Abstract
Adipose tissue is an abundant, easily accessible, and reproducible cell source for musculo-skeletal regenerative medicine applications. Initial derivation steps yield a heterogeneous population of cells collectively termed the stromal vascular fraction (SVF), which consist of endothelial cells, immune cells, pericytes, and pre-adipocytes. Subsequent selection of an adherent cell subset from the SVF results in a relatively homogeneous population of adipose-derived stromal/stem cells (ASCs). Mammalian ASCs exhibit the ability to selectively differentiate into chondrogenic, myogenic, and osteogenic lineages in response to inductive stimuli in vitro (when cultured on scaffolds in bioreactors) and in vivo (when implanted in pre-clinical animal models). Unlike hematopoietic cells, ASCs do not elicit a robust lymphocyte reaction and instead generate and release immunosuppressive factors, such as prostaglandin E2. These unique immunomodulatory features suggest that both allogeneic and autologous ASCs will engraft successfully following application for tissue regeneration purposes. The differentiation and expansion potential of ASCs can be modified by growth factors like bone morphogenetic protein 6, bio-inductive scaffolds, and bioreactors providing environmental control and biophysical stimulation. Gene therapy approaches using lentiviral transduction can also be used to direct differentiation of ASCs along particular lineage pathways. We discuss here the utility of ASCs for musculo-skeletal tissue repair and some of the technologies that can be implemented to unlock the full regenerative potential of these highly valuable cells.
Keywords: Adipose stem cells (ASCs), Bone marrow-derived mesenchymal stem cells (BMSCs), Chondrocyte, Immunophenotype, Myocyte, Osteoblast, Stromal vascular fraction (SVF) cells, Tenocyte
2. Introduction
Tissue engineering is an emerging field that integrates advances in the fields of biomaterials, cytokines/growth factors, and stem cell biology towards repair and regeneration of damaged tissues and organs. Musculo-skeletal defects due to acute trauma, congenital malformations, degenerative diseases, and neoplasia are potential targets for cell-based regenerative therapies. While scaffolds can be manufactured from either native or synthetic biomaterials, and regulatory signals (molecular and physical) can be provided using advanced bioreactors, large scale production of stem cells and the definition of environmental conditions necessary for these cells to drive the repair processes continue to present major challenges. Stem cells are living biological products whose production requires quality controls for contaminating infectious agents, post-cryopreservation functional testing, tumorigenicity, and viability. It is estimated that de novo creation of a single cubic centimeter of bone tissue will require ~7 × 107 cells (1), necessitating the derivation of ~ 109 stem cells from a single donor. Adipose tissue has the potential to meet this demand in a highly reproducible manner (2). This review contains information from recent studies surrounding the isolation and characterization of human adipose-derived cells (ASCs) for musculo-skeletal applications, scaffold and bioreactor technologies used for directed differentiation of these cells, and some of the current pre-clinical and clinical trial data.
3. A. Isolation of stromal vascular fraction and adipose-derived stem cells: frequency and yield
Subcutaneous adipose tissue is a relatively accessible reservoir for adult stem cell harvest. Plastic surgeons routinely perform >300,000 elective tumescent lipoaspiration procedures on patients in the U.S. each year, yielding liter volumes of subcutaneous adipose tissue. Routinely, this biological material has been discarded; however, new tissue engineering and regenerative medical approaches are being developed to use it as a source of stromal vascular fraction (SVF) cells and adipose-derived stromal/stem cells (ASCs) (3–6). Several companies have begun marketing closed system surgical devices to harvest and process the lipoaspirate intra-operatively (7, 8). The point of care devices are designed to minimize the risk of tissue contamination and to optimize the reproducibility and reliability of the cell product. Most isolation procedures have the same basic steps (detailed in (9)). First, the contaminating erythrocytes are removed with a phosphate buffered saline rinse. Then, the tissue is digested with collagenase type I (0.075 to 0.1%) for a period of 30 to 90 minutes at 37°C. Some investigators also include dispase and/or hyaluronidase in their digestion buffer to improve cell recovery. The SVF cells are separated from the mature, lipid-laden adipocytes by centrifugation at speeds of 300 × g (6). The resulting SVF cell pellet consists of a heterogeneous population of endothelial cells, erythrocytes, lymphocytes, macrophages, pericytes, and pre-adipocytes.
A single milliliter of human subcutaneous adipose tissue typically yields between 100,000 to 500,000 nucleated cells (10–13). A nearly identical SVF cell population can be recovered from the bloody fluid collected during the lipoaspiration procedure without the requirement of a collagenase digestion step (13). Flow cytometric analyses have determined that a significant and reproducible percentage of SVF cells express the following hematopoietic surface antigens: CD11b, CD14, CD34, CD45, HLA-AB, and HLA-DR (12, 14).
The SVF cells have been used without further processing for intraoperative tissue engineering procedures. Clinician investigators have reported excellent outcomes using SVF cells for soft tissue reconstruction of breast and facial defects (3, 4, 15). Despite this, the SVF cells frequently are innoculated onto plastic culture-ware with or without extracellular matrix coating such as collagen or fibronectin (6). Following a period of several hours to several days, the non-adherent cells are removed and the remaining adherent cells are identified as ASCs. Approximately 1 out of 30 SVF cells will adhere to the tissue culture surface and between 105 and 106 ASCs can be cultured from a single cubic milliliter of human lipoaspirate after 3 to 7 day (12). ASCs have a distinct surface immunophenotype as assessed by flow cytometric analyses. With progressive passage, the majority of ASCs express the stromal markers CD9, CD10, CD29, CD44, CD73, CD90, and CD166 while, with the exception of HLA-AB, the presence of hematopoietic markers declines relative to the original SVF cells (10, 12, 14, 16, 17). Furthermore, ASCs are positive for pericytic markers such as 3G5 and CD146 (18, 19). Several groups have begun to use combinations of surface proteins as “immune-tags” with which to enrich or isolate ASCs from the SVF cells directly (20). This has been effective in pre-clinical studies but it has yet to be determined if such approaches will prove to be sufficiently robust and cost-efficient for future clinical applications. At present, adhesion to plastic or matrix substrate remains the most common method for direct isolation of ASCs from large volumes of human lipoaspirate. The ASCs are capable of releasing a range of cytokines and growth factors, having significant paracrine effects (21–24). Furthermore, the ASCs exhibit multipotent differentiation potential in vitro and in vivo (5, 6, 25). Since these features have been covered in several excellent reviews (5, 6, 25), we focus only on those pathways most directly related to musculo-skeletal repair - Chondrogenic, Osteogenic, and Skeletal Myogenic differentiation.
3. B. Advantages and limitations relative to amnion, bone marrow, and placental derived stem cells
Human subcutaneous adipose tissue offers several advantages as a stromal/stem cell source. First, with the widespread incidence of obesity, most adult subjects have abundant adipose tissue. Second, the tissue is accessible via a relatively non-invasive harvest technique. The procedure is well accepted by the public as evidenced by the fact that hundreds of thousands of individuals incur significant costs to undergo elective liposuction procedures each year. This is a sharp contrast to the harvest of bone marrow-derived mesenchymal stromal/stem cells (BMSCs) by bone marrow aspiration, a painful procedure that is associated with significant donor site morbidity. Third, the frequency of stromal/stem cells per unit volume of adipose tissue, which are 10–100 times more abundant per unit volume than bone marrow (12), combined with the liter volumes of adipose tissue available makes the isolation and expansion of billions of ASCs within a minimal time frame feasible.
Despite these advantages, there are reported limitations to ASC use. First and foremost, human ASCs maintained in culture for extended periods of time (>3 months) cause sarcomas in immunodeficient mice (26). This underscores the risks of ex vivo stromal/stem cell manipulation and supports the need for minimal cell handling. Second, a number of investigations have compared ASCs and BMSCs side by side. While some investigators have concluded that they function equally well with respect to chondrogenic and osteogenic differentiation, others have concluded that BMSCs show some superior qualities with respect to differentiation potential (27) (28–33). While it should be noted that these studies have used inductive conditions optimized for the BMSC population, these findings suggest that for some musculo-skeletal applications, there may be certain advantages to using BMSCs until culture conditions are further optimized for ASCs. Finally, the biological age of the donor affects the telomere length of harvested ASCs and BMSCs (34). This contrasts to amniotic, umbilical cord, and placental derived adherent stromal/stem cells which retain their maximal telomere length and, through the expression of telomerase, their renewal capacity (35–41). Hence, it is likely that the relative life span and regenerative properties of stromal/stem cells from perinatal and adult tissues will vary. On the other hand, as “adult” stem cells, ASCs and BMSCs are available for harvest throughout an individual’s lifetime, whereas amniotic, umbilical cord, or placental stems cells would require perinatal cell banking and long term storage for autologous use or allogeneic transplantation. Further investigation is necessary to determine the potential effect of donor age and tissue type on the efficacy and function of stromal/stem cells.
3. C. Chondrogenic potential
Chondrogenic differentiation can be induced in ASCs in vitro by exposure to combination of factors such as dexamethasone, transforming growth factor (TGF) β1 or β3, and bone morphogenetic protein 6 (BMP6) (16, 42–46) (Table 1). Positive Alcian Blue staining or Safranin O staining confirms chondrogenesis within a 2 to 4 week period (16, 42, 43). Differentiation is most robust in 3-dimensional, as compared to 2-dimensional, culture conditions as determined by expression and synthesis of proteoglycans and chondrocyte-specific genes and proteins. A variety of 3 dimensional approaches have been employed (47–49). The simplest is the use of micromass cultures, where 2.5–5 × 105 ASCs are pelleted by centrifugation and maintained for up to 3 weeks under chondrogenic culture conditions (49). At the next level, ASCs are suspended at concentrations of 4 to 10 × 106 per ml in gels of alginate, agarose, or gelatin followed by chondrogenic induction (42, 47, 48). As a third alternative, ASCs are seeded onto porous solid scaffolds constructed from collagen, elastin, synthetic materials (polyvinylpropinate, polyglycolic/lactic acid), or cartilage extracellular matrix extracts (50). The chondrogenic potential of ASCs increases with extended passage in vitro; cells at passage 9 exhibited more robust expression of differentiation markers compared to passage 4 (51). Furthermore, the ASC’s culture expansion conditions in monolayer prior to culture in a 3-dimensional or micromass condition influences their chondrogenic potential (52).
Table 1.
| Chondrogenic Induction | DMEM (high glucose) Medium supplemented with 10 % Fetal Bovine Serum, 1 X Antibiotics (Penicillin/Streptomycin), 1 X ITS (Insulin, Transferrin, Selenium), Dexamethasone (100 nM), L-Ascorbic Acid 2-Phosphate (50 μg/ml), TGFβ1 or TGFβ3 (10 ng/ml), BMP6 (10 or 500 ng/ml). Maintain ASCs in pellet culture or suspended in a biomaterial scaffold (agarose, alginate) with inductive cocktail for 3 to 4 weeks. |
| Dimethyl-Methylene Blue Assay (DMMB) | Quantifies the content of sulfated glycosaminoglycans in the extracellular matrix, an indicator of chondrogenic differentiation. |
| Hydroxyproline Assay (OHP) | Quantifies the total collagen in the extracellular matrix following chondrogenic differentiation. |
| Safranin-O/Fast Green Histological Stain | Stains the negatively charged proteoglycans in the collagenous matrix. |
| Immunohistochemistry (IHC) | Can be used to detect cartilage protein components consistent with chondrogenesis such as aggrecan and collagen II, components of mature cartilage extracellular matrix; Collagen I, a marker of fibrous matrix; Collagen X, a marker of hypertrophic cartilage; Chondroitin sulfate, the most prevalent glycosaminoglycan; Sox 9, a transcriptional regulator of chondrogenesis. |
| Quantitative PCR (qPCR) | Quantifies the mRNA levels of chondrogenic genes associated with the extracellular matrix (aggrecan, collagens type I, II, and X) or transcriptional regulation (Sox 9) |
| In Vivo Bioassay | Implantation of human ASC chondrogenic scaffold subcutaneously in immunodeficient rodent model for periods of 4 to 12 weeks with subsequent analysis of implant graft by histochemical and/or PCR based method for detection of human cells embedded in a cartilaginous matrix |
3. D. Osteogenic potential
ASCs can be induced to undergo osteogenic differentiation in vitro by exposure to a combination of ascorbate, β glycerophosphate, various BMPs, dexamethasone, and/or vitamin D3 (16, 43, 53, 54) (Table 2). Within a 2 to 4 week period, matrix mineralization is evident based on positive Alizarin Red or von Kossa’s staining (16, 43, 53, 54). This mineral formation is accompanied by increased expression of osteogenic proteins and mRNAs, including alkaline phosphatase, bone morphogenetic proteins and their receptors, bone sialoprotein, Cbfa1/Runx2, and osteocalcin (16, 43, 53, 54). Osteoinductive or osteoconductive matrices have been found to further enhance the ASC expression of such markers (please see Section G below). In vitro, the osteogenic capacity of ASCs on scaffolds can be enhanced using a perfusion bioreactor (55). When combined with tricalcium phosphate/hydroxyapaptite matrices, human ASCs form osteoid-like structures when implanted subcutaneously in immunodeficient mice (56, 57). Furthermore, murine ASCs exposed to BMP2 and retinoic acid can repair critical sized craniofacial defects in syngeneic animals; however, the repair process is time dependent (58, 59). After extended periods of time, osteoclast cells infiltrate and resorb the newly formed bone (58, 59). Further studies are required to understand the underlying mechanism for these observations. Clinical trials have begun using ASCs for craniofacial defect repair. The REGEA Institute and Helsinki University Central Hospital in Finland reported the successful repair of a hard palate defect in a 65 year old patient (60). In successive procedures, subcutaneous autologous ASCs were harvested, expanded in culture over 3 weeks, and seeded onto tricalcium phosphate matrices in the presence of BMP2 (60). The cell product was placed within a titanium cage designed in the shape of the patient’s defect and implanted into the patient’s rectus abdominus muscle (60). After bone formation was confirmed by radiograph after 6–8 months, the construct was harvested and re-implanted into the defect site. Blood supply was reestablished by anastomosis with the facial artery (60). The construct completely integrated and the patient had full recovery of function (60). The REGEA group has treated ~20 subjects to date with >90% successful outcomes based on integration of the bone based on radiographic analyses and reconstruction of dentition (personal report, Riita Suuronen, Susanna Miettinen). Similar success in weight bearing bones with autologous or allogeneic human ASCs will significantly advance treatment of orthopedic injuries.
Table 2.
| Osteogenic Induction | DMEM (high glucose) or BGJb Medium supplemented with 10% Fetal Bovine Serum, 1 X Antibiotics (Penicillin/Streptomycin), 10 mM β-Glycerophosphate, 50 μg/ml L-Ascorbic Acid 2-phosphate, 10 nM Dexamethasone and/or 10 nM 1,25 Dihydroxy Vitamin D3 and/or BMP2 (10 to 100 ng/ml). Maintain ASC under osteogenic conditions for periods of 2 to 4 weeks. |
| Mineralization Assay | Monitor the extent of calcium phosphate mineralization of the extracellular matrix by staining the fixed cultures with Alizarin Red or von Kossa. The matrix staining with Alizarin Red can be quantified in an individual well of ASC under osteogenic conditions by elution with cetylpyridinium chloride monohydrate and subsequent optical density (OD540) determination. The level of osteogenic-induced ASCs can be normalized relative to a well of uninduced ASCs maintained and stained for an equivalent time period. |
| Alkaline Phosphatase Assay | Quantify the activity of bone alkaline phosphatase (ALP), an osteoblast associated enzyme induced transiently during matrix mineralization. This can be quantified by a liquid biochemical assay in cell lysates or monitored in fixed cultures of ASCs microscopically. |
| ELISA Assay | Monitor the secretion of osteoblast associated proteins as a function of differentiation including: Osteocalcin (bone matrix protein), Osteoprotegerin (osteo-regulatory cytokine), RANKL (osteo-regulatory cytokine). |
| Immunohistochemistry (IHC) | Can be used to detect the expression of protein biomarkers associated with osteogenesis including: Bone Sialoprotein (BSP) and Osteocalcin (OCN), osteoblast specific proteins associated with the extracellular matrix; Collagen I, the predominant collagen in bone; Cbfa1/Runx2 and Osterix, transcriptional regulators of osteogenesis; Osteonectin (ON) and Osteopontin (OSP), extracellular matrix proteins found in bone as well as other tissues; Osteoprotegerin (OPG) and RANKL, osteo-regulatory cytokines secreted by bone marrow stromal cells. |
| Quantitative PCR (qPCR) | Quantifies the mRNA levels of osteogenic genes associated with the extracellular matrix (ALP, BSP, OCN, ON, OSP), transcription (Cbfa1/Runx2, Osterix), or cytokine regulation (OPG, RANKL). |
| In Vivo Bioassay | Differentiated human ASCs can be loaded onto biomaterial scaffolds (tricalcium phosphate/hydroxyapatite ceramic ± collagen type I) and implanted subcutaneously in an immunodeficient rodent model for periods of 4 to 12 weeks with subsequent analysis of implant graft by histochemical and/or PCR based method for detection of human cells embedded in a calcified matrix. |
3. E. Skeletal muscle potential
ASCs can be induced to express biochemical features consistent with skeletal myogenesis by exposure to low serum conditions and/or horse serum (16, 43, 61–63) (Table 3). In response to these factors, ASCs express mRNAs encoding myogenic proteins, including the transcriptional regulators MyoD and myogenin as well as structural proteins such as myosin heavy chain (16, 43, 61–63). Based on histological evidence, ASCs fuse to form multinucleated myotubes in vitro. To date, pre-clinical animal and clinical trials using human ASCs for skeletal muscle myogenesis have not been reported. In contrast, there is significant and growing interest worldwide in using ASCs for cardiac myogenesis (64–69). These studies have been included in previous reviews (6, 70, 71). A comparison of the biomarkers used to distinguish the musculoskeletal differentiation of ASCs is presented in Table 4.
Table 3.
| Myogenic Conditions | DMEM (high glucose) supplemented with 10% Fetal Bovine Serum, 5% Horse Serum, 1% Antibiotic (Penicillin/Streptomycin)/Antimycotic (Amphotericin), 50 μM Hydrocortisone maintained for periods of up to 3 weeks. |
| Histochemical Assay | Toluidine blue staining to determine the presence of multinucleated myotubules in the culture. |
| Immunohistochemistry (IHC) | Detects proteins associated with myogenesis including the transcription factors MyoD, Myf5, Myf6, and Myogenin and the structural protein Myosin Light Chain. |
| Quantitative PCR (qPCR) | Quantifies the mRNA levels of myogenic genes associated with transcription (MyoD, Myf5, Myf6, Myogenin) and function (Myosin Light Chain, Myosin Light Chain Kinase). |
Table 4.
Lineage Specific Markers
| Lineage Differentiation Pathway | Transcriptional Marker | Extracellular, Structural or Cytoplasmic Marker |
|---|---|---|
| Chondrogenic | Sox 9 | Aggrecan, Collagen Type II, Chondroitin Sulfate |
| Myogenic | MyoD, Myf5, Myf6, Myogenin | Myosine Light Chain, Myosin Light Chain Kinase |
| Osteogenic | Cbfa1/Runx2, Osterix | Alkaline Phosphatase, Bone Sialoprotein, Osteocalcin, Osteonectin, Osteopontin, Osteoprotegerin, RANKL |
3. F. Immunogenic features – autologous vs allogeneic transplantation
As noted above, the immunophenotype of adipose-derived cells alters as a function of their adherence to tissue culture plastics and passage in vitro. In contrast to SVF cells, passage 2 and greater ASCs lose their expression of HLA-DR and CD86 which serve as recognition markers for T cells (14). While SVF cells elicit a robust mixed lymphocyte reaction from allogeneic peripheral blood monocytes, passaged ASCs are relatively non-reactive (14, 72). Furthermore, the presence of ASCs suppresses on-going mixed lymphocyte reactions between allogeneic peripheral blood monocytes (14, 72). The immunosuppressive properties of ASCs are due, in part, to their production of prostaglandin E2 and indoleamine 2,3-dioxygenase (73, 74). In a variety of disease models, ASCs have been found to suppress inflammatory cytokine expression by T cells and to reduce Th1/Th17 cell expansion (75–79). The presence of ASCs also stimulated the expression of interleukin 10, an anti-inflammatory cytokine (75–78). These ASC immunomodulatory features are similar to those described in BMSCs (80–84). In fact, allogeneic BMSCs are being transplanted in on-going clinical trials and similar engraftment of allogeneic ASCs may prove feasible as well. Consistent with this prediction, transplantation of allogeneic ASCs elicits minimal immune response in a spinal fusion model (85, 86). These early findings have significant implications with respect to the manufacture and quality control of a clinical ASC product that has yet to be tested in clinical trials.
3. G. Tissue engineering by using ASCs, 3D scaffolds and perfusion bioreactors
Tissue engineering (TE) methods are being increasingly adopted for translating the multi-lineage potential of ASCs into therapeutic applications. In general, tissue engineering outcomes depend on ability to predictably guide the ASC assembly into functional differentiated tissues, which in turn requires the combined application of biological, structural and mechanical cues enabling cell differentiation and patterning. Within the body, developmental and regenerative processes are orchestrated by cascades of physiological factors, which vary in space and time, yet are acting in concert to elicit coordinated cellular responses. To recapitulate some of these regulatory cascades in vitro, a ‘biomimetic’ approach has been established (87) that attempts to recapitulate in vitro (using engineering tools) the in vivo cell/tissue milieu (present during tissue development and remodeling). In an ideal case, the cellular niche is tightly regulated to mimic the native environment of a specific tissue, thereby prompting the cells (individually and collectively) to exhibit differentiated phenotypes unique to that tissue.
In a tissue engineering system, a three-dimensional (3D) scaffold provides a structural and logistic template for cell attachment and tissue formation, and a bioreactor provides adequate nutrient transfer and imparts biophysical stimuli to the cultured cells (Figure 1). The substrate properties and bioreactor conditions are directed by biological requirements, and chosen to closely mimic the native environment of the specific tissue that is being regenerated. For example, hydrogels are frequently chosen to induce chondrogenic responses as they enable the cells to adopt spherical morphologies and facilitate the distribution of compressive stresses that might be applied to the construct. In contrast, osteogenic outcomes are favored by using rigid, porous scaffolds as this structure is reflective of native bone. A recent article reviews distinct techniques that have been utilized for a number of musculo-skeletal tissues with different starting cell populations (87). Here, we review the use of scaffolds and bioreactors as they have been applied specifically to ASCs.
Figure 1. Tissue Engineering approach.
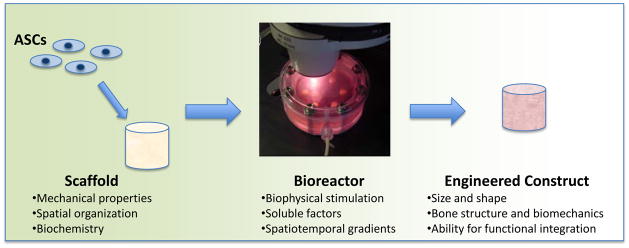
ASCs are combined with scaffolds and bioreactors that act in tandem to provide developmental cues to undifferentiated ASCs, to result in a 3D engineered construct with functional properties.
3D substrates are designed to have specific biochemical, topographical and mechanical properties, to direct the cells to spatially organize, to form functional contacts with other cells and the matrix, and to secrete and assemble tissue-specific extracellular matrix (ECM) when provided with the appropriate lineage-specific stimuli. Various groups have combined ASCs with 3D gels or solid scaffolds to form bone- (88–91), cartilage- (92, 93) and fat-like (94–96) tissues in vitro and in vivo.
The biochemical characteristics of the scaffold stimulate the developing ASCs and, together with soluble growth factors, impart lineage-specific information. It has been shown that ASCs implanted in hydroxyapatite-tricalcium phosphate (HA-TCP) scaffolds enabled osteogenic differentiation with far greater efficiency (80 %) than the same cells seeded in Collagraft™ scaffolds (20%) when both scaffolds were implanted subcutaneously into SCID mice and allowed to develop for 6 weeks (88). The mineral component of the HA-TCP scaffold (and possibly, the increased stiffness relative to Collagraft™) appeared to favor an osteogenic phenotype in ASCs. Likewise, increases in the osteogenic differentiation of human ASCs were observed when cells were cultured on different ceramics - akermite vs. β-TCP scaffolds - under otherwise similar in vitro conditions (89). The akermite scaffolds contained calcium (Ca), magnesium (Mg), and silicone (Si) ions on the surface, which are capable of dissolving into the culture medium and affect the degradation profile of the biomaterial. Other studies, not involving ASCs, have demonstrated that the surface topography and mechanical properties of the scaffolds also influence cellular characteristics and may be equally important in guiding cellular differentiation and determining functional outcomes in orthopaedic applications (97–99). Knowledge gleaned from these studies may enable the functional characteristics of the scaffolds to be determined a priori so that their properties can be tailored suit specific outcomes.
A major technical hurdle preventing the immediate clinical translation of tissue-engineered constructs has been their small size. Due to the diffusion limitations of nutrients and metabolic products, only relatively thin layers of viable tissue can be grown under static conditions. Bioreactors are employed to improve nutrient distribution and facilitate the development of homogenous tissues (100). The method for inducing convective flow and specific biophysical stimuli should correlate with the functional characteristics of the tissue being engineered. In vivo, cartilage tissues are exposed to dynamic compressive stimuli (101, 102), bone constructs (103) and blood vessels (104) require pressure and shear stresses for proper development, tendons and ligaments respond to tensile and shear stresses (105, 106), and myocardial tissues utilize electrical (107) and contractile (108) stimuli. These stresses, in addition to eliciting cell-specific responses, improve the convective mass transport parameters governing the exchange of nutrients and waste occurring at the inner regions of constructs. The application of such biophysical forces in bioreactors can be coupled with soluble growth factors as to induce lineage-specific stem cell differentiation and assembly into functional tissues.
Relatively few studies have been published thus far with ASCs cultured on scaffolds in bioreactors. In contrast, multiple studies performed with bone marrow-derived stem cells have validated the utility of bioreactor systems as a means of improving cell growth in bone (103, 109–111), cartilage (112) and ligament (105) constructs. Yet, two relatively recent studies investigating in vitro bone formation by ASCs in bioreactor configurations have been particularly instructive.
In the first study, Scherberich et al (113) employed bioreactor cultivation as a means to avoid conventional monolayer cultivation and better preserve the native multi-lineage capabilities of ASCs. The stromal vascular fraction (SVF) cells were seeded directly into hydroxyapatite scaffolds and cultured for 5 days in a perfusion bioreactor prior to implanting subcutaneously into nude mice. The osteogenic and vascular components within these constructs were assessed after 8 weeks of subcutaneous cultivation. In parallel, SVF cells were culture-expanded for 5 days using traditional monolayer methods prior to implantation into the hydroxyapatite scaffolds. These constructs were also implanted into nude mice and assessed after 8 weeks. It was found that the two methods of in vitro cultivation yielded considerably different cell numbers (monolayer cultures resulted in almost double the number of cell obtained from bioreactor cultures after only 5 days), but they had similar percentages of stromal (CD 90+) and endothelial (CD34+/CD31−) progenitors. However, a major difference was observed at the end of the subcutaneous implantation: both types of constructs demonstrated vascular formations (CD34+/CD31+), but only constructs grown in the bioreactors exhibited bone formation. Within the same study, SVF cells seeded directly into scaffolds and implanted without bioreactor cultivation also failed to form bone. This suggests that the osteogenic capabilities were not lost during monolayer expansion, but that the effects of the scaffold biochemistry and bioreactor cultivation were somehow combined to prime the cells along an osteogenic lineage.
A more recent study offers insights into the ‘priming’ of ASCs in vitro by their cultivation on osteogenic scaffolds in a bioreactor with medium perfusion, to obtain thick bone constructs (Figure 2) (114). ASCs at the 3rd passage were seeded into 4 mm thick scaffolds made of decellularized trabecular bone, at a high initial density, and cultivated either in perfusion bioreactors or in static conditions for 5 weeks to evaluate the in vitro bone-forming properties of ASCs. The same osteogenic supplements (dexamethasone, β-glycerophosphate and ascorbic acid) were used under both cultivation conditions. Medium flow through the interstitial pore spaces of the seeded constructs significantly enhanced ASC viability and tissue uniformity relative to constructs cultured without perfusion (Figure 2). It was postulated that the improved cell-cell contacts, in particular within the central region of the constructs, resulted in improved collagen matrix deposition and expression of bone-specific proteins (bone sialoprotein and osteopontin). This study further supports scientific advantages of using bioreactor systems providing environmental control and biophysical signaling for directed differentiation of stem cells in general and ASCs in particular. The related manufacturing and regulatory issues that need to be considered to make this technology clinically viable are being addressed in current studies (115, 116).
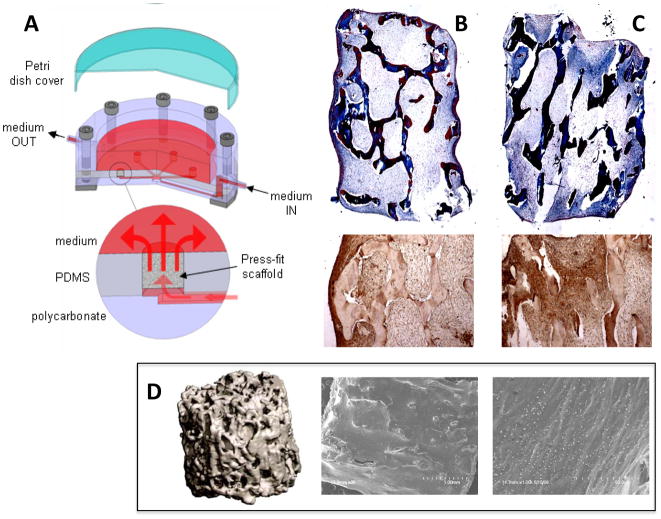
Figure 2. Engineering bone from ASCs.
(A) Schematic of bioreactor design (taken from (123) and reproduced with permission from Liebert Inc.) (B, C) Trichrome stains and osteopontin immunohistochemical stains for constructs cultured under static (B) and perfused (C) conditions. Matrix and cell distribution are greatly improved in perfused constructs. (D) μCT (left) and SEM images (middle and right) of bone constructs cultured with ASCs in perfusion bioreactors. SEMs show alignment of matrix with mineral deposits. (Images modified from (55) and reproduced with permission from Liebert Inc.)
3. H. Combination with gene transduction vectors
Human ASCs can be transduced with a variety of viral vectors to express specific genes of interest (117, 118). Comparative analyses have determined that lentiviral vectors infect ASCs more efficiently than adenoviral or retroviral vectors (117). Bone formation has been enhanced in rat models by ASC delivery vehicle of BMP2 as a virally expressed protein (118). Similarly, human ASCs with a lentiviral expression vector for MyoD displayed improved myogenic potential in a murine model of muscular dystrophy (119). Recently, human ASCs have been transduced with lentiviral vectors expressing four transcription factors (c-myc, klf4, oct-4, sox2) to create induced pluripotent stem (iPS) cells (120). Human ASC derived iPS cells express surface markers comparable to embryonic stem cells, displayed pluripotent differentiation potential in vitro, and form teratomas following in vivo implantation (120). The frequency of iPS cell induction is low, but pluripotent cell lines derived from human ASCs have direct value for musculo-skeletal discovery research and future clinical applications.
4. Perspective and conclusions
Human ASCs offer major practical and theoretical advantages for future tissue engineering and regenerative medical applications, with several challenges that are being addressed in ongoing studies. The abundance, accessibility, reproducibility, and ease of isolation of ASCs are among the major reasons for using ASCs in musculo-skeletal repair. While some studies have raised concerns about the inferiority of ASCs relative to BMSCs with respect to chondrogenic or osteogenic differentiation in vitro, it should be noted that such studies were conducted using osteoinductive conditions that were optimized for BMSCs. Work by Estes (45), Henning (44), and their colleagues has demonstrated that appropriate combinations of growth factors, different from those routinely used for BMSCs, are necessary to augment chondrogenic capacity of ASCs. Similar approaches, using growth factors and/or scaffolds, have the potential to further improve the myogenic and osteogenic differentiation of ASCs. Over the past decade, human ASC research has progressed from its focus on in vitro and pre-clinical animal models to human clinical trials. The next decade is likely to yield definitive studies supporting the safety and efficacy of using ASCs for bone, cartilage, and muscle repair. Such outcomes will most likely enhance our ability to treat disabilities that routinely impair quality of life for patients of all ages.
Acknowledgments
The authors wish to acknowledge Laura Dallam (Administrative Assistant, PBRC) for assistance in formatting and editing this manuscript; our colleagues in the Gimble, Guilak, Lopez, and Vunjak-Novakovic laboratories for their comments and input; and the following organizations for funding support: Pennington Biomedical Research Foundation (J.M.G.), NIH grants AR48852, AR48182, AG15768, and AR50245 (F.G.) and 2P41-EB002520 and R01 DE16525 (GV-N).
References
- 1.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002:66–80. doi: 10.1097/00003086-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3:705–713. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- 3.Strem BM, Hedrick MH. The growing importance of fat in regenerative medicine. Trends Biotechnol. 2005;23:64–66. doi: 10.1016/j.tibtech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Gimble JM, Guilak F, Nutall ME, Sathishkumar S, Vidal MA, Bunnell BA. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfusion Medicine and Hemotherapy. 2008;35:228–238. doi: 10.1159/000124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, Toyoshima Y, Sugimachi K, Toyoda M, Marc H, Douglas A. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10:417–426. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 8.Katz AJ, Hedrick MH, Llull R, Futrell JW. A novel device for the simple and efficient refinement of liposuctioned tissue. Plast Reconstr Surg. 2001;107:595–597. doi: 10.1097/00006534-200102000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010 Jul;5(7):1294–311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 11.Sen A, Lea-Currie YR, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YD, Gimble JM. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001;81:312–319. doi: 10.1002/1097-4644(20010501)81:2<312::aid-jcb1046>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell JBMK, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen YD, Storms RW, Goh B, Kilroy GS, Wu X, Gimble JM. The immunophenotype of human adipose derived cells: Temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Halvorsen YD, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose derived cells: Temporal changes in vitro. Stem Cells. 2006;24:1245–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell -assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatological Surgery. 2007 doi: 10.1111/j.1524-4725.2008.34256.x. In Press. [DOI] [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 18.Zannettino ACWPS, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential Human Adipose-Derived Stromal Stem Cells Exhibit a Perivascular Phenotype In Vitro and In Vivo. Journal of Cellular Physiology. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 19.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 20.Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 21.Kilroy GE, Foster S, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YDC, Cheatham B, Storms RW, Gimble JM. Cytokine Profile of Human Adipose-derived Stem Cells: Expression of Angiogenic, Hematopoietic, and Pro-inflammatory Factors. Journal of Cellular Physiology. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 22.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 23.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 24.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 25.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 26.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 27.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 28.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 29.Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 30.Liu TM, Martina M, Hutmacher DW, Hoi J, Hui P, Lee EH, Lim B. Identification of Common Pathways Mediating Differentiation of Bone Marrow and Adipose Tissues Derived Human Mesenchymal Stem Cells (MSCs) into Three Mesenchymal Lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 31.Danisovic L, Lesny P, Havlas V, Teyssler P, Syrova Z, Kopani M, Fujerikova G, Tre T, Sykova E, Jendelova P. Chondrogenic differentiation of human bone marrow and adipose tissue-derived mesenchymal stem cells. J Appl Biomed. 2007;5:139–150. [Google Scholar]
- 32.Danisovic L, Varga I, Polak S, Ulicna M, Hlavackova L, Bohmer D, Vojtassak J. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen Physiol Biophys. 2009;28:56–62. [PubMed] [Google Scholar]
- 33.Diekman BO, Rowland CR, Caplan AI, Lennon D, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage derived matrix. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1286–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 36.Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, Huang HI. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24:1759–1768. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 37.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 38.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 39.Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online. 2009;18(Suppl 1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- 40.Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, Minoo P, Atala A, De Filippo RE, Warburton D. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 42.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 43.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 44.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 45.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 46.Diekman BO, Estes BT, Guilak F. The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 48.Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 49.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 50.Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estes BT, Wu AW, Storms RW, Guilak F. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;209:987–995. doi: 10.1002/jcp.20808. [DOI] [PubMed] [Google Scholar]
- 52.Estes BT, Diekman BO, Guilak F. Monolayer cell expansion conditions affect the chondrogenic potential of adipose-derived stem cells. Biotechnol Bioeng. 2008;99:986–995. doi: 10.1002/bit.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells--their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S41–44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 54.Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 55.Frohlich M, Grayson W, Marolt D, Gimble J, Velikonja NK, Vunjak-Novakovic G. Bone Grafts Engineered from Human Adipose-Derived Stem Cells in Perfusion Bioreactor Culture. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hicok KC, Du Laney TV, Zhou YS, Halvorsen YD, Hitt DC, Cooper LF, Gimble JM. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 57.Justesen J, Pedersen SB, Stenderup K, Kassem M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004;10:381–391. doi: 10.1089/107632704323061744. [DOI] [PubMed] [Google Scholar]
- 58.Cowan CM, Aalami OO, Shi YY, Chou YF, Mari C, Thomas R, Quarto N, Nacamuli RP, Contag CH, Wu B, Longaker MT. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 59.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 60.Mesimaki K, Lindroos B, Tornwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 62.Mizuno H. The myogenic potential of human processed lipoaspirates - Part I: Morphological, immunohistochemical analysis and gene expression. J Japan Soc Plastic Recontr Surg. 2001;21:427–436. [Google Scholar]
- 63.Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. discussion 210–191. [DOI] [PubMed] [Google Scholar]
- 64.Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, MacLellan WR, Hedrick MH, Fraser JK. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 65.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 66.Madonna R, Willerson JT, Geng YJ. Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- 67.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 68.Bai X, Pinkernell K, Song YH, Nabzdyk C, Reiser J, Alt E. Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem Biophys Res Commun. 2007;353:665–671. doi: 10.1016/j.bbrc.2006.12.103. [DOI] [PubMed] [Google Scholar]
- 69.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 71.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 72.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 73.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded Adipose-Derived Stem Cells Suppress Mixed Lymphocyte Reaction by Secretion of Prostaglandin E2. Tissue Engineering. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 74.DelaRosa O, Lombardo E, Beraza A, Mancheno-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, Garcia L, Abad JL, Trigueros C, Delgado M, Buscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Buscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T-cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 79.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 80.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 81.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 82.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 83.Le Blanc K, Samuelsson H, Lonnies L, Sundin M, Ringden O. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation. 2007;84:1055–1059. doi: 10.1097/01.tp.0000285088.44901.ea. [DOI] [PubMed] [Google Scholar]
- 84.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 85.McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble JM. Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Eng Part A. 2009;15:2677–2686. doi: 10.1089/ten.TEA.2008.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez MJ, McIntosh KR, Spencer ND, Borneman JN, Horswell R, Anderson P, Yu G, Gaschen L, Gimble JM. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J Orthop Res. 2009;27:366–373. doi: 10.1002/jor.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grayson WL, Martens TP, Eng GM, Radisic M, Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20:665–673. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hicok KC, Du Laney TV, Zhou YS, Halvorsen YDC, Hitt DC, Cooper LF, Gimble JM. Human adipose-derived adult stem cells produce osteoid in vivo. 2004. pp. 371–380. [DOI] [PubMed] [Google Scholar]
- 89.Liu QH, Cen L, Yin S, Chen L, Liu GP, Chang J, Cui L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29:4792–4799. doi: 10.1016/j.biomaterials.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 90.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. Journal of Biomedical Materials Research Part A. 2008;84A:191–197. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 91.Lee JH, Rhie JW, Oh DY, Ahn ST. Osteogenic differentiation of human adipose tissue-derived stromal cells (hASCs) in a porous three-dimensional scaffold. Biochem Biophys Res Commun. 2008;370:456–460. doi: 10.1016/j.bbrc.2008.03.123. [DOI] [PubMed] [Google Scholar]
- 92.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 93.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochemical and Biophysical Research Communications. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 94.Kang JH, Gimble JM, Kaplan DL. In Vitro 3D Model for Human Vascularized Adipose Tissue. Tissue Engineering Part A. 2009;15:2227–2236. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang XL, Zhang XH, Sun L, Subramanian B, Maffini MV, Soto A, Sonnenschein C, Kaplan DL. Preadipocytes Stimulate Ductal Morphogenesis and Functional Differentiation of Human Mammary Epithelial Cells on 3D Silk Scaffolds. Tissue Engineering Part A. 2009;15:3087–3098. doi: 10.1089/ten.tea.2008.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 98.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 99.Zajac AL, Discher DE. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Current Opinion in Cell Biology. 2008;20:609–615. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends in Biotechnology. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Engineering. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 102.Hung CT, Mauck RL, Wang CCB, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Annals of Biomedical Engineering. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 103.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 105.Altman GH, Lu HH, Horan RL, Calabro T, Ryder D, Kaplan DL, Stark P, Martin I, Richmond JC, Vunjak-Novakovic G. Advanced bioreactor with controlled application of multi-dimensional strain for tissue engineering. Journal of Biomechanical Engineering-Transactions of the Asme. 2002;124:742–749. doi: 10.1115/1.1519280. [DOI] [PubMed] [Google Scholar]
- 106.Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annual Review of Biomedical Engineering. 2004;6:131–156. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 107.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Nehuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circulation Research. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 109.Bancroft GN, Sikavitsast VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteloblasts in a dose-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. Journal of Biomedical Materials Research Part A. 2005;72A:326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 111.Grayson WL, Bhumiratana S, Chao PG, Cannizzaro C, Liu XS, Guo XE, Caplan AI, Vunjak-Novakovic G. Increased Perfusion Rate and Cell-Seeding Density Enhance Tissue-Engineering of Human Bone. Orthopaedic Research Society; San Diego, CA: 2007. [Google Scholar]
- 112.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis and Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823–1829. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 114.Frohlich M, Grayson WL, Marolt D, Gimble J, Kregar-Velikonja N, Vunjak-Novakovic G. Bone Grafts Engineered from Adipose-Derived Stem Cells in Perfusion Bioreactor Culture. Tissue Eng A. doi: 10.1089/ten.tea.2009.0164. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin I, Smith T, Wendt D. Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products. Trends in Biotechnology. 2009;27:495–502. doi: 10.1016/j.tibtech.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Wendt D, Riboldi SA, Cioffi M, Martin I. Potential and Bottlenecks of Bioreactors in 3D Cell Culture and Tissue Manufacturing. Advanced Materials. 2009;21:3352–3367. doi: 10.1002/adma.200802748. [DOI] [PubMed] [Google Scholar]
- 117.Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, Benhaim P, Chen IS, Hedrick MH. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 118.Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 119.Goudenege S, Pisani DF, Wdziekonski B, Di Santo JP, Bagnis C, Dani C, Dechesne CA. Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of MyoD. Mol Ther. 2009;17:1064–1072. doi: 10.1038/mt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Estes BTDB, Gimble JM, Guilak F. Isolation of adipose derived stem cells and their induction to a chondrogenic phenotype. Nature Protocols. doi: 10.1038/nprot.2010.81. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gabbay JS, Heller JB, Mitchell SA, Zuk PA, Spoon DB, Wasson KL, Jarrahy R, Benhaim P, Bradley JP. Osteogenic potentiation of human adipose-derived stem cells in a 3-dimensional matrix. Ann Plast Surg. 2006;57:89–93. doi: 10.1097/01.sap.0000205378.89052.d3. [DOI] [PubMed] [Google Scholar]
- 123.Grayson WL, Chao PH, Marolt D, Kaplan DL, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 2008;26:181–189. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]