Abstract
Purpose
To determine the macular morphologic features that correlate best with visual outcome in eyes with surgically closed idiopathic macular hole.
Methods
Transversal observational case series of 24 eyes (22 subjects) imaged postoperatively using high-resolution Fourier domain optical coherence tomography (FD-OCT). Total and inner macular volume for central 3 mm area, central foveal thickness, and size of foveal inner segment–outer segment junction abnormality were correlated with best-corrected visual acuity. Microperimetry (MP-1) test was performed in a subset of 18 eyes.
Results
Mean postoperative best-corrected visual acuity was 20/36 (range, 20/25–20/70). Postoperative follow-up mean was 32.97 ± 24.68 months (range, 5–96 months). Eighteen eyes underwent internal limiting membrane (ILM) peeling. Among FD-OCT parameters, logarithm of the minimum angle of resolution best-corrected visual acuity and mean total microperimetry-1 sensitivity correlated best with inner macular volume in all eyes and ILM-peeled eyes (P < 0.05). Macular surface irregularities were noted in 12 eyes (66.7%) with ILM peeling but in none of the non–ILM-peeled eyes (P = 0.02). No significant correlation was found between microperimetry-1 sensitivity and other FD-OCT parameters.
Conclusion
Because inner macular volume strongly correlated with visual outcome in eyes with surgically closed macular hole, the possible effect of ILM peeling on visual outcome needs to be further investigated.
Keywords: macular hole, Fourier domain optical coherence tomography, microperimetry, internal limiting membrane, macular volume, central foveal thickness, foveal photoreceptor abnormality
Since the first introduction of vitrectomy surgery for closure of idiopathic macular hole by Kelly and Wendel in 1991, there have been several refinements in the surgical technique aimed to improve the anatomical and functional outcome of the surgery. Despite these refinements, visual acuity often does not return to normal in eyes with macular hole after surgical closure of the hole.1
To understand the factors that may limit visual recovery in eyes with surgically closed macular hole, commercial time domain (TD) or Fourier domain (FD) optical coherence tomography (OCT) imaging has been used to study the pre- and postoperative morphologic features of the macula in eyes with surgically closed macular hole.2–19 Studies using TD-OCT have shown a negative correlation between the preoperative macular hole dimensions and postoperative visual recovery.3,5 Several groups have studied postoperative macular morphologic features in eyes with surgically closed macular hole using OCT, but no clear consistent morphologic feature that strongly correlates with postoperative visual acuity has been found so far. Several studies noted foveal photoreceptor abnormalities in eyes with surgically closed macular hole that tended to be more common in eyes with poorer vision, but the correlation between the size of this abnormality and postoperative vision was not consistently found.10–19 Other studies attempted to correlate the foveal thickness measurements obtained using TD-OCT with postoperative best-corrected visual acuity (BCVA) with variable findings.7,13 Another study, analyzing the foveal morphology after macular hole closure, reported that U- and V-type of hole closures appeared to have had better visual outcome than W-type of closure.8
In recent years, internal limiting membrane (ILM) peeling has become an increasingly popular technique incorporated in macular hole surgery to increase the rate of macular hole closure.20,21 Although there were some initial concerns regarding possible trauma to the macula from ILM peeling based on persistent electroretinography abnormalities and regarding toxicity of some of the dyes used to stain ILM,22,23 to date no serious adverse effects on visual acuity from ILM peeling have been reported in the surgical management of macular hole. Nonetheless, recent histologic studies have shown that surgically removed ILM specimens from eyes with macular hole or pucker contain neuronal elements.24 These findings raised concerns of damage to inner retinal tissue during ILM peeling.
In this study, we used a high-resolution, research-grade FD-OCT instrument to perform detailed morphologic and volumetric analyses of eyes with surgically closed macular hole to identify features that correlated best with postoperative visual acuity. A research-grade FD-OCT instrument was used because it has a higher image resolution than most commercial FD-OCT instruments (axial resolution of 4.5 μm vs. 5.5 μm) and allows the use of custom-made segmentation software for a more accurate volumetric analysis of the various macular layers. Detailed macular morphologic analysis, including macular volume measurement and its correlation to visual outcome in eyes with surgically closed macular hole, has not been done previously and would be of interest especially given the risk of tissue loss or damage from ILM peeling during macular hole surgery.
Methods
This is a transversal study that enrolled all subjects with surgically closed idiopathic macular hole seen at the Retina Service at the University of California Davis Eye Center. Subjects were enrolled from April 2009 to June 2010 and provided a written informed consent. The protocol was approved by the Office of Human Research Protection at the University of California, Davis School of Medicine and carried out in accordance with the tenets of the Declaration of Helsinki.
All subjects underwent complete ophthalmic examination including Snellen BCVA and dilated fundus examination. Only subjects who had macular hole surgery at least 3 months before enrollment and without any concurrent visually significant ocular pathology were enrolled. A macular hole was defined as closed if the hole edges were flat against the underlying retinal pigment epithelium and well approximated, as described by Tornambe et al.25
Table 1 summarizes the demographic and clinical information for the 24 eyes from 22 subjects imaged in this study. The mean age was 70.64 ± 5.49 years (range, 62–83 years). Fifteen subjects were women (68%). The mean preoperative Snellen BCVA was 20/100 (range, 20/40–20/400). The preoperative macular hole staging was as follows: 8 eyes with stage II, 10 eyes with stage III, 2 eyes with stage IV, and 4 eyes with recurrent macular hole. The preoperative OCT B-scans (mostly TD-OCT) were available for 19 of 24 eyes for review. The maximum hole diameter measured at the center of the hole ranged from 160 to 614 μm (313 ± 127.93). All eyes had undergone standard pars planar vitrectomy with intraocular gas tamponade with postoperative face down positioning for 5 to 7 days. Among them, 18 eyes had concurrent ILM peeling and 6 eyes did not have ILM peeling. Of the 18 eyes that underwent ILM peeling, indocyanine dye (0.05%)-assisted ILM peeling was performed in 13 eyes, triamcinolone acetonide–assisted ILM peeling in 3 eyes and trypan blue (TB) dye–assisted ILM peeling in 2 eyes. The mean duration of follow-up after vitrectomy surgery to FD-OCT imaging was 32.97 ± 24.68 months (range, 5–96 months).
Table 1.
Summary of Demographics, Preoperative Clinical Characteristics and Surgical Technique Used for Study Eyes (N = 24)
| Feature | Mean ± SD (Range); Number |
|---|---|
| Number of eyes (subjects) | 24 (22) |
| Age, years | 70.64 ± 5.49 (62–83) |
| Macular hole stage (number of eyes) | |
| II | 8 |
| III | 10 |
| IV | 2 |
| Recurrent | 4 |
| Surgical technique (number of eyes) | |
| No ILM peeling | 6 |
| ILM peeling | 18 |
| ICG 0.05% | 13 |
| Triamcinolone acetonide | 3 |
| Tryphan blue | 2 |
| Preoperative hole size (μm) | 313 ± 127.93 (160–614) |
| Preoperative Snellen BCVA | 20/100 (20/40–20/400) |
ICG, Indocyanine green.
Fourier Domain Optical Coherence Tomography Imaging
A research-grade high-resolution FD-OCT system developed at the University of California Davis, Vision Science and Advanced Retinal Imaging Laboratory (VRSI) with an axial and transversal resolution of 4.5 μm and 1 μm to 15 μm, respectively, was used to image the macula.26 This system has an acquisition speed of 23,000 lines per second and has been used to study various retinal conditions.27–29 For each patient, a serial 6 mm horizontal line scans and at least 2 volumetric data sets covering an area of 6 mm × 6 mm and 5 mm × 5 mm of the macula (which resulted in 6 mm × 6 mm × 2 mm and 5 mm × 5 mm × 2 mm [lateral × lateral × depth] volumes) were acquired. Volumetric data sets were acquired using raster scans consisting of 100 serial B-scans, each made up of 1,000 A-scans per frame and each B-scan was separated by 60 μm and 50 μm, respectively. Small motion shifts between consecutive B-scans were corrected using a cross-correlation-based algorithm. Data sets with eye blinks or large eye motion defects were excluded from the evaluation.
Segmentation and Macular Volume Measurement Technique
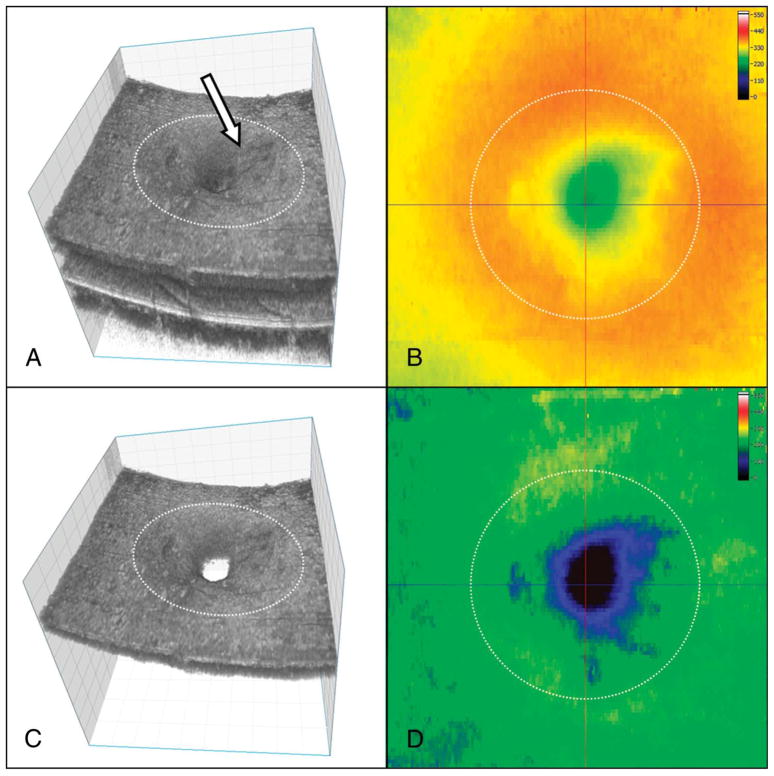
In this study, the best of volumetric data sets, without large motion artifacts, were segmented using a support vector machine–based segmentation algorithm (volume visualization and image processing software) and the thickness maps were processed in LabVIEW-based software as described by Zawadzki et al.30 Briefly, the segmentation software was trained to identify the regions of interest while disregarding the regions not of interest. The regions of interest for segmentation for total macular volume (tMV) measurement included all retinal layers (i.e., top of the nerve fiber layer to bottom of the pigmented epithelial layer), and for inner retinal macular volume (iMV) measurement, the top 3 layers (i.e., top of the nerve fiber layer to bottom of the inner plexiform layer) were included. These measurements were obtained for the central 3-mm-diameter circular area of the macula, as the ILM peeling was performed 360°, for at least 1 disk diameter area around the macular hole (Figure 1). To calculate the iMV changes, we choose to segment the top three retinal layers as inner retinal layers because macular surface irregularities noted in some eyes extended deeper than the nerve fiber layer. Additionally, a clear change in intensity between the inner plexiform and inner nuclear layers made it easy to identify the inner plexiform layer as the outer boundary for iMV, making segmentation more accurate. Because this is a semiautomatic segmentation algorithm, any obvious errors in segmentation were manually corrected by evaluating individual B-scan. All the B-scans were thoroughly evaluated before obtaining the retinal thickness maps. To determine the central foveal point around which to measure the central 3 mm area of the macula, the horizontal and vertical intersecting lines were manually placed on the B-scans in XZ (fast B-scan) and YZ (slow B-scan) planes, thereby further adding to the accuracy of the measurements obtained. Figure 1 illustrates the segmented 3-dimensional data set for tMV and iMV measurements with the corresponding color-coded thickness maps. Central foveal thickness (CFT) and horizontal foveal inner segment–outer segment (IS-OS) abnormality size was measured manually on the foveal FD-OCT B-scan, using the ImageJ software of the National Institutes of Health.31 For measuring the size of the foveal IS-OS abnormality, the linear horizontal distance between the two bright ends of the IS-OS junctional line were measured. Two independent observers (S.P. and R.J.Z.) judiciously performed these measurements and a consensus measurement was obtained for analysis. R.J. Zawadzki was blinded to the postoperative BCVA and the surgical technique used for each eye was analyzed. An irregular contour of the inner macular surface was identified as an irregular defect or a depression noted along the inner macular surface on the serial OCT B-scans.
Fig. 1.
High-resolution FD-OCT three-dimensional structure and corresponding thickness maps of the macula of an eye with surgically closed idiopathic macular hole. A. The segmented tMV volumetric data set is shown, from nerve fiber to retinal pigment epithelial layer. Note the retinal surface irregularities (solid white arrow) in this eye that had ILM peeling. B. The corresponding color-coded retinal thickness map for tMV measurement. C. The segmented three-dimensional volumetric data set for iMV, that is, nerve fiber to inner plexiform layer is shown. D. The corresponding color-coded retinal thickness map for iMV measurement. The intersection of the vertical and horizontal black lines on Figure 1B and 1D represents the central fovea. Dashed circle denotes the central 3 mm zone for which macular volume was measured.
Microperimetry-1 Test
Microperimetry-1 (MP-1) testing was performed using microperimeter (Nidek Technologies, Padova, Italy) in 18 eyes of 17 subjects. As the MP-1 test was performed on a separate scheduled visit, not all subjects were able to return for this test. The central 8° was tested in a dilated pupil, using Goldmann size III target and 4-2-1 staircase strategy. The mean total MP-1 sensitivity of the total 45 data points was correlated with the postoperative logarithm of the minimum angle of resolution (logMAR) BCVA, tMV, and iMV for the central 3 mm area obtained from the FD-OCT volumetric data sets. The foveal IS-OS abnormality size on FD-OCT was correlated with mean MP-1 sensitivity of the central nine data points.
Statistical Analysis
Postoperative Snellen BCVA was converted to the logMAR for statistical analysis. The mean postoperative logMAR BCVA to tMV (central 3 mm), iMV (central 3 mm), CFT, and foveal IS-OS abnormality size on FD-OCT was analyzed using the Pearson correlation coefficient. The difference in mean measurement for tMV, iMV, and CFT was analyzed using an unpaired t-test and difference in incidence of macular surface abnormalities was analyzed using chi-square tests among ILM-peeled and non–ILM-peeled eyes. Similarly, the correlation between mean total MP-1 sensitivity of 45 data points to mean postoperative log-MAR BCVA, tMV (central 3 mm), iMV (central 3 mm), and mean MP-1 sensitivity of central 9 data points to foveal IS-OS abnormality size was analyzed using the Pearson correlation coefficient. A P value of less than 0.05 was considered statistically significant.
Results
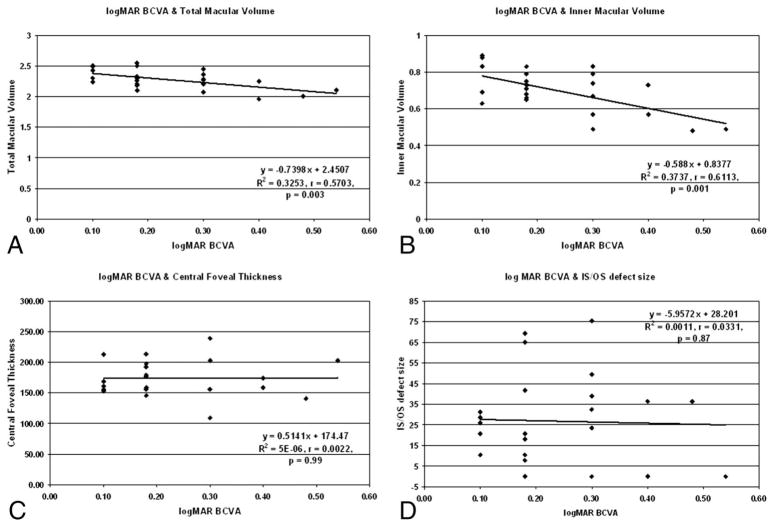
Table 2 summarizes the postoperative BCVA and FD-OCT findings of the eyes imaged. The mean postoperative Snellen BCVA was 20/36 (range, 20/25–20/70) and logMAR BCVA was 0.24 ± 0.12 (range, 0.10–0.54). All eyes except one eye were pseudophakic. The mean tMV was 2.27 ± 0.16 mm3 (range, 1.96–2.55 mm3), the mean iMV was 0.70 ± 0.12 mm3 (range, 0.48–0.89 mm3), the mean CFT was 174.60 ± 28.96 μm (range, 109.20–239.20 μm), and the mean foveal IS-OS abnormality size was 26.78 ± 22.25 μm (range, 0–75.4 μm). Figure 2 shows the scatter plots correlating logMAR BCVA with tMV, iMV, CFT, and foveal IS/OS abnormality size. We found a statistically significant correlation between the postoperative logMAR BCVA and tMV (r = −0.57, P = 0.003) and iMV (r = −0.61, P = 0.001). However, the correlation between postoperative logMAR BCVA and CFT (r = 0.0022, P = 0.99) and foveal IS-OS abnormality size (r = −0.03, P = 0.87) was not statistically significant (Table 3). Furthermore, the size of the foveal IS-OS defect did not correlate with duration of follow-up after vitrectomy surgery (r = −0.32, P = 0.12).
Table 2.
Summary of Postoperative Visual Acuity, Macular Volume, CFT and Foveal IS-OS Defect on FD-OCT (N = 24)
| Mean ± SD (Range) | |
|---|---|
| Snellen BCVA | 20/36 (20/25–20/70) |
| LogMAR BCVA | 0.24 ± 0.12 (0.10–0.54) |
| tMV (mm3) | 2.27 ± 0.16 (1.96–2.55) |
| iMV (mm3) | 0.70 ± 0.12 (0.48–0.89) |
| CFT (μm) | 174.60 ± 28.96 (109.20–239.20) |
| Foveal IS-OS defect (μm) | 26.78 ± 22.25 (0–75.4) |
iMV, inner retinal macular volume for central 3 mm area; tMV, tMV for central 3 mm area.
Fig. 2.
Scatterplots showing the correlation between logMAR BCVA and FD-OCT parameters for 24 eyes: A. tMV, B. iMV, C. CFT, D. foveal photoreceptor IS-OS junction abnormality size.
Table 3.
Summary of FD-OCT Parameters and Their Correlation With logMAR Visual Acuity in Eyes With Surgically Closed Macular Holes With and Without ILM Peeling
| FD-OCT Parameter
|
||||
|---|---|---|---|---|
| iMV | tMV | CFT | Foveal IS-OS Abnormality Size | |
| All eyes (N = 24) | r = −0.61; P = 0.001 | r = −0.57; P = 0.003 | r = 0.0022; P = 0.99 | r = −0.03; P = 0.87 |
| ILM peeled eyes (N = 18) | r = −0.61; P = 0.006 | r = −0.56; P = 0.01 | r = −0.07; P = 0.78 | r = −0.18; P = 0.46 |
| Non–ILM-peeled eyes (N = 6) | r = −0.47; P = 0.34 | r = −0.52; P = 0.29 | r = 0.79; P = 0.05 | r = 0.37; P = 0.46 |
iMV, Inner macular volume for central 3 mm area; P, statistical value; r, correlation coefficient; tMV, tMV, total macular volume for central 3 mm area; CFT, central foveal thickness; IS-OS, photoreceptor inner segment-outer segment; ILM, internal limiting membrane.
To ensure that the variation in postoperative macular volume was not a result of variations in preoperative visual acuity or stage of the macular hole, further analysis was performed to correlated iMV and tMV with these two preoperative clinical features. No statistical difference was found in mean iMV and mean tMV for stage II and stage III macular hole eyes (unpaired t-test, P > 0.21). The analysis did not include Stage IV and recurrent macular hole eyes because the number of eyes in these groups were small. Similarly, when correlating the preoperative visual acuity with postoperative iMV and tMV measurements for all eyes, no significant correlation (r < 0.10, P > 0.64) was noted.
Comparison Between the ILM-Peeled Versus Non–ILM-Peeled Eyes
Table 4 summarizes the BCVA and FD-OCT findings of eyes with ILM peeling when compared with eyes without ILM peeling. Among the 18 eyes that had ILM peeling, the majority of eyes had stage III or IV macular hole before surgery. In contrast, among the six eyes that did not have ILM peeling, all but one eye had stage II macular hole. As such, the preoperative macular hole size tended to be smaller in the group of eyes that did not have ILM peeling and the preoperative BCVA also tended to be better in the group that did not have ILM peeling when compared with the group that had ILM peeling. However, the difference was not statistically significant, probably because of the small sample size. Similarly, the mean postoperative BCVA for the group that had ILM peeling was slightly lower but not significantly different from the group without ILM peeling (20/38 for the ILM-peeled group vs. 20/29 for the non–ILM-peeled group).
Table 4.
Comparison Between the ILM-Peeled Eyes and Non–ILM-Peeled Eyes
| Characteristics Preoperative MH (n) | ILM-Peeled Eyes (N = 18) | Non–ILM-Peeled Eyes (N = 6) | P |
|---|---|---|---|
| Stage II | 3 | 5 | |
| Stage III | 9 | 1 | |
| Stage IV | 2 | 0 | |
| Recurrent hole | 4 | 0 | |
| Preoperative MH size (μm), mean ± SD (range) | 331.38 ± 127.72 (180–614) | 216 ± 90.15 (160–320) | 0.12 |
| Preoperative BCVA, mean (range) | 20/112 (20/40–20/400) | 20/67 (20/40–20/80) | 0.22 |
| Postoperative BCVA, Mean (range) | 20/38 (20/25–20/70) | 20/29 (20/25–20/40) | 0.09 |
| tMV (mm3), mean ± SD (range) | 2.26 ± (0.17) (2.01–2.51) | 2.32 ± 0.12 (2.30–2.49) | 0.46 |
| iMV (mm3), mean ± SD (range) | 0.68 ± 0.12 (0.48–0.88) | 0.74 ± 0.10 (0.63–0.89) | 0.31 |
| CFT (μm), mean ± SD (range) | 172.61 ± 30.09 (149–239) | 180.56 ± 26.87 (155–213) | 0.57 |
| IS-OS defect size (μm), mean ± SD (range) | 29.63 ± 24.01 (0–75.4) | 18.20 ± 14.15 (0–28.6) | 0.28 |
iMV, inner retinal macular volume for central 3 mm area; MH, macular hole; P value, comparing the mean values using unpaired t-test; tMV, tMV for central 3 mm area.
Table 3 summarizes the correlation between BCVA and FD-OCT morphologic features of ILM-peeled eyes when compared with non–ILM-peeled eyes. Among eyes with ILM peeling, a significant correlation between the logMAR BCVA and tMV (r = −0.56, P = 0.01) and iMV (r = −0.61, P = 0.006) was noted. In contrast, logMAR BCVA did not correlate with CFT (r = −0.07, P = 0.78) and foveal IS-OS abnormality size (r = −0.18, P = 0.46).
On the contrary, among eyes that did not undergo ILM, there was no statistically significant correlation between the postoperative logMAR BCVA and tMV (r = −0.52, P = 0.29), iMV (r = −0.47, P = 0.34), and foveal photoreceptor IS-OS defect size (r = 0.37, P = 0.46). However, a statistically significant correlation was noted between postoperative logMAR BCVA and CFT (r = 0.79, P = 0.05) despite the small sample size.
When comparing the FD-OCT parameters between the ILM-peeled group and non–ILM-peeled group (Table 4), the mean postoperative tMV, iMV, and CFT tended to be slightly lower in eyes with ILM peeling when compared with eyes without ILM peeling, but this difference was not statistically significant using an unpaired t-test. The mean size of the foveal IS-OS abnormality was larger in the group with ILM peeling, but the difference was again not statistically significant.
When comparing the macular surface contour between the group of eyes that had ILM peeling with eyes without ILM peeling, varying degrees of macular surface abnormalities were noted in 12 (66.7%) of the eyes with ILM peeling (Figure 3) but in none of the eyes without ILM peeling (Figure 4). This difference was statistically significant (P = 0.02, chi-square with Yates correction for continuity = 5.556, df = 1).
Fig. 3.
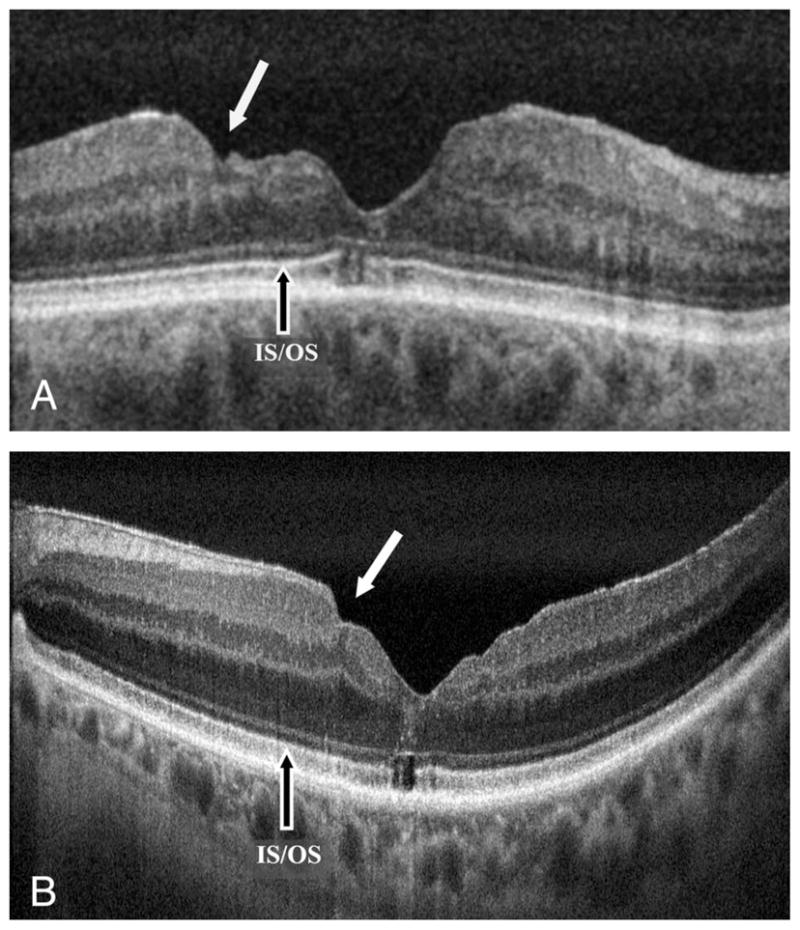
High-resolution FD-OCT B-scan images of eyes that had ILM peeling for surgical closure of macular hole. A. An eye with BCVA of 20/60 showing more severe macular surface irregularity (solid white arrow). This eye underwent a second vitrectomy with ILM peeling for recurrent macular hole. Note also the foveal photoreceptor IS-OS abnormality. B. An eye with BCVA of 20/30 with more subtle macular surface irregularity (solid white arrow) and mild epiretinal membrane peripheral to the irregularity. Foveal photoreceptor IS-OS abnormality is present again. Image A is a single frame image and Image B is an average of 10 frames.
Fig. 4.
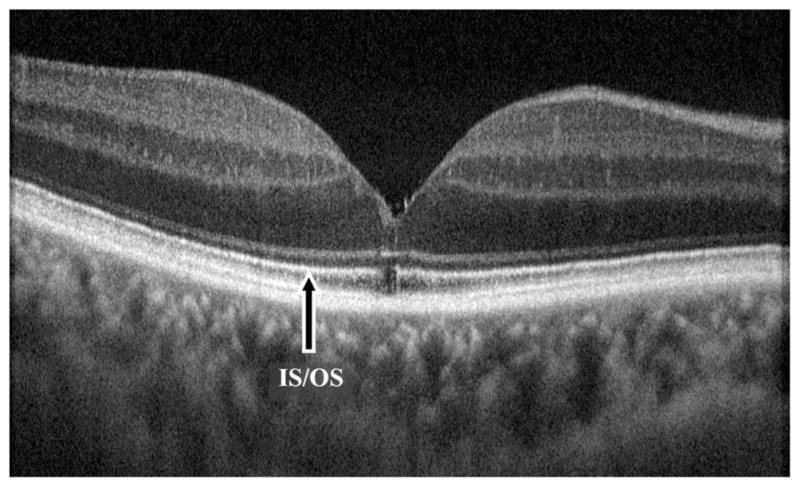
High-resolution FD-OCT B-scan image of an eye with surgically closed macular hole without ILM peeling. BCVA is 20/25 and no macular surface irregularity is noted. A small foveal photoreceptor IS-OS defect is present. This image is an average of 10 frames.
Correlation Between MP-1, BCVA, and OCT
Table 5 summarizes the MP-1 findings for the subset of 18 eyes (17 eyes) that underwent this additional test (8°, 10 dB). Thirteen of these eyes had ILM peeling. The mean MP-1 sensitivity of 45 data points was 17.46 ± 2.32 dB (range, 12.65–19.92 dB) and the mean MP-1 sensitivity of the central 9 data points was 14.38 ± 4.41 dB (range, 5.33–19.22 dB) for all eyes.
Table 5.
Summary of MP-1 Sensitivity and Correlation With logMAR Visual Acuity and FD-OCT Morphologic Features in Eyes With Surgically Closed Macular Holes With and Without ILM Peeling
| LogMAR BCVA and Mean Total MP-1 Sensitivity (45 Data Points) | tMV and Mean Total MP-1 Sensitivity (45 Data Points) | iMV and Mean Total MP-1 Sensitivity (45 Data Points) | IS-OS Defect Size and Mean Central MP-1 Sensitivity (9 Data Points) | |
|---|---|---|---|---|
| All eyes (N = 18) | r = −0.75; P = 0.0004 | r = 0.47; P = 0.05 | r = 0.71; P = 0.001 | r = −0.42; P = 0.086 |
| ILM peeled eyes (N = 13) | r = −0.71; P = 0.0067 | r = 0.46; P = 0.11 | r = 0.71; P = 0.0071 | r = −0.32; P = 0.29 |
| Non–ILM-peeled eyes (N = 5)* | r = −0.94 | r = 0.22 | r = 0.46 | r = −0.57 |
iMV, Inner macular volume for central 3 mm area; P, statistical value; r, correlation coefficient; tMV, tMV for central 3 mm area.
P value for non–ILM-peeled eyes was not calculated because of fewer number of eyes.
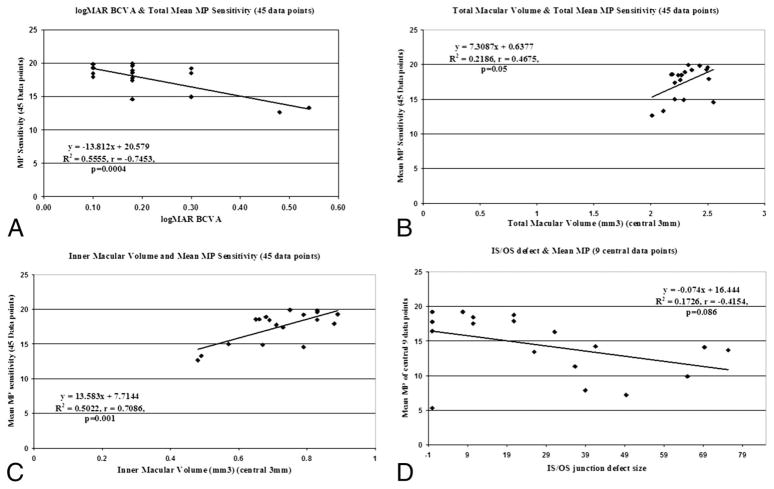
As shown in Figure 5, a significant correlation was noted between the postoperative logMAR BCVA and the mean total MP-1 sensitivity of the 45 data points (r = −0.75, P = 0.0004). The mean total MP-1 sensitivity for the 45 data points also correlated positively with tMV (r = 0.47, P = 0.05) for all eyes. A much stronger correlation was noted between mean total MP-1 sensitivity and iMV (r = 0.71, P = 0.001) for all eyes. This correlation extended to the subclass of eyes with ILM peeling (r = 0.71; P = 0.0071) but not to non–ILM-peeled eyes. A trend toward correlation was noted between foveal IS-OS abnormality size and mean MP-1 sensitivity of central 9 points among all MP-1 tested eyes, but the correlation was not statistically significant (r = −0.42, P = 0.086).
Fig. 5.
Scatterplots showing the correlation between MP-1 sensitivity and logMAR BCVA and FD-OCT parameters for 18 eyes: A. Mean total MP-1 sensitivity (45 data points) and logMAR BCVA, B. Mean total MP-1 sensitivity (45 data points) and tMV, C. Mean total MP-1 sensitivity (45 data points) and iMV, D. Mean central MP-1 sensitivity (9 data points) and foveal photoreceptor IS-OS junction abnormality size.
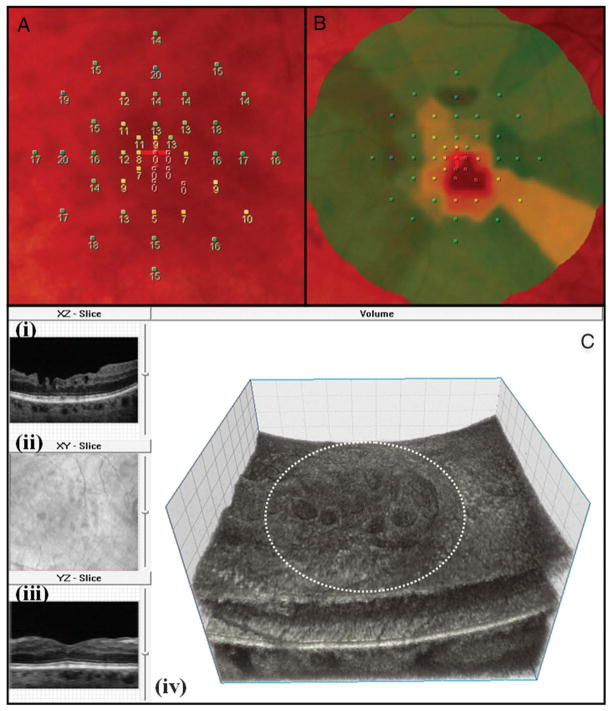
Figure 6 shows an example of an eye that underwent peeling with poorer postoperative MP-1 sensitivity. As demonstrated, a correlation is noted between the regions of the macula with surface irregularity on FD-OCT and the zones of the macula corresponding to the decreased macular sensitivity on MP-1. Correlation between MP-1 data and FD-OCT macular surface finding was noted in 16 of 18 eyes tested with MP-1.
Fig. 6.
MP-1 (8° 10 dB strategy) and high-resolution FD-OCT image of an eye with surgically closed macular hole with ILM peeling. A. MP-1 sensitivity with numerical values for the 45 data points, B. MP-1 sensitivity color map (green denotes normal sensitivity, whereas yellow and orange denotes progressive loss of sensitivity, respectively) and C. the three-dimensional FD-OCT volumetric data set as viewed on volume visualization and image processing software; (i) the B-scan in XZ plane showing macular surface irregularity, (ii) the en face projection view of the volume showing the extent of the macular surface irregularity relative to the retinal vessels, (iii) the “virtual” B-scan in YZ plane reconstructed from volume through section of macula with less surface irregularity and (iv) the three-dimensional FD-OCT volume with surface irregularities. The dashed white circle represents the approximate area over which the MP-1 sensitivity assessment was done. For this eye, the BCVA postoperatively was 20/70, mean total MP-1 sensitivity of 45 data points was 13.30 dB and mean central MP-1 sensitivity of 9 data points was 5.33 dB and tMV and iMV for central 3 mm was 2.11 mm3 and 0.49 mm3, respectively. This is an example of an eye with poor MP-1 sensitivity and macular surface abnormality on FD-OCT B-scan. As shown, there is some correlation between the area of the macula with decreased sensitivity on MP-1 and the area of the macula with surface irregularity on FD-OCT.
Discussion
In this study, a research-grade high-resolution FD-OCT instrument was used to perform detailed morphology and volumetric analysis of the macula of eyes with surgically closed macular hole to determine macular morphologic features that correlated best with visual outcome. Among the parameters studied, that is, iMV and tMV for the central 3 mm, CFT, and size of the foveal photoreceptor IS-OS abnormality, iMV correlated the best with postoperative visual acuity and macular sensitivity on MP-1 in all eyes imaged. This correlation between the iMV and postoperative BCVA and macular sensitivity on MP-1 was again noted in the subgroup of eyes that had ILM peeling. Among the small group of eyes that did not have ILM peeling, postoperative BCVA correlated instead with CFT despite the small sample size. This observation raises the question of whether different factors may limit visual acuity outcome in eyes with ILM peeling when compared with eyes without ILM peeling. In particular, among eyes with ILM peeling, our findings raise the question of whether there could be possible damage to inner macular layers during ILM peeling that may have some adverse effect on visual outcome after surgery. Consistent with this hypothesis is the presence of macular surface irregularities noted on FD-OCT in varying degrees in 66.7% of eyes that underwent ILM peeling. Although most of these surface irregularities were mild among ILM-peeled eyes, they were absent in eyes that did not have ILM peeling in our series. In a recent case report by Chae et al,32 a similar inner retinal surface irregularity was noted on commercial FD-OCT in an eye after ILM peeling for macular hole in the region of the macula not subjected to any frank surgical trauma. In this case report, a beaten bronze discoloration was noted on funduscopy correlating to this region of the macula, but the authors do not comment on the visual outcome of this eye. No such abnormality on funduscopy was noted in any of the eyes enrolled in our study on funduscopy on routine postoperative examination. Because no significant differences in visual outcome after macular hole surgery have been reported to date after ILM peeling when compared with eyes without ILM peeling, if there is a potential adverse effect of ILM peeling on visual outcome, it is possible that it may be subtle or offset by the positive effect of ILM peeling on hole closure.15,33 Various studies have shown that ILM peeling during macular hole surgery is associated with increased primary hole closure rate.20,21,34 A recent randomized prospective study found a significantly higher macular hole closure rate using dye-assisted ILM peeling for both stage 2 and 3 macular hole.21 This study also found dehiscence of the nerve fiber layer in 50% of the eyes that underwent ILM peeling that corresponded with nerve fiber layer irregularities seen on OCT and likely resulted from the ILM procedure itself. It did not appear to be detrimental to functional outcome. Nonetheless, a review of the literature reveals various studies that raise concerns regarding possible adverse effects of ILM peeling. A study of microperimetry testing of eyes with closed macular hole showed asymptomatic paracentral scotoma in some eyes after ILM peeling but not in eyes without ILM peeling.35 A multifocal macular electroretinography study showed b-wave abnormalities after ILM peeling suggestive of Muller cell damage.22 Recent immunohistochemical and electron microscopy studies of surgically removed ILM specimen showed neuronal elements, direct evidence of retinal trauma during ILM removal.24 Finally, there are preclinical studies showing histologic evidence of damage to retinal surface tissue layers by indocyanine green dye upon halogen light exposure, suggesting a possible retinal toxic effect of indocyanine green dye on retinal surface tissue.36 Other possible toxic effects of various dyes on retinal neurons and retinal pigment epithelium also have been noted and concerns remain regarding the safety of these dyes in macular surgery.37–39
With the advent of high-resolution FD-OCT imaging systems, detailed morphologic and three-dimensional volumetric analysis of the macula is possible.26–30 The research-grade FD-OCT used in this study is technically similar to commercial FD-OCT but has distinct advantages by offering higher axial resolution (4.5 μm vs. 5.5 μm) to an extent and allowing the use of custom semiautomatic segmentation software to obtain volume measurement of the various retinal layers with accuracy that is not possible currently with commercial instruments.
In this study, quantitative volumetric analysis of surgically closed macular hole eyes found a statistically significant correlation between a decrease in macular volume, in particular iMV, with a decrease in postoperative BCVA and macular sensitivity on MP-1. This correlation extended to eyes with ILM peeling. Furthermore, postoperative iMV and tMV seem to be independent of preoperative macular hole stage or visual acuity. Among the small group of eyes without ILM peeling, postoperative BCVA correlate most with central foveal thickness. These findings are consistent with the hypothesis that the loss of iMV may be a visually limiting factor among eyes with ILM peeling and may result from the ILM peeling technique rather than from the effect of the macular hole itself. However, a larger study will be needed to further validate our findings and impressions.
Many of the previous studies have found a significant correlation between the extent of the foveal IS-OS defect and postsurgical visual outcome; however, this was not observed in all studies.10–19 In line with few previous studies that used FD-OCT, our series similarly did not find a correlation between the size of the foveal IS-OS defect and visual outcome whether the eyes had ILM peeling. Our analysis of IS-OS abnormality size was based on measuring the horizontal linear separation between two bright ends of the IS-OS defect on a carefully chosen, most centered foveal B-scan and did not consider parameters such as magnitude of the IS-OS signal and IS-OS defect area as done by Oh et al.18 Another possible relevant clinical feature that we did not analyze is the correlation between the preoperative IS-OS abnormality size with postoperative IS-OS abnormality size. This was because most of the preoperative OCT imaging was performed using TD-OCT with lower axial resolution.
In our current study, a minimum follow-up period of 5 months was chosen to allow postoperative recovery of the photoreceptors. As such, the follow-up duration did not influence the size or appearance of the IS-OS junction, a contrast to a previous study using Zeiss OCT-3.14 A recent study by Chung et al19 using MP-1 test found a correlation between poor postoperative visual acuity and a large mean difference in MP-1 sensitivity among study and fellow eyes. The IS-OS defect size did not correlate with postoperative visual acuity, measured MP-1 parameters, and degree of fundus autofluorescence. Our observations using MP-1 test showed that iMV correlated best with mean total MP-1 sensitivity. This correlation was stronger among ILM-peeled eyes, suggesting that ILM peeling associated inner retinal morphologic changes had affected the MP-1 sensitivity. Consistent with this hypothesis is the qualitative correlation between the pattern of macular surface irregularity noted in ILM-peeled eyes and MP-1 defect (Figure 6). The linear IS-OS defect size showed a trend toward correlating with mean central MP-1 sensitivity (central 9 points), but this correlation was not statistically significant. Thus, a possible contribution of foveal photoreceptor changes on postoperative visual function cannot be ruled out but does not seem to be the major determinant of visual outcome after macular hole surgery among ILM-peeled eyes in our series.
The mean final postoperative BCVA of the subjects in our series is comparable with reported visual outcome after macular hole surgery.15,17,18 Thus, our observations are unlikely to be unique to our study population despite our small sample size. There are, however, several limitations of our study. Because there was no significant difference in BCVA between the ILM-peeled group and non–ILM-peeled group in our series, it is difficult to speculate on the magnitude of the adverse effect of ILM peeling on postoperative vision, if any. This may be because our sample size was small especially for the non–ILM-peeled group, but it may also be because the benefit of ILM peeling in increasing the success of macular hole closure may be offsetting any negative effect the trauma of ILM peeling may have on visual outcome. The few subjects enrolled in this study without ILM peeling was because of the limited number of subjects identified that had this surgical technique. A more equal number of subjects in the two groups may have allowed a more balanced analysis. A second limitation of the study is that there were some selection biases in determining which eyes had ILM peeling and which eyes did not have ILM peeling. As shown in Table 3, ILM-peeled eyes tended to have larger holes with poorer vision preoperatively than non–ILM-peeled eyes, but the difference was not statistically significant probably because of the small sample size. Last, ILM peeling was done by various surgeons using various techniques, each with potentially different adverse effects on the retina. This study was too small to compare the effects of the various ILM techniques used by the different surgeons.
Nonetheless, our report presents new observations correlating iMV with visual outcome after macular hole surgery. Further studies are needed to validate our preliminary findings and to determine whether this correlation indeed reflects the result of adverse effect of ILM peeling on macular surface. As ILM peeling has become increasingly popular among retinal surgeons, and now used for surgeries other than macular hole, it becomes important to further investigate the potential adverse effects of ILM peeling on macular health and morphology. A larger prospective randomized multicenter study using FD-OCT may be helpful to answer these questions. Although it may be difficult to replicate our findings using commercial FD-OCT instruments because of the unavailability of the semiautomated segmentation software of the various retinal layers currently, correlation between tMV and visual outcome can be made even with the current commercial FD-OCT instruments to determine whether changes in macular volume has a significant effect on visual outcome in surgically closed macular hole eyes as was found in our study.
Acknowledgments
Supported by Research to Prevent Blindness, New York, NY (unrestricted departmental grant and a Senior Scientist Award to J.S.W.) and the National Eye Institute, Bethesda, MD (Grant 014743 to J.S.W.).
The authors acknowledge Bioptigen Inc Research Triangle Park, NC for sharing real-time FD-OCT acquisition software. Special thanks also to the members of Vision Science and Advanced Retinal Imaging laboratory, VSRI, and the Institute for Data Analysis and Visualization, IDAV, Department of Computer Science, University of California, Davis, for their help.
Footnotes
Presented in part as a paper at the Macula Society Meeting, Boca Raton, Florida, March 10, 2011; and at World Ophthalmology Congress, Berlin, Germany, June 2010; and as a poster at the American Society of Retina Specialists Meeting, Vancouver, August 2010.
The authors declare no conflicts of interest.
References
- 1.Richter-Mueksch S, Sacu S, Osarovsky-Sasin E, et al. Visual performance 3 years after successful macular hole surgery. Br J Ophthalmol. 2009;93:660–663. doi: 10.1136/bjo.2008.154963. [DOI] [PubMed] [Google Scholar]
- 2.Imai M, Iijima H, Gotoh T, Tsukahara S. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol. 1999;128:621–627. doi: 10.1016/s0002-9394(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemoto R, Yamamoto S, Aoki T, et al. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with and without internal limiting membrane peeling. Br J Ophthalmol. 2002;86:1240–1242. doi: 10.1136/bjo.86.11.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Moreno JM, Staicu C, Pinero DP, et al. Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol. 2008;92:640–644. doi: 10.1136/bjo.2007.136176. [DOI] [PubMed] [Google Scholar]
- 6.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015–1019. doi: 10.1136/bjo.87.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusuhara S, Teraoka Escano MF, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence in eyes with idiopathic macular holes. Am J Ophthalmol. 2004;138:709–716. doi: 10.1016/j.ajo.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 8.Michalewska Z, Michalewski J, Cisiecki S, et al. Correlation between foveal structure and visual outcome following macular hole surgery: a spectral optical coherence study. Graefes Arch Clin Exp Ophthalmol. 2008;246:823–830. doi: 10.1007/s00417-007-0764-5. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi T, Fujiwara M, Sakaguchi H, et al. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–1824. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Kitaya N, Hikichi T, Kagokawa H, et al. Irregularity of photoreceptor layer after successful macular hole surgery visual acuity improvement. Am J Ophthalmol. 2004;138:308–310. doi: 10.1016/j.ajo.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Villate N, Lee JE, Venkatraman A, Smiddy WE. Photoreceptor layer features in eyes with closed macular holes: optical coherence tomography findings and correlation with visual outcomes. Am J Ophthalmol. 2005;139:280–289. doi: 10.1016/j.ajo.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Ko TH, Witkin AJ, Fujimoto JG, et al. Ultrahigh-resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol. 2006;124:827–836. doi: 10.1001/archopht.124.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haritoglou C, Neubauer AS, Reiniger IW, et al. Long-term functional outcome of macular hole surgery correlated to optical coherence tomography measurements. Clin Experiment Ophthalmol. 2007;35:208–213. doi: 10.1111/j.1442-9071.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 14.Baba T, Yamamoto S, Arai M, et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina. 2008;28:453–458. doi: 10.1097/IAE.0b013e3181571398. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Watanabe Y, Arakawa A, et al. Spectral-domain optical coherence images of inner/outer segment junctions and macular hole surgery outcomes. Graefes Arch Clin Exp Ophthalmol. 2009;247:325–330. doi: 10.1007/s00417-008-0999-9. [DOI] [PubMed] [Google Scholar]
- 16.Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2009;147:313–318. doi: 10.1016/j.ajo.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Chang LK, Koizumi H, Spaide RF. Disruption of the photoreceptor inner segment-outer segment junction in eyes with macular holes. Retina. 2008;28:969–975. doi: 10.1097/IAE.0b013e3181744165. [DOI] [PubMed] [Google Scholar]
- 18.Oh J, Smiddy WE, Flynn HW, Jr, et al. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci. 2010;51:1651–1658. doi: 10.1167/iovs.09-4420. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Shin CJ, Kim JG, et al. Correlation of microperimetry with fundus autofluorescence and spectral-domain optical coherence tomography in repaired macular holes. Am J Ophthalmol. 2011;151:128.e3–136.e3. doi: 10.1016/j.ajo.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Tognetto D, Grandin R, Sanguinetti G, et al. Internal limiting membrane removal during macular hole surgery: results of a multicenter retrospective study. Ophthalmology. 2006;113:1401–1410. doi: 10.1016/j.ophtha.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 21.Christensen UC, Kroyer K, Sander B, et al. Value of internal limiting membrane peeling in surgery for idiopathic macular hole stage 2 and 2: a randomized clinical trial. Br J Ophthalmol. 2009;93:1005–1015. doi: 10.1136/bjo.2008.151266. [DOI] [PubMed] [Google Scholar]
- 22.Teraski H, Miyake Y, Nomura R, et al. Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Invest Ophthalmol Vis Sci. 2001;42:229–234. [PubMed] [Google Scholar]
- 23.Ferencz M, Somfai GM, Farkas A, et al. Functional assessment of the possible toxicity of indocyanine green dye in macular hole surgery. Am J Ophthalmol. 2006;142:765–770. doi: 10.1016/j.ajo.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 24.Kenawy N, Wong D, Stappler T, et al. Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology. 2010;117:320–323. doi: 10.1016/j.ophtha.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Tornambe PE, Poliner LS, Cohen RG. Definition of macular hole surgery end points: elevated/open, flat/open, flat/closed. Retina. 1998;18:286–287. doi: 10.1097/00006982-199803000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Alam S, Zawadzki RJ, Choi SS, et al. Clinical application of rapid serial Fourier-domain optical coherence tomography macular imaging. Ophthalmology. 2006;113:1425–1431. doi: 10.1016/j.ophtha.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troung SN, Alam S, Zawadzki RJ, et al. High-resolution Fourier-domain optical coherence tomography of retinal angiomatous proliferation. Retina. 2007;27:915–925. doi: 10.1097/IAE.0b013e31805468fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SS, Troung SN, Zawadzki RJ, et al. High-resolution Fourier-domain optical coherence tomography of choroidal neovascular membranes associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4200–4206. doi: 10.1167/iovs.09-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AJ, Telander DG, Zawadzki RJ, et al. High-resolution Fourier-domain optical coherence tomography and microperimetric findings after macula-off retinal detachment repair. Ophthalmology. 2008;115:1923–1929. doi: 10.1016/j.ophtha.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zawadzki RJ, Fuller AR, Wiley DF, et al. Adaptation of a support vector machine algorithm for segmentation and visualization of retinal structures in volumetric optical coherence tomography data sets. J Biomed Opt. 2007;12:041206-1–041206-8. doi: 10.1117/1.2772658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasband WS. Image J. Bethesda, MD: U.S. National Institute of Health; 1997–2006. Available at: http://rsb.info.nih.gov/ij. [Google Scholar]
- 32.Chae JB, Choi DK, Kim J-G. Nerve fibre layer irregularity after internal limiting membrane peeling, seen by spectral domain optical coherence tomography. Eye (Lond) 2011;25:1515–1516. doi: 10.1038/eye.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen UC, Kroyer K, Sander B, et al. Macular morphology and visual acuity after macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol. 2010;94:41–47. doi: 10.1136/bjo.2009.159582. [DOI] [PubMed] [Google Scholar]
- 34.Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:769–777. doi: 10.1016/s0002-9394(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 35.Haritoglou C, Ehrt O, Gass CA, et al. Paracentral scotomata: a new finding after vitrectomy for idiopathic macular hole. Br J Ophthalmol. 2001;85:231–233. doi: 10.1136/bjo.85.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haritoglou C, Priglinger S, Gandorfer A, et al. Histology of the vitreoretinal interface after indocyanine green staining of the ILM, with illumination using a halogen and xenon light source. Invest Ophthalmol Vis Sci. 2005;46:1468–1472. doi: 10.1167/iovs.04-0838. [DOI] [PubMed] [Google Scholar]
- 37.Schumann RG, Gandorfer A, Priglinger SG, et al. Vital dyes for macular surgery. A comparative electron microscopy study of the internal limiting membrane. Retina. 2009;29:669–676. doi: 10.1097/IAE.0b013e318196b1c8. [DOI] [PubMed] [Google Scholar]
- 38.Gale JS, Proulx AA, Gonder JR, et al. Comparison of the in vitro toxicity of indocyanine green to that of tryphan blue in human retinal pigment epithelium cell cultures. Am J Ophthalmol. 2004;138:64–69. doi: 10.1016/j.ajo.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 39.Jackson TL, Hillenkamp J, Knight BC, et al. Safety testing of indocynaine green and tryphan blue using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci. 2004;45:2778–2785. doi: 10.1167/iovs.04-0320. [DOI] [PubMed] [Google Scholar]