Abstract
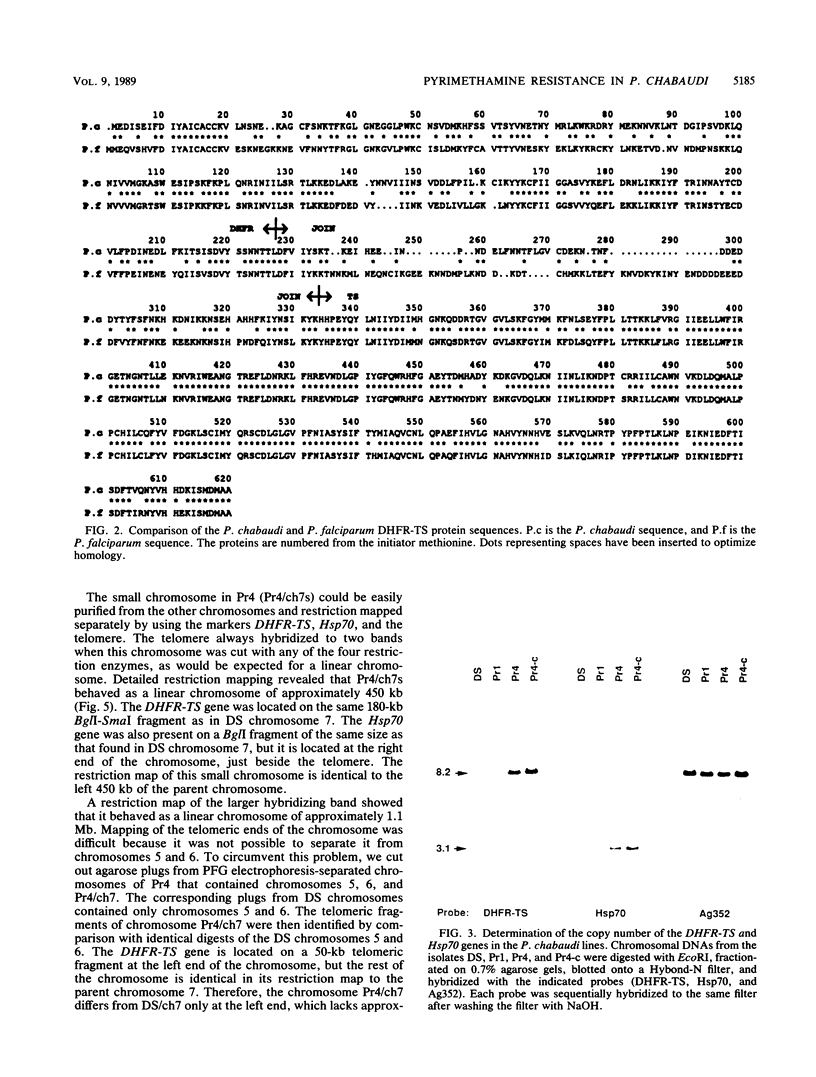
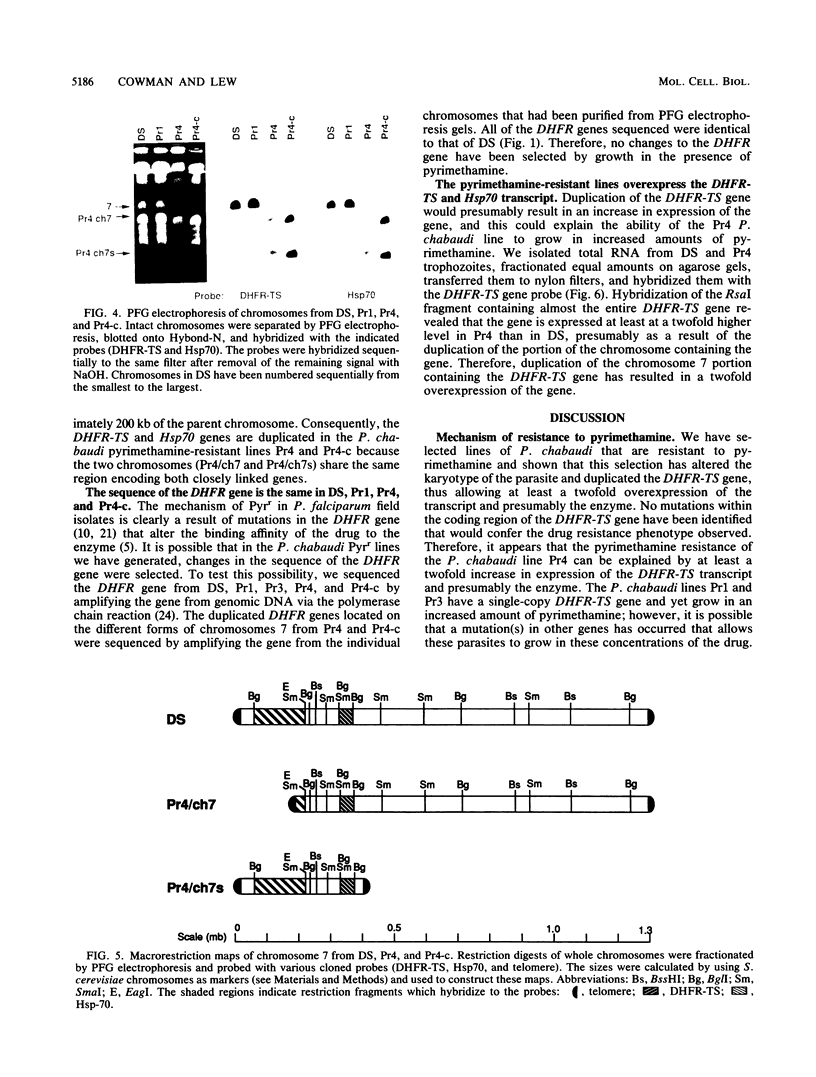
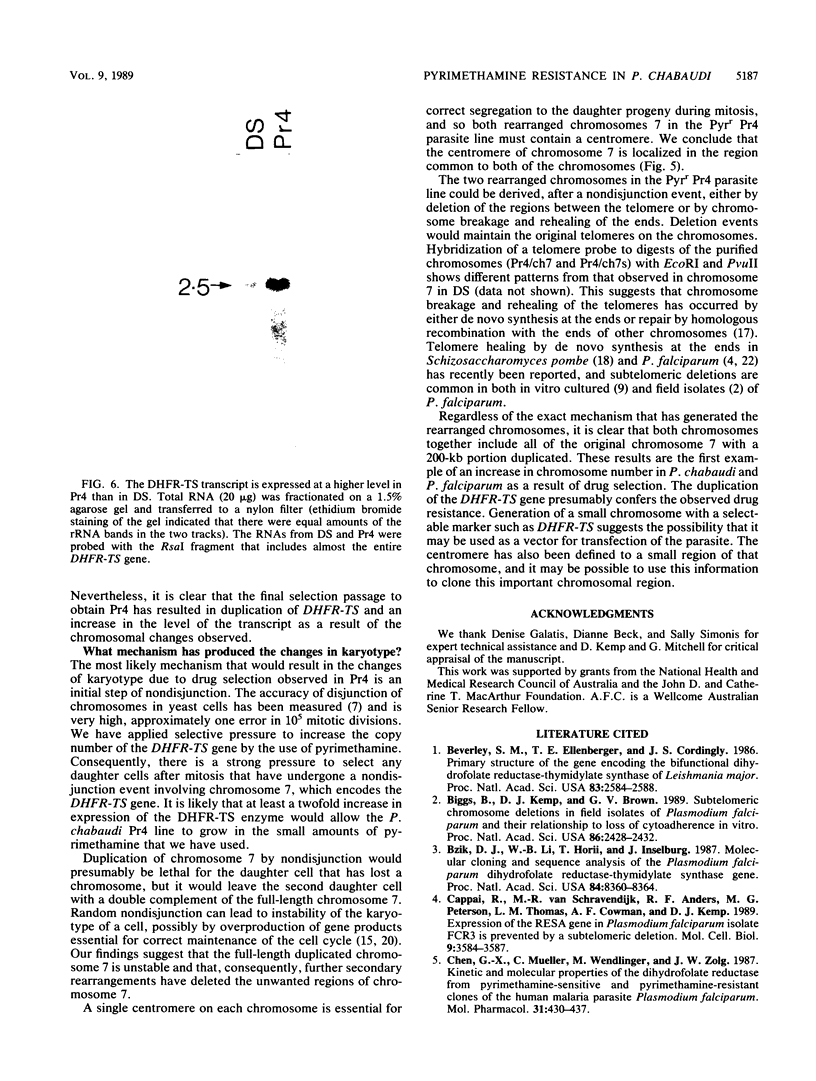
We selected lines of Plasmodium chabaudi that are resistant to high levels of the antifolate drug pyrimethamine and have shown that rearrangement and duplication of a portion of chromosome 7 has occurred in the resistant lines. This chromosomal duplication results in an increase in the chromosome number from 14 to 15: two derived chromosomes (450 kilobases and 1.1 megabases) were smaller than the original chromosome 7 (1.3 megabases), so that essentially only a 200-kilobase region was duplicated. This region contained the DHFR-TS gene and the closely linked Hsp70 gene. We have macrorestriction mapped chromosome 7 from the pyrimethamine-susceptible line (DS) and also the duplicated chromosome 7s in the resistant line. From these maps, we have proposed a process for the karyotype changes. Sequencing of the DHFR gene from the parent and derived chromosomes showed that there were no mutations in the coding sequence. As a result of the duplication of the DHFR-TS gene, there is at least a twofold increase in expression of the DHFR-TS gene, and this may explain the ability of the pyrimethamine-resistant lines to grow in increased amounts of the drug.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs B. A., Kemp D. J., Brown G. V. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. X., Mueller C., Wendlinger M., Zolg J. W. Kinetic and molecular properties of the dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant clones of the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987 Apr;31(4):430–437. [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980 Oct 9;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Morry M. J., Biggs B. A., Cross G. A., Foote S. J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone R., Burchall J. J., Hitchings G. H. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969 Jan;5(1):49–59. [PubMed] [Google Scholar]
- Ferone R., Roland S. Dihydrofolate reductase: thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5802–5806. doi: 10.1073/pnas.77.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C. E., Coderre J. A., Meek T. D., Garvey E. P., Claman D. M., Beverley S. M., Santi D. V. A bifunctional thymidylate synthetase-dihydrofolate reductase in protozoa. Mol Biochem Parasitol. 1984 Apr;11:257–265. doi: 10.1016/0166-6851(84)90070-7. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Wong M. L., Ruiz-Perez L., Santi D. V. Electron microscopy of amplified DNA forms in antifolate-resistant Leishmania. J Biol Chem. 1987 Oct 25;262(30):14618–14624. [PubMed] [Google Scholar]
- Holliday R. Chromosome error propagation and cancer. Trends Genet. 1989 Feb;5(2):42–45. doi: 10.1016/0168-9525(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Bzik D. J., Horii T. Pyrimethamine resistant Plasmodium falciparum: overproduction of dihydrofolate reductase by a gene duplication. Mol Biochem Parasitol. 1987 Nov;26(1-2):121–134. doi: 10.1016/0166-6851(87)90136-8. [DOI] [PubMed] [Google Scholar]
- Jäger D., Philippsen P. Stabilization of dicentric chromosomes in Saccharomyces cerevisiae by telomere addition to broken ends or by centromere deletion. EMBO J. 1989 Jan;8(1):247–254. doi: 10.1002/j.1460-2075.1989.tb03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Fukui K., Niwa O., Sugawara N., Szostak J. W., Yanagida M. Identification of healed terminal DNA fragments in linear minichromosomes of Schizosaccharomyces pombe. Mol Cell Biol. 1987 Dec;7(12):4424–4430. doi: 10.1128/mcb.7.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Welsh J. A., Dame J. B., Quakyi I. A., Graves P. M., Drake J. C., Allegra C. J. Mechanism of pyrimethamine resistance in recent isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1984 Nov;26(5):656–659. doi: 10.1128/aac.26.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks-Wagner D., Hartwell L. H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 1986 Jan 17;44(1):43–52. doi: 10.1016/0092-8674(86)90483-6. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Walliker D., Wellems T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Pace T., Dore E., Frontali C. Identification of a telomeric DNA sequence in Plasmodium berghei. EMBO J. 1985 Nov;4(11):2991–2995. doi: 10.1002/j.1460-2075.1985.tb04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sheppard M., Kemp D. J., Anders R. F., Lew A. M. High level sequence homology between a Plasmodium chabaudi heat shock protein gene and its Plasmodium falciparum equivalent. Mol Biochem Parasitol. 1989 Feb;33(1):101–103. doi: 10.1016/0166-6851(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Sheppard M., Thompson J. K., Anders R. F., Kemp D. J., Lew A. M. Molecular karyotyping of the rodent malarias Plasmodium chabaudi, Plasmodium berghei and Plasmodium vinckei. Mol Biochem Parasitol. 1989 Apr;34(1):45–52. doi: 10.1016/0166-6851(89)90018-2. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W., Yuthavong Y. Kinetic and molecular properties of dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant Plasmodium chabaudi. Mol Biochem Parasitol. 1984 Mar;10(3):355–367. doi: 10.1016/0166-6851(84)90033-1. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D., Carter R., Sanderson A. Genetic studies on Plasmodium chabaudi: recombination between enzyme markers. Parasitology. 1975 Feb;70(1):19–24. doi: 10.1017/s0031182000048824. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Walliker D., Sanderson A., Yoeli M., Hargreaves B. J. A genetic investigation of virulence in a rodent malaria parasite. Parasitology. 1976 Apr;72(2):183–194. doi: 10.1017/s0031182000048484. [DOI] [PubMed] [Google Scholar]