Abstract
Immunosenescence is the term commonly used to describe the multifaceted phenomenon encompassing all changes occurring in the immune system during aging. It contributes to render older adults more prone to develop infectious disease and main age-related diseases. While age clearly imposes drastic changes in immune physiology, older adults have heterogeneous health and immune phenotypes. This confronts scientists and researcher to develop more age-specific interventions rather than simply adopting intervention regimes used in younger people and this in order to maintain immune protection in older adults. Thus, this review provides evidences of the central role played by cell-mediated immunity in the immunosenescence process and explores the means by which senescent state of the cell-mediated immune function could be identified and predicted using biomarkers. Furthermore considerations are given to recent advances made in the field of age-specific immune interventions that could contribute to maintain immune protection, to improve quality of life, and/or to promote healthy aging of the growing part of the population.
Keywords: Cell-mediated immunity, Healthy aging, Immunosenescence, TREC ratio, Thymus TREC
Introduction
Over the last 50 years, the number of individual older than 65 years has tripled. By 2025–2030, projections indicate that the population aged over 65 will be growing 3.5 times as rapidly as the total population (Lutz et al. 1997; Oeppen and Vaupel 2002). The optimism created by the ever increasing life expectancy and the expectation of many individuals that they will live longer and healthier should be balanced by the reality of health care burden placed on medical and social welfare services by the increased number of older individuals (Lang and Aspinall 2012).
Indeed, the age-related changes of the immune system, commonly termed immunosenescence (Weiskopf et al. 2009; Ongrádi and Kövesdi 2010), contribute to the increased susceptibility of older adults to develop not only infectious diseases, but cancer, Alzheimer’s diseases, osteoporosis, and autoimmunity (Ginaldi et al. 2005; 2008; Lang et al. 2010b; Fulop et al. 2011).
Although individuals’ age is a major contributor, there is no single cause of immunosenescence, which is the consequence of a compilation of events (Govind et al. 2012) including thymic involution (Aspinall et al. 2010), the continuous reshaping of the immune repertoire by persistent antigenic challenges (Virgin et al. 2009), the dysregulation of Toll-like receptor functions (Shaw et al. 2011), the reduced production of naïve B cells and the intrinsic defects arising in resident B cells (Frasca et al. 2011), and the impact of nutritional status and dysregulation of hormonal pathways (Kelley et al. 2007; Lang et al. 2012; Lang and Samaras 2012). Moreover, human aging is also inextricably linked with an ever increasing incidence of chronic-comorbid health conditions (e.g., diabetes and heart failure) which contribute to increase the age-related chronic low-grade inflammation and therefore further impinge the immune system (Fulop et al. 2010; Franceschi et al. 2007).
Therefore, while age clearly imposes drastic changes in immune physiology, older adults have heterogeneous health and immune phenotypes. This poses new challenges to scientists and researchers as well because research on the immunology of aging needs to go beyond the characterization of age-related immune deficiencies. Thus, after demonstrating the central role played by cell-mediated immunity in the immunosenescence process, this review will explore the means by which immunosenescent state could be identify through the interesting question whether cell-mediated immune competences could be predicted using biomarkers. Furthermore, considerations will be given to recent advances made in the field of age-specific immune interventions that could contribute to maintain immune protection, to improve quality of life, and/or to promote healthy aging of the growing part of the population.
What are the main features of the T cell-mediated immunity senescence?
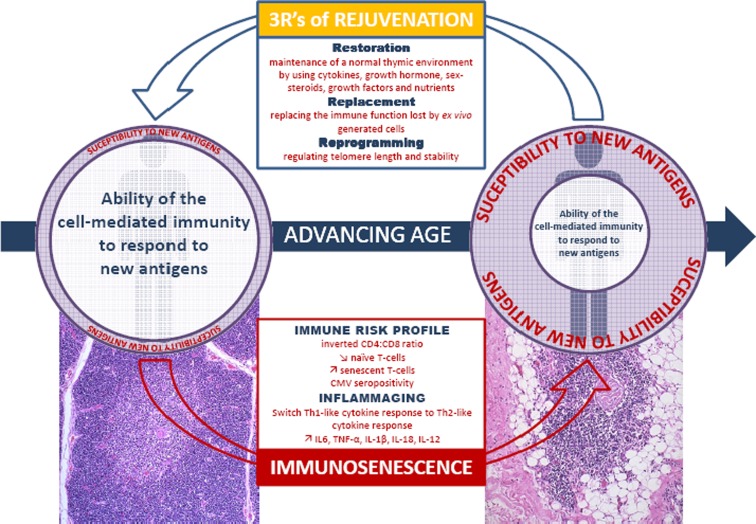
Quantification of T cell numbers throughout the life span shows that they are maintained in human beings (Aspinall et al. 2010) even in their tenth decades at levels which are comparable to those found in younger individuals (Mitchell et al. 2010). This would imply that there is no decline in the homeostatic mechanisms which preserve the size of the peripheral T cell pool within defined boundaries (Lang et al. 2011b). As showed in Fig. 1, the age-related changes in cell-mediated immunity are characterized by two major patterns: the reduction in thymic output (i.e.,  naïve T cells) and the increase in the number of antigen-experienced memory and in particular effector cells (i.e.,
naïve T cells) and the increase in the number of antigen-experienced memory and in particular effector cells (i.e.,  senescent cells) (Weiskopf et al. 2009). In addition, but not further detailed thereafter, thymic involution also leads to a decreased output of regulatory T cells (i.e., Tregs) which has been reported to decline after the age of 50 and might contribute to age-related phenomena such as autoimmunity and increased inflammation as well (Tsaknaridis et al. 2003; Weiskopf et al. 2009; Franceschi et al. 2007).
senescent cells) (Weiskopf et al. 2009). In addition, but not further detailed thereafter, thymic involution also leads to a decreased output of regulatory T cells (i.e., Tregs) which has been reported to decline after the age of 50 and might contribute to age-related phenomena such as autoimmunity and increased inflammation as well (Tsaknaridis et al. 2003; Weiskopf et al. 2009; Franceschi et al. 2007).
Fig. 1.
Schematic representation of the main features observed within the T cell-mediated immune system with advancing age. Thymic atrophy is characterized by a progressive, age-related reduction in the size of the thymus due to profound changes in its anatomy (i.e., progressive loss of thymic mass and replacement of thymocytes with adipocytes). This is a key contributory factor in the reduced ability of the immune system to respond to new antigen. While the quantification of T cell numbers shows that they are maintained throughout the life span, with the age-associated reduction in thymic output (i.e., naïve T cells), the constituent of the T cell pool progress towards their replicative limit (i.e., senescent cells). Potential beneficial impacts of the 3Rs of Rejuvenation are also represented
The decreased number of naïve T cells
Production and maintenance of the diverse peripheral naïve T cell repertoire are critical to the normal function of the immune system (Weiskopf et al. 2009; Ongrádi and Kövesdi 2010). In the older adults, there is a decrease both in the diversity and functional integrity of the CD4+ and CD8+ T cells subsets which contribute to a decreased ability to respond adequately to reinfection (Naylor et al. 2005) and a poorer vaccine effectiveness (Lang et al. 2011a). Age-associated changes in cell-mediated immunity strongly depend on thymic function (Aspinall et al. 2010). Thymic involution is one of the major feature of human immunosenescence because it is the single preceding event in all cases (Ostan et al. 2008; Aspinall et al. 2010). It is characterized by a progressive, reduction in size, due to profound changes in its anatomy with reducing the active areas of thymopoiesis related to fat accumulation throughout life.
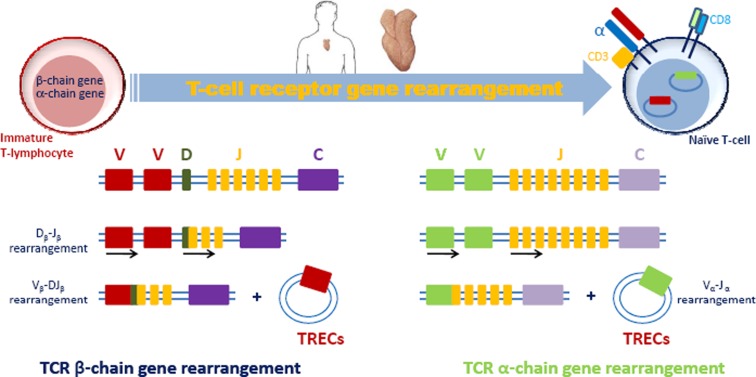
Thymic atrophy and decreased thymopoiesis are active processes mediated by the upregulation of thymosupressive cytokines (i.e., interleukin—IL-6, leukemia inhibitory factor—LIF, and oncostatin M—OSM) in aged human being and mice thymus tissue (Sempowski et al. 2000; Ongrádi and Kövesdi 2010), while IL-7 production by stromal cell is significantly decreased (Andrew and Aspinall 2002; Ortman et al. 2002). IL-7 is necessary for thymopoiesis, promoting cell survival by maintaining the anti-apoptotic protein Bcl-2 and inducing V-DJ recombination (see Fig. 2) (Kim et al. 1998; Aspinall and Andrew 2000; Jiang et al. 2005). The above changes result in decreased thymic output, in diminished number of circulating naïve T cells (i.e., CD45RA+CD28+ and CD45RA+CD28+CD26L) in the blood stream and lymph nodes (Aspinall et al. 2010; Ongrádi and Kövesdi 2010). Naïve T cells from aged individuals exhibit numerous functional defects, including shorter telomeres, a restricted TCR repertoire, reduced cytokine production, and impaired expansion and differentiation into effector cells following antigen stimulation (Weiskopf et al. 2009; Ferrando-Martinez et al. 2011).
Fig. 2.
Schematic representation of the somatic rearrangement process undergoing in every immature T cell TCR loci during the development from hematopoietic stem cell to mature naïve T cells. During the rearrangement process, the intervening DNA sequences, both for α- and β-chain, are deleted and circularized into episomal DNA molecules, called TCR excision circles (TRECs) (Adapted from Lang et al. (2011b))
The age-related expansion of dysfunctional terminally differentiated T cells
Consequently to decreasing thymopoiesis, a shift in the ratio of naïve to memory T cells in order to maintain peripheral T cell homeostasis is observed with advancing age. Repeated exposure to antigens directly shapes the T cell pool, and certain pathogens directly contribute to immunosenescence (Virgin et al. 2009; Ongrádi and Kövesdi 2010). While some reports suggest that localized, niche limited, latent herpes virus (HHV1) may not have any impact, evidence implicates chronic cytomegalovirus (CMV or HHV5) infection in the age-dependent expansion of dysfunctional terminally differentiated T cells (CD8+CD28−) (Pawelec et al. 2009; Lang et al. 2010a; Brunner et al. 2011). In older adults with CMV seropositivity, up to 25% of the total CD8+ T cells pool is specific for CMV immunodominant epitopes (Pawelec et al. 2009; Virgin et al. 2009). This expansion of CMV-specific CD8+ is associated with the loss of the costimulatory molecule CD28 which has been reported as key predictor of immune incompetence in older individuals (Vallejo 2005; Frasca et al. 2011). CD28 marker is expressed constitutively on >99% of human T cells at birth. With advancing age, a progressive increase in the proportion of CD28− T cells is observed and particularly within the CD8+ T cell subset (Lang et al. 2010a). CD28-mediated costimulation is needed for effective primary T cell expansion and for the generation and activation of regulatory T cells (Hünig et al. 2010). CD28 signal transduction results in IL-2 gene transcription, expression of IL-2 receptor, and the stabilization of a variety of cytokines messenger RNAs. Consequently, memory CD8+CD28− T cells generated from aged naïve cells, compared to memory cell produced from young naïve cells produced much less cytokine as well (Th1 IL-2 and Th2 IL-4 and IL-5) (Ongrádi and Kövesdi 2010). Aged CD4+CD28− produced from aged naïve cells also expressed decreased CD40L (CD154) maker. The CD154 ligand has been shown to induce cytokine production, costimulate proliferation of activated T cells and this accompanied by production of IFN-γ, TNF-α, and IL-2. Hence the capacity of these cells to help in B cell proliferation and antibody production is considerably reduced contributing to the impairment of humoral response in the aged (Haynes 2005; Lang et al. 2010b; Frasca et al. 2011).
Globally, the proliferative capacity of CD28− T cells is also limited; these cells have shortened telomeres and show increased resistance to apoptosis and restricted T cell diversity and are named senescent cells (Vallejo 2005). These cells are also able to secrete proinflammatory cytokines with a switch from Th1- to Th2-like cytokines response that contributes to the ongoing age-related inflammatory process termed inflammaging (Franceschi 2007; Franceschi et al. 2007). Senescent cells also exert regulatory roles in vivo that further impinge the immune system capacities such as poorer immune responses to influenza vaccination (Goronzy et al. 2001; Saurwein-Teissl et al. 2002) and higher autoreactive immunologic memory (Weiskopf et al. 2009).
Is T cell-mediated immunity senescence a quantifiable disorder?
Predicting individual immune responsiveness using biological markers that easily distinguish between healthy and immunosenescent states is a desirable challenge. Since the single preceding event in all cases of immunosenescence is thymic involution (Aspinall et al. 2010), can we identify a specific T cell-mediated immunity makers which are linked to a state of immunosenescence? The pioneering OCTO and NONA studies have resulted in the emerging concept of an immune risk profile (IRP) (Wikby et al. 2005; Strindhall et al. 2007; Wikby et al. 2008). This immune condition consists of (1) a depleted number of naïve T cells; (2) a high CD8+, low CD4+ numbers characterized by an inverted CD4+/CD8+ ratio; (3) a poor mitogen response to concavanavalin (ConA) stimulation; and (4) the expansion of dysfunctional terminally differentiated CD8+CD28− T cells (i.e., senescent cells) (Pawelec et al. 2009; Brunner et al. 2011). This IRP identified from healthy octogenarians and nonagenarians when 2-, 4-, and 6-year mortality was predicted. Hirokawa et al. have thus proposed a T cell immune score expressing the immune status as a simple score combining five T cell-related parameters (Hirokawa et al. 2009): total number of T cells, CD4+/CD8+ ratio, number of naïve T cells (CD4+CD45RA+), ratio of naïve to memory (CD4+CDRO+) T cells, and T cell proliferative index. In patients with colorectal cancer compared to healthy age-matched controls, this T cell immune score of patients in stages I–IV was significantly decreased. Furthermore, the complex remodeling of immune system observed during aging also includes profound modifications within the cytokine network (Larbi et al. 2011). The typical feature of this phenomenon is a general increase in plasma cytokines levels and cell capability to produce proinflammatory cytokines, including a chronic, low-grade, proinflammatory condition usually termed inflammaging (Franceschi 2007; Macaulay et al. 2012). This results from a shift from a CD4+ T helper cells, Th1-like cytokine response to a Th2-like response, and furthermore an increase in levels of proinflammatory cytokines (i.e., IL-6, tumor necrosis factor (TNF-α), as well as IL-1β, IL-18, and IL-12). While a wide range of factors has been claimed to contribute to this state (i.e., increased amount of adiposity, decreased production of sex steroid, and chronic health comorbid disorders) (Ostan et al. 2008; Fulop et al. 2010), this altered inflammatory response has also been attributed to the continuous exposure to CMV antigen stimulation and/or reactive oxygen species (Pawelec et al. 2009; Brunner et al. 2011; Larbi et al. 2011). However, whether these parameters could provide a robust set of criteria for the determination of an individual’s immunological status in the older old adults, further studies are still required in order to identify biomarkers that are identifiable earlier in life so that intervention strategies can be administered sooner rather than later (Govind et al. 2012).
With this aim, genomic may help to identify factors usable not only as a measure of biological aging but that may also be useful as a tool for predicting immune capabilities within the population (Ostan et al. 2008). Studies that tracked the changes in thymic output have attempted to establish the number of naïve cells and thereby provide an assessment of immune status by using an excisional by-product of T cell receptor (TCR) genes rearrangement (Douek et al. 1998; 2000; Hazenberg et al. 2002; 2003; Mitchell et al. 2010; Govind et al. 2012). These products are termed TCR-rearrangement excisions circles (TRECs) (Takeshita et al. 1989; Livak and Schatz 1996; Kong et al. 1998).
Are TRECs a biomarker of effective aging?
TRECs: episomal DNA sequences generated during the TCR genes rearrangement
The ability of T lymphocytes to recognize a specific region of a particular antigen is driven by the presence of antigen receptors on the surface of each cell. The TCR is a heterodimer that consists in 95% of T cells of an alpha (α) and beta (β) chain, whereas in 5% of T cells this consists of gamma and delta (γ/δ) chains. In order to create a border repertoire of TCR, an intricate process of cutting and splicing undergoes during the complex transition from hematopoietic stem cell to mature naïve T lymphocyte that leads to random joining of DNA segments from the TRC locus (Chain et al. 2005). In T cells expressing TCR-αβ, rearrangements of both TCR-α and TCR-β genes produce TRECs, as depicted in Fig. 2, by VJ gene recombination and by V(D)J gene recombination, respectively (Bogue and Roth 1996). Both involve a somewhat random joining of gene segments to generate the complete TCR chain, and the two rearrangement events that occur during this process are identical in 70% of αβ T cells (Verschuren et al. 1997). The α-chain rearrangement produces a signal joint TREC (sj-TREC) and the β-chain, a coding joint TREC (Douek et al. 1998). Thus the TRECs generated are common to most αβ T lymphocytes and are detectable exclusively in phenotypically naïve T cells (i.e., undetectable in memory/effector T cells, B cells, and other peripheral mononuclear cells) (Aspinall et al. 2000; Hazenberg et al. 2003; Kohler et al. 2005). Because of the enormous diversity of TCR-α VJ and TCR-β VDJ recombination events (Siu et al. 1984; Arden et al. 1985), and thus the number of TRECs produced, no single TREC can be used as a marker to assess the overall thymic function (Douek et al. 1998; Hazenberg et al. 2003). While α- and β-TRECs possess an identical DNA sequences respectively and are both stable (Livak and Schatz 1996), not duplicated during subsequent mitosis (Takeshita et al. 1989), TRECs generated during α-chain rearrangement are generally preferred (Aspinall et al. 2000). Indeed they are generated after β-TRECs and are therefore less diluted out with each subsequent cellular division. Moreover, a common requirement for productive rearrangement of the TCR-α locus is the deletion of the TCR-δ locus (see Fig. 2). Sj-TREC generated during the α-chain rearrangement can be easily quantified in clinic samples (Aspinall et al. 2000; Douek et al. 2000; Hazenberg et al. 2000; Patel et al. 2000; Hazenberg et al. 2002; 2003; Murray et al. 2003; Kohler et al. 2005; Zubakov et al. 2010; Lang et al. 2011b).
sj-TREC: a biomarker of the resting naïve T cell pool rather than of thymic outputs
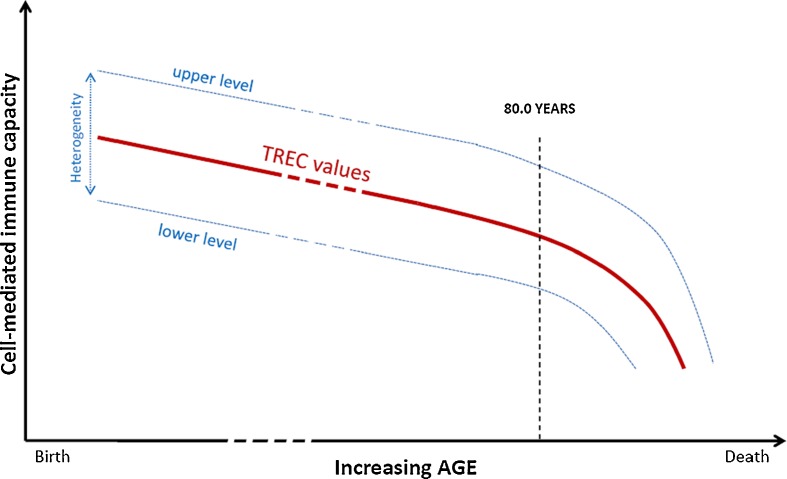
Phenotypic analyses have confirmed that the exhaustion of thymic output with advancing age was the basis of the deficient replacement of naïve T cells lost in the periphery (i.e., by death or conversion to memory/effector cells) (Kohler et al. 2005; Ostan et al. 2008; Haines et al. 2009; Weiskopf et al. 2009). Whether this contributes to the inability of maintaining the T cell repertoire breadth in older adults, TREC values could not be immediately interpreted to reflect continuous thymic output of naïve T cells (Hazenberg et al. 2003). While, as shown in Fig. 3, some reports have shown age-associated decline in the sj-TREC values (Mitchell et al. 2010; Zubakov et al. 2010), Chen et al has demonstrated that TREC were still readily detectable in healthy nonagenarians (Chen et al. 2010). This suggests, as demonstrated by Hazenberg, that TREC T cells content should be finally more considered as a biomarker of the resting naïve T cell pools rather than a record of thymic output (Hazenberg et al. 2003). This is well illustrated by findings from studies performed in individuals suffering from different health conditions (Douek et al. 1998; Markert et al. 1999; Douek et al. 2000; Patel et al. 2000). Two major biological parameters that complicate the interpretation of TREC data explain this assertion: longevity of naïve T cells and TREC dilution within the two daughter cells after each round of cell division (Hazenberg et al. 2003). Indeed, estimating that healthy adult has a steady state of 1011 naïve T cells and a thymic output of 107–108 naïve cells per day, it was estimated that naïve T cells have a life span of 1,000–10,000 days (Sprent and Tough 1994). Consistently, thymectomy should not lead to rapid decline in naïve T cell numbers, and in a group of adults thymectomized 3 to 39 years prior to analysis, TRECs were still clearly present (Douek et al. 1998). It was thus assumed that naïve T cell division would be too low to significantly affect the TREC content (Douek et al. 1998). Whether that is true in healthy adults, it is not the case in HIV-infected individuals or in lymphopenic cancer adults (Hazenberg et al. 2000; 2002). In these two populations, TREC values are significantly lower compared to healthy age-matched control, but TREC increased rapidly with highly active antiretroviral therapy and during T cell reconstitution with stem cell transplantation, respectively, and even TREC values reached supranormal levels (Hazenberg et al. 2000; 2002). In individuals with severe combined immunodeficiency or in congenitally athymic patients (i.e., DiGeorge syndrome), TRECs became detectable after either hematopoietic stem cell transplantation or transplantation of cultured postnatal thymic tissue (Markert et al. 1999; Patel et al. 2000). Finally, in any case, in clinical conditions involving or influencing the cell-mediated immune system or with advancing age, the number of TREC and the T cell TREC content are not only determined by thymic output but also by peripheral events such as homeostatic proliferation of existing naïve T cells which replace those cells lost by death or conversion to memory/effector cells (Hazenberg et al. 2003). Thus, analyzing TREC numbers in healthy individuals, Murray et al. found a marked change in the source of naive T cells before and after 20 years of age (Murray et al. 2003). The bulk of the naive T cell pool was sustained primarily from thymic output for individuals younger than 20 years of age whereas proliferation within the naïve phenotype was dominant for older individuals. Over 90% of phenotypically naïve T cells in middle age were not of direct thymic origin. Similarly, but as regard to humoral immunosenescence, developing B cell receptor excision circles assay could be probably of high interest in order to study the age-related changes occurring within the naïve B lymphocyte pool (Jasper et al. 2003).
Fig. 3.
Schematic representation of the age-related changes in TREC values across the life span based on Zubakov et al. (2010) and Mitchell et al. (2010) study results. The red line depicts the decline in TREC value in healthy individuals, and the two dashed lines on either side are the upper and lower TREC values for a given age observed within this population. The whole figure shows the age-related decrease in TREC values but also demonstrates the convergence of the overall spread of the TREC values with advancing age
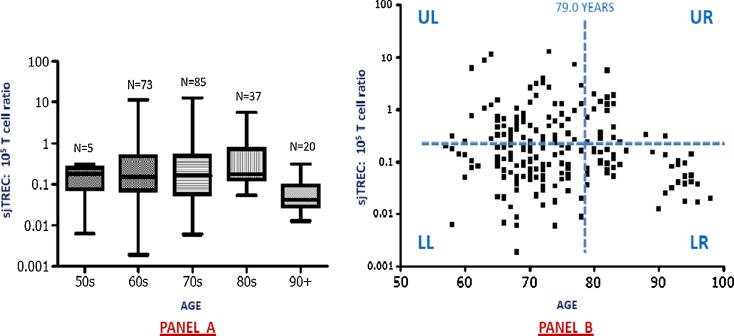
Could we identify different trends of aging when analyzing sj-TREC values?
In a possibly clearer picture, the TREC decline in the oldest old was recently shown in a study analyzing blood samples from 215 healthy individuals ranging in age from 60 to 104 years (Mitchell et al. 2010). The number of donors aged ≥70 years were 66% and ≥80 years, 27%. Changes in thymic output were quantified using TREC/105 T cells ratio. TREC measurements were obtained by quantitative polymerase chain reaction, and the number of T cells was determined using a fluorescence activated cell sorter analysis. Thus, while the absolute number of leucocytes and T lymphocytes did not change significantly across the age range studied, the authors demonstrated a slowly accelerated curvilinear decline of the TREC ratio between sixth and ninth decade of life. As showed with Fig. 4, the most pronounced decline was seen in those individuals more than 90 years of age. Moreover, samples from earlier decades showed a wide range of TREC values with a convergence of the sample heterogeneity observed in the TREC levels with increasing age (see Fig. 4a). These findings contribute to speculate for a number of interesting hypotheses presented in Fig. 4b. First, are low TREC measurements reflective of an individual’s immunosenescence status; if so, are the individuals in the lower left quadrant (low TREC level at younger age) at a more advanced stage of immunosenescence? The converse argument could also be inferred for individuals with the highest TREC levels (upper left quadrant). These individuals may therefore be more likely to progress to become the long-lived healthy individuals observed in the low right quadrant. This concept lends itself to the argument that immunosenescence is not merely a measurement of chronological age but points towards immune exhaustion arising at different ages (i.e., physiological age) (Lang et al. 2010b; Mitchell et al. 2010). The downward trajectory of an individual’s thymic output profile over time has been demonstrated previously by Kilpatrick et al. (2008) and could be considered as part of longitudinal studies similar to the OCTA and NONA studies to investigate further the potential role of sj-TREC as predictive marker of aging (Wikby et al. 2005; Strindhall et al. 2007; Wikby et al. 2008). Thus, whether predicting human phenotypes from genotypes is relevant both for personalized medicine and applying preventive strategies (Janssens and van Duijn 2008), additional clinical and translational studies at population, clinical, cellular, and molecular levels are still needed in order to elucidate the exact implications of the TREC values on the age-related senescence of the cell-mediated immune response (Lang et al. 2011a).
Fig. 4.
Graphic representation of the age-related changes in TREC/105 T cells ratio. a Demonstrates (1) the slow decline in the ratio values between the sixth and ninth decades of life with a more pronounced decline seen in individuals more than 90 years of age and (2) a convergence of the sample heterogeneity observed in the TREC levels with increasing age. b Shows an annotated diagram of the age-related changes observed in TREC measurement. The dashed horizontal line indicates the median TREC/105 T cell ratio in the sample population and the dashed vertical line is the average life expectancy across the study population (79.0 years). Upper left (UL), lower left (LL), upper right (UR), and lower left (LR) quadrants refer to different quadrants formed by the bisection of the data horizontal and vertical lines (Adapted from Mitchell WA 2010)
How to rejuvenate the T cell-mediated immune system?
Different ways have been already explored regarding how best to rejuvenate the peripheral T cell pool (Govind et al. 2012; Lang and Aspinall 2012). The different approaches can be categorized in to the 3Rs of rejuvenation as presented in Fig. 1. Two of three approaches (i.e., restoration and reversion) have recently demonstrated their effectiveness in reversing age-related changes of the B cell population (Keren et al. 2011a; 2011b).
The 3Rs of rejuvenation
Replacement strategies aim to restore immune functions lost by several techniques including the transfusion of autologous blood derived from an individual during their early life and transfused when they are much older and adoptive transfer procedures (Oelke et al. 2003; Cobbold et al. 2005). Alternatively it also involves transferring ex vivo generated naïve T cells (Hare et al. 1999; de Pooter et al. 2003) or to physically remove senescent cells from the circulation with the aim of inducing the homeostatic expansion of more functional population of memory T cells (Trzonkowski et al. 2003; Hadrup et al. 2006; Lang et al. 2011b). Reprogramming strategy is probably the most “revolutionary” one. To date, there is general consensus regarding the idea that telomeres represent an inherent biological clock (Mera 1998; Westin et al. 2007). Thus, pharmacologic approaches have been developed in order to enhance telomerase activity and restore telomere length as possible means for the prevention or retardation of replicative senescent cells or to significantly extend cellular lifespan (McElhaney and Effros 2011; Govind et al. 2012). Interestingly, some authors have demonstrated that the idea of rejuvenating a self-tolerant immune system (i.e., cell-mediated and humoral immune system) is also clinically feasible and safe (de Kleer et al. 2006; Alexander et al. 2009). Indeed, clinical trials have indicated that immunoablation followed by autologous hematopoietic stem cell transplantation (ASCT) had the potential to induce remission in subjects suffering from refractory autoimmune diseases (Rosen et al. 2000; Burt et al. 2006). Indeed, with ASCT, it induced not only depletion of autoreactive immunologic memory cells but also immunologic self-tolerance by reprogramming autoreactive T cells and profoundly resetting the adaptive immune system and this by restoring the CD4+CD25+ immune regulatory network (de Kleer et al. 2006; Alexander et al. 2009). Finally, restoration strategies aim to maintain a normal thymic environment by using growth hormone, sex steroids, growth factors, nutrients, and cytokines. While some reports that IL-7 introduced into the thymus is unable to reverse thymic involution (Phillips et al. 2004), animal studies provide promising results. These findings suggest that IL-7 could have significant potential in the clinic for assisting in the treatment of viral infections (Aspinall et al. 2007; Levy et al. 2009), boosting immune recovery after bone marrow transplantation, or to improve the immune system (Rosenberg et al. 2006; Sportes et al. 2008; 2010).
Interleukin-7: a promising treatment to improve the cell-mediated immune system
IL-7 is a γ-chain cytokine produced by stromal cells and the thymus. As previously mentioned, it plays a pivotal role in supporting thymocytes development, as well as peripheral T cells survival and proliferation (Kim et al. 1998; Aspinall et al. 2000; Jiang et al. 2005). Some studies carried out in old animals have reported that IL-7 reversed thymic atrophy, increased thymopoiesis improved thymic output, and boosting immune function (Aspinall and Andrew 2001; Henson et al. 2005; Pellegrini et al. 2011). Thus, old female rhesus macaques injected with recombinant IL-7 subcutaneously (60 g/kg) for a 14-day period, compared to animals receiving saline vehicle alone, showed an increase not only in the number of CD4+CD3+ and CD8+CD3+ T cells and in the number of naïve T cells (CD45RA+) for both CD4+ and CD8+ subsets, but also in TREC levels (Aspinall et al. 2007). Moreover, these same old female rhesus macaques vaccinated with inactivated influenza vaccine (strain A/PR/8/34) elicited increase in specific hemagglutination inhibition (HAI) titer. In addition, treated animals showed higher numbers of influenza-specific memory CD8+ T cells compared to pretreatment levels with numbers greater than in saline-treated group. Animals with the higher HAI titers and the best proliferation against influenza antigen were among those with the highest TREC ratio levels. In addition, it has been recently demonstrated in old C57BL/6 female mice that intratracheal instillation provided an effective route for delivering IL-7 into the blood stream and from there into the lymphoid tissues when compared with injected IL-7 subcutaneously (Mitchell et al. 2012). In functional assessment studies, pulmonary administration demonstrated to significantly improve intrathymic T cell development when compared with controls receiving saline vehicle by instillation or animals receiving IL-7 by subcutaneous injection.
Conclusion
Immunosenescence contributes to render aging and older adults more prone to develop infectious diseases and unable to mediate immune response against new antigens. This review demonstrates the central role played by T cell-mediated immunity both related to intrinsic defects and its reduced capacity to help in B cells proliferation and specific antibodies production. However, immunosenescence also affects B cell and innate immunity as well. While research is already very active and more and more growing regarding how to best rejuvenate the peripheral T and B cell pool, robust methods for identifying and measuring immunosenescence and strong biological makers that distinguish between healthy and immunosenescent states are still lacking. With these perspectives and based on recent animal and human studies, the sj-TREC measurement appears as an interesting biomarker of the resting naïve T cell pool. However, complementary clinical and translational studies at clinical and population levels are still needed in order to demonstrate that the TREC ratio could be used as a predictive maker of optimal cell-mediated immune response to new antigens.
References
- Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, Mei H, Radtke H, Gromnica-Ihle E, Burmester GR, Arnold R, Radbruch A, Hiepe F. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–223. doi: 10.1182/blood-2008-07-168286. [DOI] [PubMed] [Google Scholar]
- Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37:455–463. doi: 10.1016/S0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- Arden B, Klotz JL, Siu G, Hood LE. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. Nature. 1985;316:783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/A:1006611518223. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D. Age-associated thymic atrophy is not associated with a deficiency in the CD44(+)CD25(−)CD3(−)CD4(−)CD8(−) thymocyte population. Cell Immunol. 2001;212:150–157. doi: 10.1006/cimm.2001.1848. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Pido J, Andrew D. A simple method for the measurement of sjTREC levels in blood. Mech Ageing Dev. 2000;121:59–67. doi: 10.1016/S0047-6374(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Pido-Lopez J, Imami N, Henson SM, Ngom PT, Morre M, Niphuis H, Remarque E, Rosenwirth B, Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10(1):5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142(Suppl 1):S111–S115. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Bogue M, Roth DB. Mechanism of V(D)J recombination. Curr Opin Immunol. 1996;8:175–180. doi: 10.1016/S0952-7915(96)80055-0. [DOI] [PubMed] [Google Scholar]
- Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematous. JAMA. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- Chain JL, Joachims ML, Hooker SW, Laurent AB, Knott-Craig CK, Thompson LF. Real-time PCR method for the quantitative analysis of human T-cell receptor gamma and beta gene rearrangements. J Immunol Methods. 2005;300:12–23. doi: 10.1016/j.jim.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Lim FC, Wu Q, Douek QW, Scott DK, Ravussin E, Hsu HC, Jazwinski SM, Mountz JD. Maintenance of naïve CD8 T-cells in nonagerians by leptine, IGFBP3 and T3. Mech Ageing Dev. 2010;131:29–37. doi: 10.1016/j.mad.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- de Pooter RF, Cho SK, Carlyle JR, Zuniga-Pflucker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102:1649–1653. doi: 10.1182/blood-2003-01-0224. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstruction. Lancet. 2000;355:1875–1878. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Ruiz-Mateos E, Hernandez A, Gutierrez E, Rodriguez-Mendez MM, Ordonez A, Leal M. Age-related deregulation of naive T cell homeostasis in elderly humans. Age. 2011;33:197–207. doi: 10.1007/s11357-010-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1301/nr.2007.dec.S173-S176. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11:537–550. [PubMed] [Google Scholar]
- Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J. Inflammaging as a prodrome to Alzheimer’s disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind S, Lapenna A, Lang PO, Aspinall R (2012) Immunotherapy of immunosenescence: Who, How and When? Open Longev Sci (in press)
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human CD4+ T cells recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare KJ, Jenkinson EJ, Anderson G. In vitro models of T cell development. Semin Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- Haynes LES. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- Hazenberg MD, Otto SA, de Pauw ES, Roelofs H, Fibbe WE, Hamann D, Miedema F. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood. 2002;99:3449–3453. doi: 10.1182/blood.V99.9.3449. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Borghans JAM, Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4:97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- Henson SM, Snelgrove R, Hussell T, Wells DJ, Aspinall R. An IL-7 fusion protein that shows increased thymopoietic ability. J Immunol. 2005;175:4112–4118. doi: 10.4049/jimmunol.175.6.4112. [DOI] [PubMed] [Google Scholar]
- Hirokawa K, Utsuyama M, Ishikawa T, Kikuchi Y, Kitagawa M, Fujii Y, Nariuchi H, Uetake H, Sugihara K. Decline of T cell-related immune functions in cancer patients and an attempt to restore then through infusion of activated autologus T cells. Mech Ageing Dev. 2009;130:86–91. doi: 10.1016/j.mad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Hünig T, Lücher F, Elfein K, Gogishvili T, Frölich M, Guler R, CulterA BF. CD28 and IL-4: two heavyweights controlling the balance between immunity and inflammation. Med Microbiol Immunol. 2010;199:239–246. doi: 10.1007/s00430-010-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens ACJW, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum Mol Genet. 2008;17:R166–R173. doi: 10.1093/hmg/ddn250. [DOI] [PubMed] [Google Scholar]
- Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor b cells and waning of b lymphopoiesis 1. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21:384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren Z, Averbuch D, Shahaf G, Zisman-Rozen S, Golan K, Itkin T, Lapidot T, Mehr R, Melamed D. Chronic B cell deficiency from birth prevents age-related alterations in the B lineage. J Immunol. 2011;187:2140–2147. doi: 10.4049/jimmunol.1100999. [DOI] [PubMed] [Google Scholar]
- Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, Schmidt-Supprian M, Lapidot T, Melamed D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011;117:3104–3112. doi: 10.1182/blood-2010-09-307983. [DOI] [PubMed] [Google Scholar]
- Kilpatrick RD, Rickabaugh T, Hultin LE, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Le CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2 and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–5741. [PubMed] [Google Scholar]
- Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Möwes B, Jülke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- Kong FK, Chen CL, Cooper M. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/S1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- Lang PO, Aspinall R. Immunosenescence and herd immunity: with an ever increasing aging population do we need to rethink vaccine schedules? Expert Rev Vaccines. 2012;11:167–176. doi: 10.1586/erv.11.187. [DOI] [PubMed] [Google Scholar]
- Lang PO, Samaras D. Aging adults and seasonal influenza: does the vitamin D status (h)arm the body? J Aging Res. 2012;2012:806198. doi: 10.1155/2012/806198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PO, Govind S, Mitchell WA, Kenny N, Lapenna A, Pitts D, Aspinall R. Influenza vaccine effectiveness in aged individuals: the role played by cell-mediated immunity. Eur Geriatr Med. 2010;1:233–238. doi: 10.1016/j.eurger.2010.07.002. [DOI] [Google Scholar]
- Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R. Immunological pathogenesis of main age-related diseases and frailty: role of immunosenescence. Eur Geriatric Med. 2010;1:112–121. doi: 10.1016/j.eurger.2010.01.010. [DOI] [Google Scholar]
- Lang PO, Govind S, Mitchell WA, Siegrist CA, Aspinall R. Vaccine effectiveness in older individuals: what has been learned from the influenza-vaccine experience. Ageing Res Rev. 2011;10:389–395. doi: 10.1016/j.arr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Lang PO, Mitchell WA, Govind S, Aspinall R. Real time-PCR assay estimating the naive T-cell pool in whole blood and dried blood spot samples: pilot study in young adults. J Immunol Methods. 2011;369:133–140. doi: 10.1016/j.jim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Lang PO, Mendes A, Socquet J, Assir N, Aspinall R (2012) Effectiveness of influenza vaccine in aging and older adults: a comprehensive analysis of the evidence. Clin Interv Aging 7 (in press) [DOI] [PMC free article] [PubMed]
- Larbi A, Pawelec G, Wong SC, Goldeck TJJY, Fulop T. Impact of age on T-cell signaling: a general defect or specific alteration? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Vettand-Fenoel V, Croughs T, Beq S, Thiebaut R, Chene G, Morre M, Delfraissy JF. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak F, Schatz D. T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T-cells. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. Doubling of world population unlikely. Nature. 1997;387:803–805. doi: 10.1038/42935. [DOI] [PubMed] [Google Scholar]
- Macaulay R, Akbar AN, Henson SM (2012) The role of the T-cell in a aged-related inflammation. Age. doi:10.1007/s11357-012-9381-2 [DOI] [PMC free article] [PubMed]
- Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM, Batchvarova MN, Douek DC, Koup RA, Kostyu DD, Ward FE, Rice HE, Mahaffey SM, Schiff SE, Buckley RH, Haynes BF. Transplantation of thymus tissue in complete DiGeorge syndrome. N Eng J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera SL. The role of telomeres in ageing and cancer. Br J Biomed Sci. 1998;55:221–225. [PubMed] [Google Scholar]
- Mitchell WA, Lang PO, Aspinall R. Tracing thymic output in older individuals. Clin Exp Immunol. 2010;161:497–503. doi: 10.1111/j.1365-2249.2010.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WA, Castells A, Lang PO, Matas E, Lapenna A, Aspinall R (2012) Pulmonary delivery of interleukine 7 provides efficient and safe delivery to the aging immune system. Rejuvenation Res (in press) [DOI] [PMC free article] [PubMed]
- Murray JM, Kaufmann GR, Hodgkin PD, Lewin SR, Kelleher AD, Davenport MP, Zaunders JJ. Naive T cells are maintained by thymic output in ealry ages but by proliferation without phenotype change after age twenty. Immunol Cell Biol. 2003;81:487–495. doi: 10.1046/j.1440-1711.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Ongrádi J, Kövesdi V. Factors that may impact on immunosenescence: a appraisal. Immun Ageing. 2010;7:7. doi: 10.1186/1742-4933-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- Ostan R, Bucci L, Capril M, Salvioli S, Scurti M, Pini E, Monti D. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–240. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- Patel DD, Gooding ME, Parrott RE, Curtis KM, Haynes BF, Buckley RH. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Eng J Med. 2000;342:1325–1332. doi: 10.1056/NEJM200005043421804. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- Rosen O, Thiel A, Massenkeil G, et al. Autologus stem-cell transplantation in refractory autoimmune diseases after in vivo immunoablation and ex-vivo depletion of mononuclear cells. Arthritis Res. 2000;2:327–336. doi: 10.1186/ar107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, et al. Leukemia inhibitory factor, oncostatin M, Il-6 and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR (2011). Dysregulation of human Toll-like receptor function in aging. Ageing Res Rev 10(346-353) [DOI] [PMC free article] [PubMed]
- Siu G, Kronenberg M, Strauss E, Haars R, Mak TW, Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. Nature. 1984;311:344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, Stetler-Stevenson M, Engel J, Buffet R, Morre M, Amato RJ, Pecora A, Mackall CL, Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- Strindhall J, Nilsson BO, Lofgren S, Ernerudh J, Pawelec G, Johansson B, et al. No Immune Risk Profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007;42:753–761. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Toda M, Ymagishi H. Excision products of the T cell receptor gene support a progressive rearrangement model of the α/δ locus. EMBO J. 1989;8:3261–3270. doi: 10.1002/j.1460-2075.1989.tb08486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of antihemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/S0264-410X(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Tsaknaridis L, Spencer L, Culbertson N, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age- dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T-cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Verschuren MC, Wolvers-Tettero IL, Breit TM, Noordzij J, van Wering ER, van Dongen JJ. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J Immunol. 1997;159:4341–4349. [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, AHmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- Westin ER, Chavez E, Lee KM, Gourronc FA, Riley S, Lansdorp PM, Goldman FD, Klingelhutz AJ. Telomere restoration and extension of proliferative lifespan in dyskeratosis congenita fibroblasts. Aging Cell. 2007;6:383–394. doi: 10.1111/j.1474-9726.2007.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- Zubakov D, Liu F, van Zelm MC, Vermeulen J, Oostra BA, van Duijn CM, Driessen GJ, van Dongen JJ, Kayser M, Langerak AW. Estimating human age from T-cell DNA rearrangements. Curr Biol. 2010;20:R970–R971. doi: 10.1016/j.cub.2010.10.022. [DOI] [PubMed] [Google Scholar]