Abstract
We have synthesized a series of LnIII-containing polyethylene glycol conjugates and studied the structural and electronic properties of these complexes. These studies demonstrate that polyethylene glycol can be used to fine-tune water-exchange rates of LnIII-containing polyaminopolycarboxylate-type complexes; this control is desirable in developing LnIII-containing contrast agents for magnetic resonance imaging.
The efficiency of LnIII-containing complexes as conventional (T1-reducing) and paramagnetic chemical exchange saturation transfer (PARACEST) contrast agents for magnetic resonance imaging (MRI) is governed by the interactions among the structural and electronic properties of these complexes and by the interactions of these complexes with the environment.1,2 The optimal value for a specific parameter is a moving target that varies as a function of magnetic field strength and the other parameters.1–4 Of these parameters, the exchange rate between coordinated and bulk water, kex, plays a critical role in establishing contrast-enhancing efficiency.1,2 Hence, the ability to precisely control the water-exchange rates of LnIII-containing complexes is imperative to achieving optimal efficiencies of contrast agents for both conventional and PARACEST imaging.
For example, conventional, GdIII-containing, small molecular-weight agents require a fast water-exchange rate (~108 s−1) to achieve optimal efficiency at the clinically relevant field strength of 1.5 T,3,5 and macromolecular GdIII-based agents require a faster water-exchange rate (~1010 s−1).4 However, these target values change with changes in field strength. Different from conventional agents, LnIII-based PARACEST agents require slow-to-intermediate water-exchange rates on the NMR time scale, and the optimal value for the water-exchange rate depends on the frequency of the pulse used to presaturate the exchangeable protons.6 The estimated optimal water-exchange rate for PARACEST agents is between 0.3 × 103 and 1 × 103 s−1 at clinically relevant pulse frequencies (50–100 Hz).6
The water-exchange rates of clinically approved GdIII-containing small molecular-weight contrast agents are slower (1 × 106 to 4 × 106 s−1) than the optimal value,7 and the water-exchange rates of most LnIII-based complexes developed as potential PARACEST agents are faster (0.3 × 104 to 1 × 104 s−1) than the optimal value.6 Consequently, there is a need to tune the water-exchange rates of LnIII-containing complexes to achieve optimum efficiencies for conventional and PARACEST agents. This need has been the focus of a great deal of research. Tuning water-exchange rates of LnIII-containing complexes has been achieved by modifying (1) the charge of the LnIII-based complex;8 (2) the accessibility of the metal center to bulk water;7,9–11 (3) the mechanism of water exchange;12 and (4) the ratio between twisted square anti-prism and square anti-prism isomers for LnIII-based complexes with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-type ligands.13 These studies demonstrate that small modifications in ligand structure can have large impacts on the water-exchange rates of LnIII-containing complexes, but most of these studies are specific to the system being studied.
We envisioned that a modular and tuneable system of modifications could be incorporated into LnIII-based DOTA-type systems to tune water-exchange rates. We hypothesized that hydrophilic oligomers of polyethylene glycol (PEG) could be used to modulate water-exchange rates by altering the accessibility of bulk water to the LnIII-center through both steric interactions and hydrogen bonding in a systematic fashion based on oligomer length. Here, we report the influence of PEG length on the molecular parameters, including water-exchange rate, of LnIII-containing DOTA-type complexes.
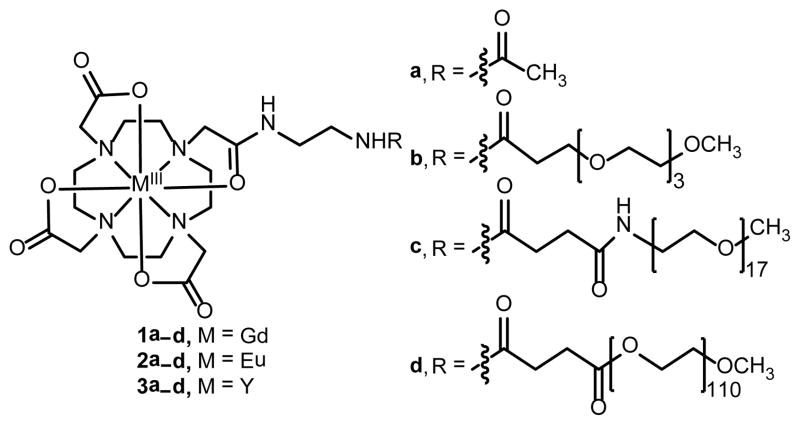
To investigate the influence of PEG oligomers on the water-exchange rates of LnIII-containing complexes, we designed and synthesized a PEG-containing model system (1a–d and 2a–d in Fig. 1) to modulate the water-exchange rates of LnIII-DOTA-based complexes. We expected that PEG would hydrogen bond to water through the large number of oxygen-based hydrogen-bond acceptors, thereby changing the extent of the hydrogen-bonding network as PEG length is varied. Our hypothesis is consistent with reports of changes in water-exchange rates being observed as the length of PEG was varied with PEG-conjugates of GdIII-containing hydroxypyridonate (HOPO) complexes.11 The HOPO-based systems have a water-coordination number of two and display fast water-exchange rates (~108 s−1). The PEG moiety in those systems led to slower water-exchange rates and a decrease in water-coordination number from two to one. We expected that PEG could be used to fine-tune the water-exchange rates of other systems including LnIII-containing DOTA-type complexes with water-coordination numbers of one and relatively slow water-exchange rates (~106 s−1).
Fig. 1.
Structures of LnIII-containing PEG conjugates 1a–d and 2a–d and YIII-containing conjugates 3a–d.
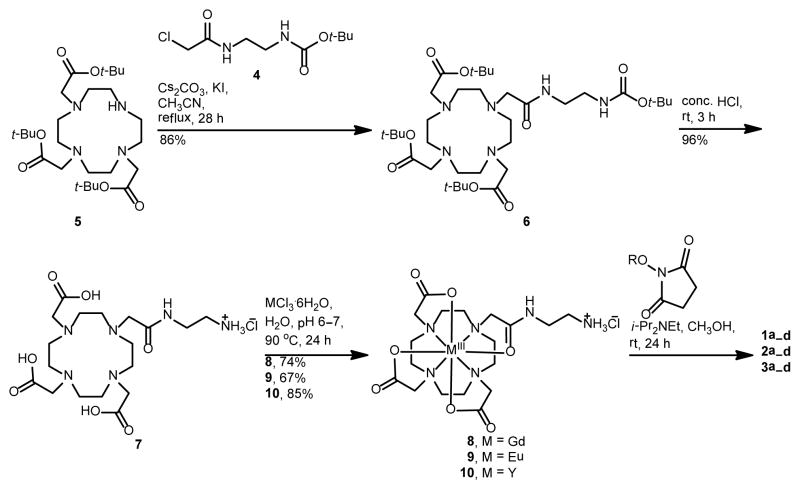
Our model system includes LnIII-containing complexes without PEG (1a and 2a) as well as three complexes with different length oligomers of PEG (1b–d and 2b–d). The synthetic route to complexes 1a–d and 2a–d is shown in Scheme 1. Briefly, the α-chloroamide derivative of tert-butoxycarbonyl-protected ethylenediamine, 4, was reacted with tri-tert-butyl-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate, 5, to obtain protected ligand 6 that was treated with HCl to yield deprotected ligand 7. Metallation was carried out using metal chlorides to obtain GdIII-and EuIII-containing complexes 8 and 9, respectively. Complexes 8 and 9 were reacted with succinimidyl ester derivatives of PEG or acetate (a, b, c, or d) to yield 1a–d or 2a–d. The yttrium analogues, 3a–d, were synthesized as diamagnetic controls for the variable-temperature 17O NMR experiments following the same procedures.
Scheme 1.
Synthetic route to LnIII-containing PEG conjugates 1a–d and 2a–d and YIII-containing conjugates 3a–d.
The water-coordination number, q, was obtained for EuIII-containing complexes 2a–d using luminescence-decay measurements (Table 1).14 Values of q were close to 1, and these values agree with what is expected for LnIII-complexes coordinated to octadentate ligands. Water-coordination number data for 2a–d suggest that steric blocking has little if any influence on the properties of the complexes.
Table 1.
Molecular parameters of complexes 1a–d.
| Parameter | 1a | 1b | 1c | 1d |
|---|---|---|---|---|
| qa,bc | 0.90 ±0.01 | 0.89 ± 0.01 | 0.96 ±0.02 | 0.76 ± 0.02 |
| τm298 × 10−6 (s)a | 0.37 ± 0.01 | 0.66 ± 0.01 | 1.2 ± 0.1 | 1.5 ± 0.07 |
| kex298 ×106 (s−1)d | 2.7 ±0.03 | 1.5 ±0.02 | 0.83 ±0.08 | 0.67 ± 0.05 |
| T1e298 ×10−9 (s)a | 440 ± 30 | 61 ± 0.7 | 12 ± 0.3 | 4.0 ± 0.04 |
| ΔH (kJ mol−1)a | 36 ± 0.8 | 50. ± 0.6 | 71 ± 4 | 90. ± 1.5 |
| r1 (mM−1 s−1)a | 2.5 ±0.1 | 4.0 ±0.1 | 5.3 ±0.1 | 6.3 ±0.1 |
| MW (Da) | 641 | 817 | 1463 | 5222 |
| gL | 1.98 | 1.98 | 1.99 | 1.98 |
| 1/T2e × 108 (s−1) | 17.8 | 8.11 | 6.51 | 5.03 |
| τR × 10−12 (s) | 46 | 79 | 110 | 220 |
reported as mean ± standard error,
from complexes 2a–d,
the error associated with q determination is ±0.1 water molecules,14
error represents relative uncertainty
To investigate the influence of PEG on tuning water-exchange rates, we performed variable-temperature 17O NMR experiments for GdIII-containing conjugates 1a–d, and the fitted molecular parameters are summarized in Table 1: the residence lifetime of coordinated water, τm298 (water-exchange rate, kex298, is 1/τm298; the longitudinal electronic relaxation time, T1e298; and the enthalpy change for the water-exchange process, ΔH. Based on the results of the 17O NMR experiments, a gradual decrease in water-exchange rates was observed with increasing length of PEG from 1a (control without PEG) to 1d (long PEG). Moreover, 1.8-, 3.3-, and 4.0-fold decreases in water-exchange rates were observed from 1a to 1b, 1c, and 1d, respectively. The observed decrease in water-exchange rates with increasing length of PEG was supported by an increase in ΔH from 1a to 1d. This increase in enthalpy suggests that the exchange between coordinated and bulk water molecules becomes difficult as PEG length is increased, possibly due to a larger hydrogen-bond network. The increase in ΔH and decrease in water-exchange rates from 1a to 1d support our hypothesis that PEG is able to modify the water-exchange properties of GdIII-containing DOTA-type complexes.
To investigate the influence of PEG on the efficiency of complexes 1a–d as conventional contrast agents, we carried out relaxivity, r1, measurements of 1a–d at 37 °C and 1.4 T in phosphate-buffered saline (pH = 7.4). The r1 values of 1a–d were obtained from the slopes of the plots of 1/T1 versus Gd concentration (Table 1). A 2.5-fold increase in relaxivity was observed as the length of PEG was increased from 1a to 1d. This increase in relaxivity corresponds to the increase in molecular weight (MW, Table 1) from 1a to 1d. As the molecular weight increases, complexes tend to tumble more slowly in solution leading to higher relaxivity values.15
To more rigorously unite our 17O NMR and relaxivity data, we performed electron paramagnetic resonance spectroscopy measurements to obtain the electronic Landé g factor, gL, and the transverse electronic relaxation rate, 1/T2e, of 1a–d. These parameters together with r1, kex, T1e, and q were used with the Solomon–Bloembergen–Morgan equations, which describe the factors affecting the efficiency of contrast agents for MRI,3 to obtain estimated rotational correlation times, τR, for 1a–d (Table 1 and calculations in the ESI). The values of τR seem lower than expected likely due to the internal motion of the PEG moieties as suggested for the HOPO-based system,11 due to the ineffective coupling between the motion of Gd-water vector and rotational motion of the entire molecule,16 or a combination of the two. An increase in the τR values was observed from 1a to 1d, which is consistent with the increase in molecular weight as the length of PEG is increased. This increase in τR is likely the cause of the observed increase in relaxivity from 1a to 1d. However, the increase in relaxivity observed from 1a to 1d is lower-than-expected based solely on the variation in τR from 1a to 1d (see ESI for calculations), and this observation is a likely result of the decrease in water-exchange rates as PEG length increases from 1a to 1d.
We have studied the influence of PEG oligomer length on the water-exchange rates and other properties that contribute to the efficiency of LnIII-containing DOTA-type contrast agents. Based on our findings, PEG can be used to fine-tune, toward slower rates, the water-exchange rates of LnIII-containing DOTA-type complexes. Our results demonstrate a similar magnitude of slowing of water-exchange rates as was reported with HOPO-based systems,11 but without the associated change in water coordination number. Consequently, PEG is able to slow the water-exchange rates of LnIII-containing complexes regardless of water-coordination number, demonstrating that conjugation of PEG represents a modular and tuneable strategy for slowing water-exchange rates that is general for LnIII-containing complexes. We expect that these findings will be useful in the design of LnIII-based contrast agents that require slow water-exchange rates on the NMR time scale.
Supplementary Material
Acknowledgments
This research was supported by a Schaap Faculty Scholar Award, Wayne State University, and a Pathway to Independence Career Transition Award (R00EB007129) from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health. We thank Ellen Inutan and Chamara Senevirathne for assistance with acquiring matrix-assisted laser desorption ionization mass spectrometry data and Corey Lambert for assistance with performing inductively coupled plasma optical emission spectroscopy.
Footnotes
Electronic Supplementary Information (ESI) available: general experimental and synthetic procedures; water-proton relaxation rate, 17O NMR, and luminescence-decay data; 1H and 13C NMR and electron paramagnetic resonance spectra; estimations of rotational correlation time and expected relaxivity; and high performance liquid chromatography chromatograms. See DOI: 10.1039/b000000x/
Notes and references
- 1.(a) Avedano S, Tei L, Lombardi A, Giovenzana GB, Aime S, Longo D, Botta M. Chem Commun. 2007:4726. doi: 10.1039/b714438e. [DOI] [PubMed] [Google Scholar]; (b) Aime S, Botta M, Bruce JI, Mainero V, Parker D, Terreno E. Chem Commun. 2001:115. [Google Scholar]; (c) Caravan P. Accounts Chem Res. 2009;42:851. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]; (d) Nonat A, Giraud M, Gateau C, Fries PH, Helm L, Mazzanti M. Dalton Trans. 2009:8033. doi: 10.1039/b907738c. [DOI] [PubMed] [Google Scholar]; (e) Laurent S, Henoumont C, Elst LV, Muller RN. Eur J Inorg Chem. 2012;2012:1889. [Google Scholar]; (f) Torres S, Martins JA, André JP, Geraldes CFGC, Merbach AE, Tóth É. Chem Eur J. 2006;12:940. doi: 10.1002/chem.200500551. [DOI] [PubMed] [Google Scholar]; (g) Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Anal Chem. 2012;84:6278. doi: 10.1021/ac300527z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Green KN, Viswanathan S, Rojas-Quijano FA, Kovacs Z, Sherry AD. Inorg Chem. 2011;50:1648. doi: 10.1021/ic101856d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miller KJ, Saherwala AA, Webber BC, Wu Y, Sherry AD, Woods M. Inorg Chem. 2010;49:8662. doi: 10.1021/ic101489t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mani T, Tircsó G, Togao O, Zhao P, Soesbe TC, Takahashi M, Sherry AD. Contrast Media Mol Imaging. 2009;4:183. doi: 10.1002/cmmi.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 4.Aime S, Botta M, Fasano M, Terreno E. Chem Soc Rev. 1998;27:19. [Google Scholar]
- 5.Que EL, Chang CJ. Chem Soc Rev. 2010;39:51. doi: 10.1039/b914348n. [DOI] [PubMed] [Google Scholar]
- 6.Opina ACL, Wu Y, Zhao P, Kiefer G, Sherry AD. Contrast Media Mol Imaging. 2011;6:459. doi: 10.1002/cmmi.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jászberényi Z, Sour A, Tóth É, Benmelouka M, Merbach AE. Dalton Trans. 2005:2713. doi: 10.1039/b506702b. [DOI] [PubMed] [Google Scholar]
- 8.Tóth É, Burai L, Brücher E, Merbach AE. J Chem Soc, Dalton Trans. 1997:1587. [Google Scholar]
- 9.Congreve A, Parker D, Gianolio E, Botta M. Dalton Trans. 2004:1441. doi: 10.1039/b402230k. [DOI] [PubMed] [Google Scholar]
- 10.(a) Aime S, Barge A, Batsanov AS, Botta M, Castelli DD, Fedeli F, Mortillaro A, Parker D, Puschmann H. Chem Commun. 2002:1120. doi: 10.1039/b202862j. [DOI] [PubMed] [Google Scholar]; (b) Thompson AL, Parker D, Fulton DA, Howard JAK, Pandya SU, Puschmann H, Senanayake K, Stenson PA, Badari A, Botta M, Avedano S, Aime S. Dalton Trans. 2006:5605. doi: 10.1039/b606206g. [DOI] [PubMed] [Google Scholar]
- 11.(a) Doble DMJ, Botta M, Wang J, Aime S, Barge A, Raymond KN. J Am Chem Soc. 2001;123:10758. doi: 10.1021/ja011085m. [DOI] [PubMed] [Google Scholar]; (b) Thompson MK, Doble DMJ, Tso LS, Barra S, Botta M, Aime S, Raymond KN. Inorg Chem. 2004;43:8577. doi: 10.1021/ic048607u. [DOI] [PubMed] [Google Scholar]; (c) Pierre VC, Botta M, Aime S, Raymond KN. Inorg Chem. 2006;45:8355. doi: 10.1021/ic061262q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Werner EJ, Kozhukh J, Botta M, Moore EG, Avedano S, Aime S, Raymond KN. Inorg Chem. 2009;48:277. doi: 10.1021/ic801730u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen SM, Xu J, Radkov E, Raymond KN, Botta M, Barge A, Aime S. Inorg Chem. 2000;39:5747. doi: 10.1021/ic000563b. [DOI] [PubMed] [Google Scholar]
- 13.(a) Aime S, Barge A, Botta M, De Sousa AS, Parker D. Angew Chem Int Ed. 1998;37:2673. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673::AID-ANIE2673>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; (b) Dunand FA, Aime S, Merbach AE. J Am Chem Soc. 2000;122:1506. [Google Scholar]
- 14.Supkowski RM, Horrocks WD., Jr Inorg Chim Acta. 2002;340:44. [Google Scholar]
- 15.Caravan P, Cloutier NJ, Greenfield MT, McDermid SA, Dunham SU, Bulte JWM, Amedio JC, Jr, Looby RJ, Supkowski RM, Horrocks WD, Jr, McMurry TJ, Lauffer RB. J Am Chem Soc. 2002;124:3152. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 16.Caravan P, Farrar CT, Frullano L, Uppal R. Contrast Media Mol Imaging. 2009;4:89. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.