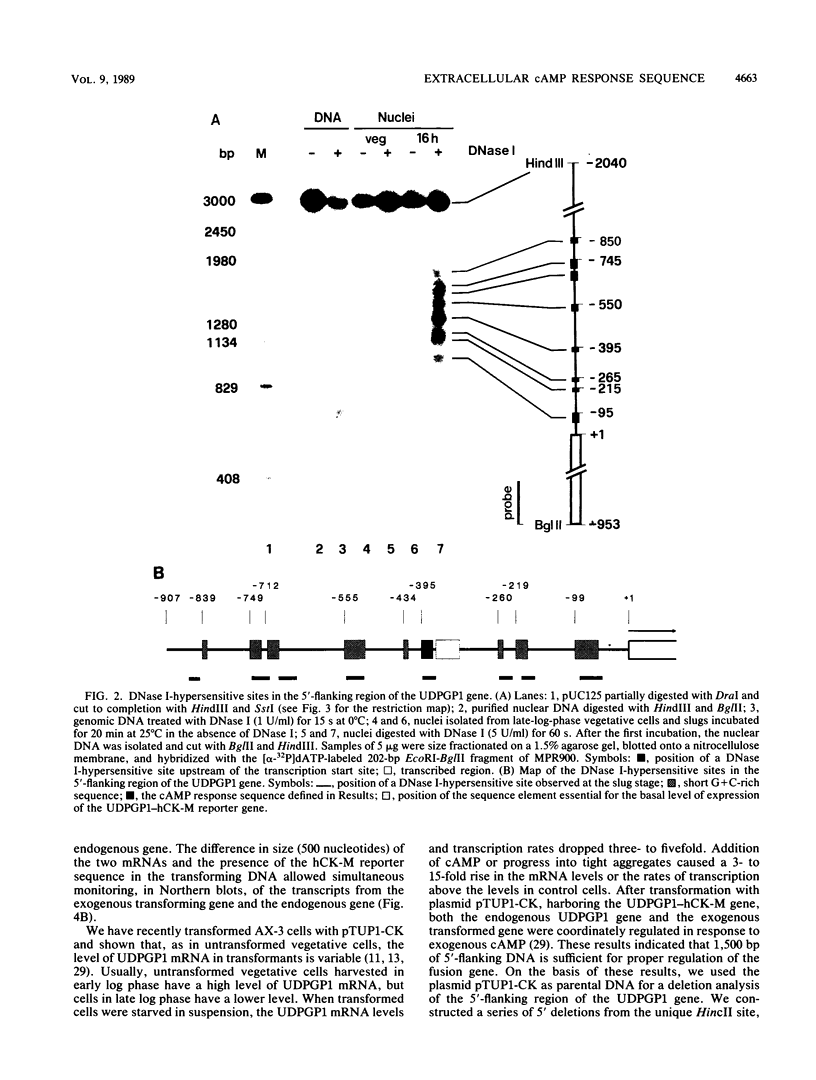
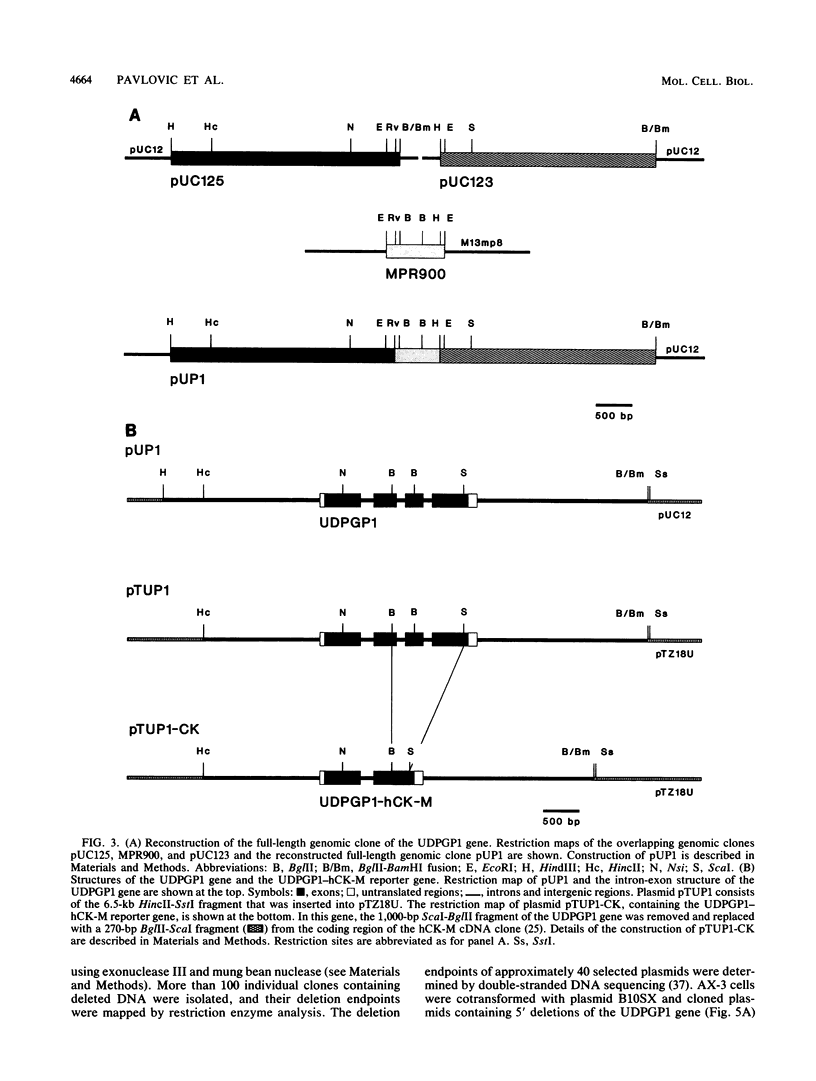
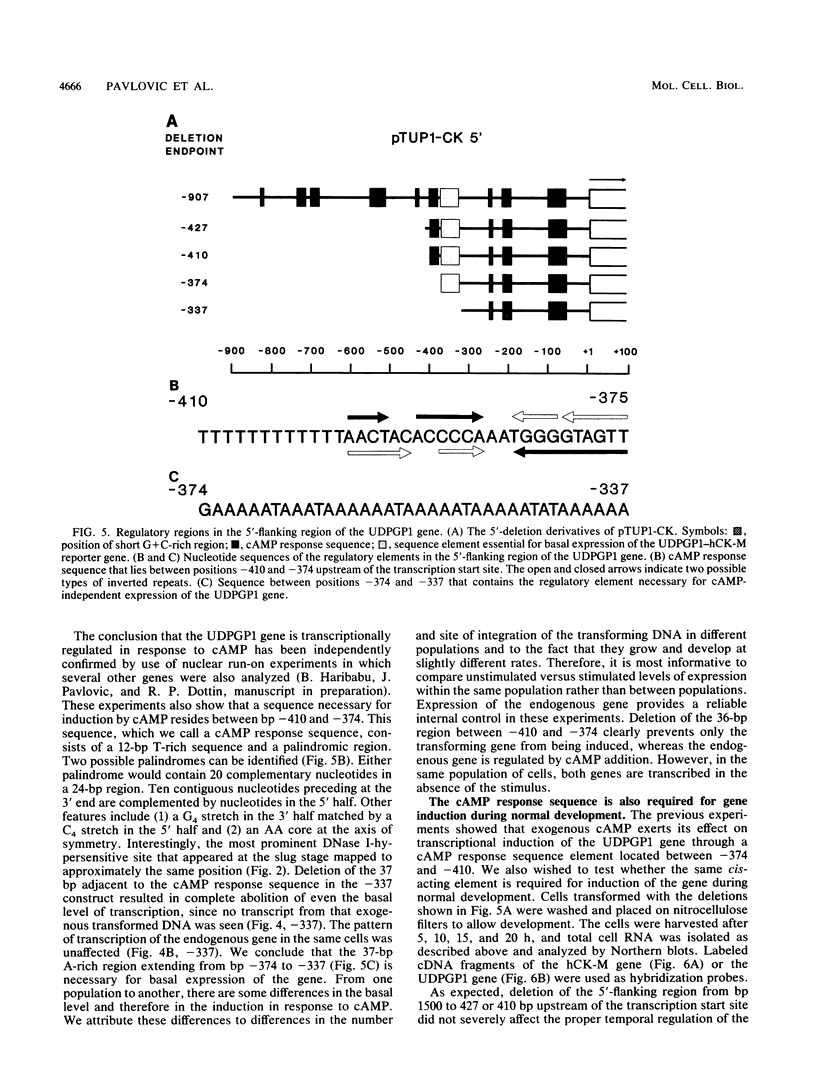
The signal transduction pathways that lead to gene induction are being intensively investigated in Dictyostelium discoideum. We have identified by deletion and transformation analysis a sequence element necessary for induction of a gene coding for uridine diphosphoglucose pyrophosphorylase (UDPGP1) of D. discoideum in response to extracellular cyclic AMP (cAMP). This regulatory element is located 380 base pairs upstream of the transcription start site and contains a G+C-rich partially palindromic sequence. It is not required for transcription per se but is required for induction of the gene in response to the stimulus of extracellular cAMP. The cAMP response sequence is also required for induction of the gene during normal development. A second A+T-rich cis-acting region located immediately downstream of the cAMP response sequence appears to be essential for the basal level of expression of the UDPGP1 gene. The position of the cAMP response element coincides with a DNase I-hypersensitive site that is observed when the UDPGP1 gene is actively transcribed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres K., Neuman W., Rowekamp W. G., Chung S. Developmental regulation of DNase I-hypersensitive sites in Dictyostelium discoideum. Mol Cell Biol. 1987 May;7(5):1823–1829. doi: 10.1128/mcb.7.5.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Knecht D., Lodish H. F., Loomis W. F. DNA sequences required for expression of a Dictyostelium actin gene. EMBO J. 1986 Dec 1;5(12):3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., Firtel R. A. Identification of the sequences controlling cyclic AMP regulation and cell-type-specific expression of a prestalk-specific gene in Dictyostelium discoideum. Mol Cell Biol. 1987 Jan;7(1):149–159. doi: 10.1128/mcb.7.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Williams J. G. Two divergently transcribed genes of Dictyostelium discoideum are cyclic AMP-inducible and coregulated during development. Mol Cell Biol. 1987 Dec;7(12):4482–4489. doi: 10.1128/mcb.7.12.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fishel B. R., Ragheb J. A., Rajkovic A., Haribabu B., Schweinfest C. W., Dottin R. P. Molecular cloning of a cDNA complementary to a UDP-glucose pyrophosphorylase mRNA of dictyostelium discoideum. Dev Biol. 1985 Aug;110(2):369–381. doi: 10.1016/0012-1606(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Cyclic AMP and other signals controlling cell development and differentiation in Dictyostelium. Annu Rev Biochem. 1987;56:853–879. doi: 10.1146/annurev.bi.56.070187.004225. [DOI] [PubMed] [Google Scholar]
- Gomer R. H., Armstrong D., Leichtling B. H., Firtel R. A. cAMP induction of prespore and prestalk gene expression in Dictyostelium is mediated by the cell-surface cAMP receptor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8624–8628. doi: 10.1073/pnas.83.22.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribabu B., Dottin R. P. Pharmacological characterization of cyclic AMP receptors mediating gene regulation in Dictyostelium discoideum. Mol Cell Biol. 1986 Jul;6(7):2402–2408. doi: 10.1128/mcb.6.7.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribabu B., Rajkovic A., Dottin R. P. Cell-cell contact and cAMP regulate the expression of a UDP glucose pyrophosphorylase gene of Dictyostelium discoideum. Dev Biol. 1986 Feb;113(2):436–442. doi: 10.1016/0012-1606(86)90178-8. [DOI] [PubMed] [Google Scholar]
- Hatch C. L., Bonner W. M. Direct analysis of RNA in whole cell and cytoplasmic extracts by gel electrophoresis. Anal Biochem. 1987 Apr;162(1):283–290. doi: 10.1016/0003-2697(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R. Different molecular mechanisms for cAMP regulation of gene expression during Dictyostelium development. Dev Biol. 1987 Jul;122(1):163–171. doi: 10.1016/0012-1606(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Lefebvre P., Chung S., Lodish H. F. Transcriptional control of gene expression during development of Dictyostelium discoideum. Mol Cell Biol. 1982 Nov;2(11):1417–1426. doi: 10.1128/mcb.2.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Ceccarelli A., Lodish H. F. Cyclic AMP stabilizes a class of developmentally regulated Dictyostelium discoideum mRNAs. Nature. 1983 Feb 17;301(5901):616–618. doi: 10.1038/301616a0. [DOI] [PubMed] [Google Scholar]
- Manrow R. E., Jacobson A. mRNA decay rates in late-developing Dictyostelium discoideum cells are heterogeneous, and cyclic AMP does not act directly to stabilize cell-type-specific mRNAs. Mol Cell Biol. 1988 Oct;8(10):4088–4097. doi: 10.1128/mcb.8.10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Georgiev G. P. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Biochem Biophys Res Commun. 1980 Jan 29;92(2):532–539. doi: 10.1016/0006-291x(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Nellen W., Firtel R. A. High-copy-number transformants and co-transformation in Dictyostelium. Gene. 1985;39(2-3):155–163. doi: 10.1016/0378-1119(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen W., Silan C., Saur U., Firtel R. A. Regulatory sequences in the promoter of the Dictyostelium Actin 6 gene. EMBO J. 1986 Dec 1;5(12):3367–3372. doi: 10.1002/j.1460-2075.1986.tb04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness P. J., Labhart P., Banz E., Koller T., Parish R. W. Chromatin structure along the ribosomal DNA of Dictyostelium. Regional differences and changes accompanying cell differentiation. J Mol Biol. 1983 May 25;166(3):361–381. doi: 10.1016/s0022-2836(83)80090-4. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Schweinfest C. W., Rajkovic A., Pavlovic J., Jamal S., Dottin R. P., Hart J. T., Kamarck M. E., Rae P. M., Carty M. D. cDNA cloning and mapping of the human creatine kinase M gene to 19q13. Am J Hum Genet. 1987 Feb;40(2):115–125. [PMC free article] [PubMed] [Google Scholar]
- Oyama M., Blumberg D. D. Changes during differentiation in requirements for cAMP for expression of cell-type-specific mRNAs in the cellular slime mold, Dictyostelium discoideum. Dev Biol. 1986 Oct;117(2):550–556. doi: 10.1016/0012-1606(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Oyama M., Blumberg D. D. Interaction of cAMP with the cell-surface receptor induces cell-type-specific mRNA accumulation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4819–4823. doi: 10.1073/pnas.83.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Banz E., Parish R. W. Hypersensitive sites in the 5' and 3' flanking regions of the cysteine proteinase I gene of Dictyostelium discoideum. Nucleic Acids Res. 1986 Nov 25;14(22):8703–8722. doi: 10.1093/nar/14.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Haribabu B., Dottin R. P. Transmembrane signal transduction regulates gene expression in Dictyostelium discoideum. Dev Genet. 1988;9(4-5):371–382. doi: 10.1002/dvg.1020090418. [DOI] [PubMed] [Google Scholar]
- Pears C. J., Williams J. G. Identification of a DNA sequence element required for efficient expression of a developmentally regulated and cAMP-inducible gene of Dictyostelium discoideum. EMBO J. 1987 Jan;6(1):195–200. doi: 10.1002/j.1460-2075.1987.tb04738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragheb J. A., Dottin R. P. Structure and sequence of a UDP glucose pyrophosphorylase gene of Dictyostelium discoideum. Nucleic Acids Res. 1987 May 11;15(9):3891–3906. doi: 10.1093/nar/15.9.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Reymond C. D. A rapid method for the preparation of multiple samples of eucaryotic DNA. Nucleic Acids Res. 1987 Oct 12;15(19):8118–8118. doi: 10.1093/nar/15.19.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap P., van Driel R. V. Induction of post-aggregative differentiation in Dictyostelium discoideum by cAMP. Evidence of involvement of the cell surface cAMP receptor. Exp Cell Res. 1985 Aug;159(2):388–398. doi: 10.1016/s0014-4827(85)80012-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Tsang A. S., Mahbubani H. A change in the rate of transcription of a eukaryotic gene in response to cyclic AMP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7171–7175. doi: 10.1073/pnas.77.12.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]