Abstract
Beta-cell transplantation is considered to be the most effective approach to cure type 1 diabetes (T1D). Unfortunately, the scarce availability of donor tissue limits the applicability of this therapy. Recent stem cell research progress shows stem cell therapy may be a potential means to solve this problem. Bone marrow-derived mesenchymal stem cells (MSCs) are self-renewable and multipotent adult stem cells which can differentiate into the three germ layers. Here we aimed to investigate whether MSCs could be reprogrammed into insulin-producing cells (IPCs). We isolated and characterized MSCs obtained from rat bone marrow. Then MSCs were induced to transdifferentiate into IPCs under specific conditions containing high concentrations of glucose, activin A, all-trans retinoic acid, and other maturation factors. The induced cells expressed multiple genes related to pancreatic beta-cell development and function, such as insulin1, glucagon, Pdx1, Pax6, and Glut-2. Insulin1 and C-peptide production were identified by immunocytochemistry. In vitro glucose challenge studies showed the induced cells secreted insulin in a glucose-dependent manner, as do normal pancreatic beta-cells. Transplantation of these MSC-derived insulin-positive cells could reverse the hyperglycemia of streptozotcin (STZ)-induced diabetic rats. These results demonstrated that MSCs could be reprogrammed into IPCs and might be a potential autologous cell source for transplantation therapy of T1D.
Keywords: Reprogramming, bone marrow, mesenchymal stem cells, insulin-producing cells
Introduction
In the year 2011, The WHO reported that 346 million people worldwide had diabetes. Type 1 diabetes (T1D) is an insulin-dependent, autoimmune disorder characterized by the destruction of insulin-producing beta-cells [1]. Current therapeutic options for individuals with T1D include insulin replacement therapy, pancreas transplantation, and beta-cell transplantation. Concerning insulin replacement therapy, it is almost impossible to maintain euglycemia consistently, resulting in aberrantly fluctuating blood glucose levels that can lead to acute and long-term complications. Pancreas transplantation often establishes an exogenous insulin-free euglycemic state, reduces long-term complications, and improves neural and vascular function [2-4]. The limitations of this procedure include a shortage of human pancreases and a long-term application of immunosuppressants [5]. Beta-cell replacement is considered to be the most promising approach for treatment of T1D. However, its application on a large scale is hampered by a shortage of cells for transplantation. Because beta-cells cannot be expanded significantly in vitro, efforts are under way to identify stem or progenitor cells that potentially could be grown and differentiated into beta-cells in vitro. Recent studies have shown that embryonic stem cells (ESCs) [6-8], induced pluripotent stem cells (iPSCs) [9], pancreatic ductal cells [10], hepatic stem cells [11], cord blood-derived cells [12], neural progenitor cells [13], and bone marrow-derived cells [14] may be used as alternative sources for IPCs. Adult stem cells are self-renewable and found in many adult tissues. Many researchers previously thought that they had less developmental potential than ESCs. However, recent research has shown that MSCs are multilineage potential cells [15-20]. MSCs have the potential for differentiating into tissues normally derived from the mesenchymal, ectodermal [15-19], and even endodermal germ layers[20]. Therefore, we investigated whether MSCs could be reprogrammed into IPCs. In the current study, we isolated MSCs from rat bone marrow and analyzed their biological characteristics. MSCs were subsequently induced to transdifferetiate into IPCs under culture conditions containing high concentrations of glucose, activin A, all-trans retinoic acid (RA), and other beta-cell-stimulating factors. The functionality of these cells was confirmed by insulin production and expression of islet-related genes. These cells could rescue streptozotocin (STZ)-induced diabetic rats after transplantation. Taken together, our results indicate that under specific conditions, MSCs could be induced to transdifferentiate into IPCs. This study provides support for utilizing MSCs as a surrogate beta-cell source for islet transplantation.
Materials and methods
Isolation and culture of rat bone marrow MSCs
Sprague-Dawley (SD) rats were purchased from the Laboratory Animal Center, Beijing Institute of Biotechnology. All procedures were performed under protocols approved by the Institutional Animal Care and Use Committee at Beijing Institute of Biotechnology. Bone marrow was obtained from the femurs and tibias of 4 male SD rats. The bones were sterilized by immersion in 70% ethanol, followed by removal of the remaining skin and muscles. Bone marrow was exposed by cutting the ends of the bones and extruded by inserting a needle and forcing cell culture medium with 15% fetal bovine serum (FBS, Gibco, USA) through the bone shaft. A single-cell suspension was generated by gentle pipetting and then layered over about 5 ml of ficoll (Ficoll-Paque, Amersham Pharmacia Biotech, Sweden). After centrifugation at 2500×g for 30 min, the mononuclear cell layer was removed from the interface and suspended in Hank’s balanced salt solution (HBSS, Gibco, USA). The cells were centrifuged at 1500×g for 15 min and resuspended in Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG, Gibco, USA) containing 15% FBS. All of the cells were plated in about 5 ml of the DMEM-LG medium in a 25-cm2 culture flask and incubated at 37°C with 5% humidified CO2. After 24 h, nonadherent cells were discarded, and adherent cells were thoroughly washed twice with PBS. Newly prepared DMEM-LG was added and replaced every 3 or 4 days for about 10 days. The cells were harvested with a solution containing 0.25% trypsin and 1 mmol/l EDTA (Sigma Aldrich, USA), replated, and recultured.
Flow cytometric analysis
MSCs at 3 to 4 passages were released by trypsinization. The cells were analyzed for the following markers: CD34, CD29, CD44, CD105, and CD166. For flow cytometric analysis, 5×105 cells were incubated with 10 μl fluorescenceconjugated antibodies (CD34, CD29, CD44, CD105, and CD166 from BD Biosciences, USA) for 20 min in the dark. Labeled cells were thoroughly washed with PBS. In all experiments, the corresponding isotype-matched antibodies (BD Biosciences, USA) were used as negative controls. The labeled cells were analyzed on a FACS Caliber (BD Biosciences, USA) by collecting 10,000 events with the Cell Quest software program (BD Biosciences, USA).
Differentiation of MSCs into IPCs
The induced differentiation was based on published protocols with minor modifications [21,22]. First, MSCs were passaged on Matrigel (BD Biosciences, USA) -coated 24-well culture plates in DMEM-LG containing 15% FBS for 24 h. Next, the culture medium was changed to Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG, Gibco, USA) containing 2% FBS and 100 ng/ml activin A (Sigma Aldrich, USA) for 24 h, and then changed to DMEM-HG containing 2% FBS and 10-6 mol/l RA (Sigma Aldrich, USA) for an additional 24 h. Thereafter, the cells were induced with DMEM-HG containing 2% FBS and 10 ng/ml basic fibroblast growth factor (bFGF, Sigma Aldrich, USA). After 3 days of bFGF induction, the culture medium was changed to DMEM-HG containing 2% FBS and 10 mmol/l nicotinamide (Sigma Aldrich, USA) for 3 days. Finally, the cells were induced with DMEM-HG containing 2% FBS and 10 mmol/l nicotinamide in the presence of 10 mmol/l exendin4 (Sigma Aldrich, USA) for 3 days.
Immunocytochemistry assay
A standard immunocytochemistry protocol was used. The final induced cells were fixed with 4% paraformaldehyde for 20 min, washed twice with PBS, and then incubated with PBS containing 0.3% Triton X-100 (Sigma Aldrich, USA) and 10% normal serum for 30 min. The cells were then incubated with the primary antibody: mouse insulin monoclonal antibody (1:250, Sigma Aldrich, USA) and guinea pig C-peptide polyclonal antibody (1:100, Linco, USA). Next, the cells were incubated with the respective secondary antibody. For insulin detection, the cells were incubated with the biotinylated anti-mouse secondary antibody (1:50, Vector Laboratories, USA) and VECTASTAIN® ABC Reagent, respectively. The color reaction was developed using 3, 3’-diaminobenzidine tetrachloride (DAB, Sigma Aldrich, USA). For C-peptide detection, the cells were incubated with FITC-conjugated anti-guinea pig secondary antibody (1:200, Santa Cruz, USA) and nuclei were detected by DAPI (Sigma Aldrich, USA) staining. In all immunocytochemistry assays, negative staining controls were carried out by omitting the primary antibody.
Reverse transcription polymerase chain peaction (RT-PCR)
Total RNA was extracted with TRIzol reagent (Invitrogen, USA) from MSCs and the induced cells on days 2, 3, 6, 9, and 12 after induction. Total RNA was digested with DNase to remove any contaminating genomic DNA. Total RNA (1 μg) was reverse transcribed and subsequent amplification was carried out using a one-step RT-PCR kit (Invitrogen, USA). The reactions were carried out at 50°C for 30 min for cDNA synthesis, and 94°C for 2 min for pre-denaturation. The samples were amplified for 30 cycles with denaturation at 94°C for 30 s, annealing at 64°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. The primers are shown in the Table 1.
Table 1.
List of rat gene-specific primers in RT-PCR analysis of cells.
| Genes | Size of PCR Product (bp) | GenBank accession No. | Primers |
|---|---|---|---|
| Sox17 | 359 | NM_001107902 | Forward: GGCGCCAGCCGGGACCTC |
| Reverse: GGCCGCCCTCGGGACCAA | |||
| Foxa2 | 400 | NM_012743 | Forward: AGGGCACGAGCCATCCGACTGGA |
| Reverse: GCCCGCCTGCCCGTACATAGGACT | |||
| Pdx1 | 377 | NM_022852 | Forward: GAGAATAAGAGGACCCGTACAGC |
| Reverse: CTGTGGGGACGCACTAAGG | |||
| Pax6 | 561 | NM_013001 | Forward: CTTCAACAGGACTCATTTCACCT |
| Reverse: GCAAAGGAATGATACAACTTGG | |||
| Insulin1 | 165 | NM_019129 | Forward: CCTGCTGGCCCTGCTCGTCCTCT |
| Reverse: GGCACTTGCGGGTCCTCCACTTCA | |||
| Glucagon | 359 | NM_012707 | Forward: TCACCAGTGACTACAGCAAATACC |
| Reverse: CAGTGATCTTGGTTTGAATCAGC | |||
| Glut-2 | 363 | NM_012879 | Forward: CATCGGCGTTGGTGCCATCA |
| Reverse: GGCCCGAGGAAGTCCGCAATGT | |||
| GAPDH | 400 | NM_017008 | Forward: AAGTTCAACGGCACAGTCAAG |
| Reverse: CAGTCTTCTGAGTGGCAGTGAT |
Insulin secretion assay
For static incubation, the final induced cells (day 12) were washed 5 times and incubated in Krebs-Ringer bicarbonate (KRB) buffer containing 3.3 mmol/l glucose for 1 h. The culture supernatants were collected after 1 h of incubation. The cells were then washed 3 times and incubated in KRB buffer containing 25 mmol/l glucose for 1 h. Following incubation, supernatants were collected again. Insulin assay was performed with Rat/mouse Insulin Enzyme-Linked Immunosorbent Assay Kit (Linco, USA) according to the manufacturer’s instructions. Total protein from the cells in each experiment was measured using the BCA Protein Assay Kit (Pierce Biotechnology, USA) to normalize for the amount of insulin secretion. Each experiment was performed in triplicate.
Transplantation into STZ-diabetic rats
Hyperglycemia was induced in male rats through intraperitoneal injection of 40 mg/kg of STZ once a day for 5 consecutive days. Blood glucose levels were determined using a GlucoTREND2 (Roche, USA) on snipped tails. Only rats with blood glucose levels stably above 13.9 mmol/l after the STZ injections were used subsequently for transplantation. Under general anesthesia, rats received a left renal capsule transplant of 1×106 final induced cells or a sham transplant of saline solution as a control. The nonfasting blood glucose levels were monitored every 2 days after transplantation.
Results
Biological properties of bone marrow-derived rat MSCs
Adherent MSCs were derived from the culture of bone marrow mononuclear cell suspension. The nonadherent cells were removed following 2-7 days of culture. After 10-15 days of culture, the cells had acquired three different morphological phenotypes: (1) spindle-shaped cells, which were the most abundant, (2) triangular-shaped cells, and (3) large flattened cells. After passage, most of the cells displayed a fibroblastic phenotype (Figure 1), and the surface markers of these cells were assessed by flow cytometric analysis. The phenotype of these cells was predominantly positive for CD29, CD44, CD105, and CD166 and negative for CD34 (Figure 2). We also observed that these cells could be induced to differentiate into osteoblasts or adipocytes (data not shown). These findings are consistent with the notion that MSCs possess stem cell properties.
Figure 1.
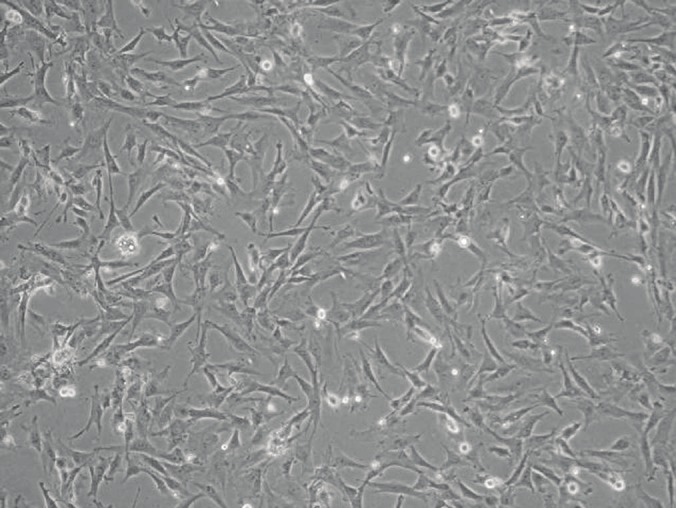
Morphological analysis of rat bone marrow-derived MSCs at day 10. Most of the MSCs displayed a fibroblastic phenotype. Magnification: 100×.
Figure 2.

The flow cytometric analysis of MSCs. The phenotype of these cells was predominantly positive for CD29, CD44, CD105, and CD166, and negative for CD34.
In vitro differentiation of MSCs into IPCs
To induce cell differentiation, we adopted an induction strategy based on high concentrations of glucose, activin A, RA, and other maturation factors. First, MSCs were plated on Matrigel-coated plates and then induced in 2% FBS DMEM-HG containing acitivin A and RA, sequentially. Next, the cells were cultured in 2% FBS DMEM-HG containing bFGF for the proliferation of pancreatic progenitor cells. During this stage, the cells morphology changed into round or oval appearance with confluence. Finally, the cells were switched to a medium containing nicotinamide and exendin4 for further maturation. At this stage, some cell clusters began to appear which were similar to pancreatic islet clusters.
To determine whether MSCs had undergone pancreatic differentiation, gene expression profiles for pancreatic beta-cell differentiation markers and hormones were assessed using RT-PCR. After the addition of activin A and RA, the expression of definitive endoderm markers Sox17 and Foxa2 was detected on day 2, but then disappeared at the end of day 9 (Figure 3). Pdx1 and Pax6, two key transcription factors for pancreatic differentiation, were first detected at the end of day 3 and were still detectable at the end of day 12. Glucagon was also detectable on day 3, but downregualted over time. Importantly, insulin1 and glut-2 were detected on day 6, and upregulated over time.
Figure 3.
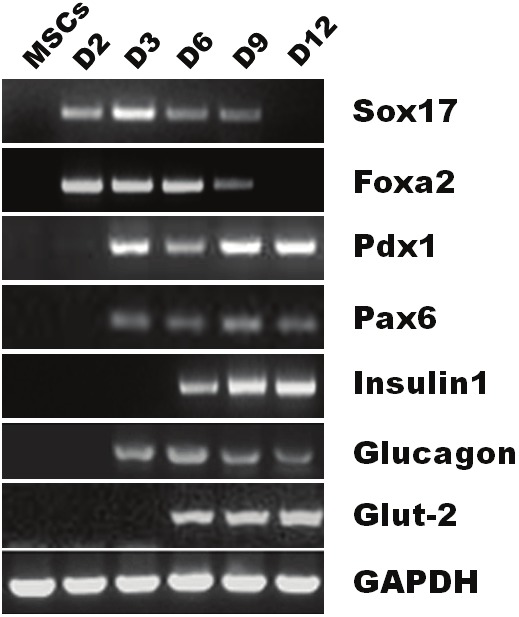
RT-PCR analysis of gene expression in MSCs during in vitro differentiation. Total RNA was extracted from MSCs and induced cells on days 2, 3, 6, 9, and 12 after induction. Lane 1, undifferentiated MSCs. Lane 2 (D2), cells were cultured in DMEM-HG containing 2% FBS and 100 ng/ml activin A for 24h. Lane 3 (D3), cells were cultured in DMEM-HG containing 2% FBS and 10-6 mol/l RA for 24h. Lane 4 (D6), cells were cultured in DMEM-HG containing 2% FBS and bFGF for 3 days. Lane 5 (D9), cells were cultured in DMEM-HG containing 2% FBS and 10 mmol/l nicotinamide for 3 days. Lane 6 (D12), cells were cultured in DMEM-HG containing 2% FBS and 10 mmol/l nicotinamide in the presence of 10 mmol/l exendin4 for 3 days.
To test whether the final induced cells actually synthesize insulin protein and release C-peptide, we detected insulin and C-peptide expression in the induced cells. The results showed that MSC-derived cell clusters expressed both insulin and C-peptide (Figure 4 and Figure 5). The transdifferentiation efficiency of MSCs into IPCs is about 20-25% by morphological observation. In addition, we found that Matrigel could increase the efficiency of IPCs and the size of cell clusters, which played an important role during the cell induction. With the absence of Matrigel, cell clusters could not form well (data not shown).
Figure 4.
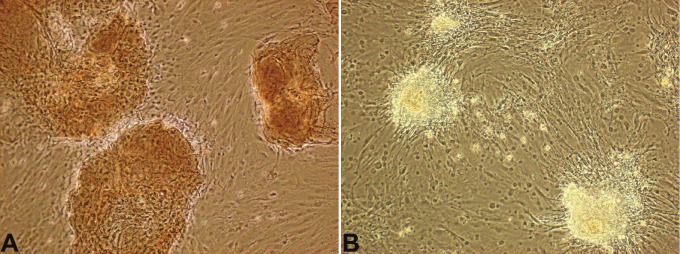
Insulin expression of MSC-derived cell clusters. The induced cells were stained with primary antibody to insulin. A, the induced cells were positive for insulin. B, negative control, the primary antibody was omitted. Magnification: 100×.
Figure 5.
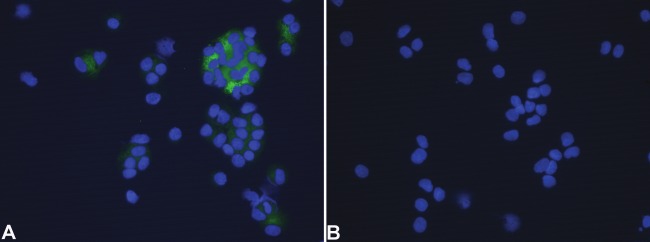
C-peptide expression of MSC-derived cells. Cytospin slides were prepared from the induced cells and stained with primary antibody to C-peptide. Nuclei were counterstained with DAPI. A, the induced cells were positive for C-peptide. B, negative control, the primary antibody was omitted. Magnification: 400×.
Glucose-induced insulin secretion from the induced cells
To determine whether the MSC-derived cells were responsive to a glucose challenge, we examined the final induced cells for insulin secretion (Figure 6). The insulin release in the high-glucose medium (25 mmol/l) was 5 times higher than that in the low-glucose medium (3.3 mmol/l). This result suggested that MSC-derived insulin-positive cells secreted insulin in a glucose-dependent manner, as do normal pancreatic beta-cells.
Figure 6.
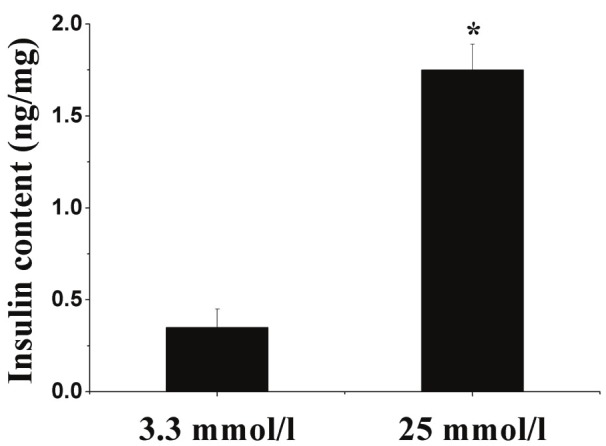
The induced cells release insulin in response to glucose. Data presented are means ± SD of the triplicate wells. Insulin level after treatment with 25 mmol/l glucose was nearly 5 times higher than that in the 3.3 mmol/l glucose. Statistical significance was tested by Student’s t-test: *P < 0.0l compared with the 3.3 mmol/l glucose treatment.
Reversal of hyperglycemia in STZ-induced diabetic rats
To determine whether the MSC-derived cells possessed the capacity to correct hyperglycemia in diabetic rats, rats were induced to become diabetic before cellular transplantation. Diabetic rats received sham surgery without cellular implantation as a control. Glucose levels of cell-implanted rats decreased and normalized within 1 week following transplantation (Figure 7). However, blood glucose levels in the diabetic control rats remained elevated. In addition, after removal of the left kidney transplanted with MSC-derived cells on day 14, the blood glucose level of diabetic rats reversed to greater than 13.9 mmol/l within 2 days. These results showed that MSC-derived IPCs, when transplanted into the renal capsule, were functional in vivo and capable of reversing hyperglycemia in diabetic rats.
Figure 7.
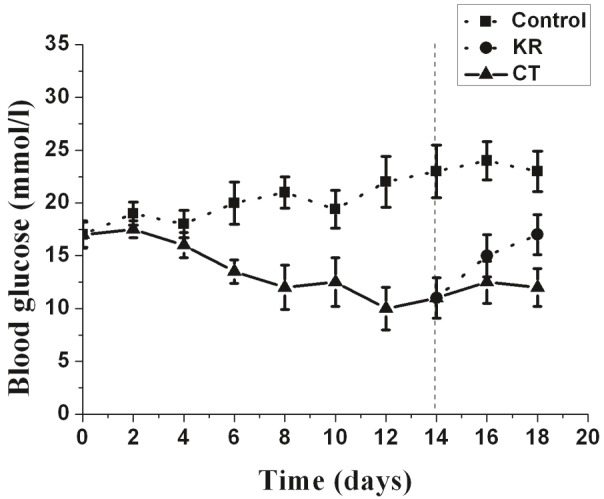
Transplantation of induced insulin-secreting cells into STZ-treated diabetic rats normalizes blood glucose levels. Glucose levels were monitored every 2 days after transplantation. CT, glucose levels in MSC-derived cells implanted in rats (n=6); KR, glucose levels in MSC-derived cells implanted in rats after the left kidney was removed at day 14 (n=5). Control, glucose levels in sham surgery diabetic rats (n=6). Data presented are means ± SD.
Discussion
Recent studies have shown the generation of IPCs from progenitor cells of various cellular sources, including the pancreas [10,23], liver [11], cord blood-derived cells [12], neural progenitor cells [13], intestinal epithelium [24], iPSCs [9], as well as ESCs [6-8]. Bone marrow MSCs have been shown to have a higher versatility to differentiate into more cell types than previously believed. However, there are still considerable controversies on the differentiation of MSCs into IPCs. Tang et al. reported that they could isolate mouse bone marrow-derived stem cells and obtain single-cell-derived cell clones that could be subsequently induced to transdifferentiate into IPCs under culture conditions containing high concentrations of glucose and the addition of beta-cell-stimulating factors after 4 months [25]. Using a cell-trapping system, Soria et al. produced an insulin-secreting cell derived from mouse ESCs that normalized blood glucose when transplanted into STZ-induced diabetic mice [8]. Bone marrow-derived cell transdifferentiation into IPCs has been challenged by the observation of cell fusion in vitro of pluripotent cells with differentiated cells thus adapting a differentiated phenotype [26,27]. Complementary information is provided by experiments from Hess et al. by using bone marrow cells expressing c-kit [28]. They claimed that the transplanted bone marrow cells most likely stimulated endogenous pancreatic tissue regeneration rather than contributing directly to beta-cell neogenesis. However, Ianus et al. reported bone marrow-derived cells could differentiate into beta-cells in vivo using an elegant Lox/Cre-GFP system and demonstrated that fusion is not a mechanism in beta-cell regeneration [29]. Altogether, these data suggest the possibility that MSC transdifferentiation into IPCs needs further investigation.
In the current study, we devised a protocol for efficiently reprogramming MSCs into IPCs within 12 days. MSCs were isolated and induced into functional IPCs under specific in vitro conditions based on a combination of high concentrations of glucose, activin A, RA, and other maturation factors. The differentiation process was characterized at the RNA and protein levels by RT-PCR and immunocytochemistry. Both insulin mRNA and C-peptide expression were measured to demonstrate de novo synthesis of insulin. Furthermore, the functionality of the IPCs generated from MSCs was tested by measuring insulin release in response to a glucose challenge and by demonstrating a reversal of diabetes upon subsequent implantation of these cells into diabetic rats.
Activin A, a member of the transforming growth factor-beta (TGF-β) superfamily, is critical for mesoderm and endoderm formation during gastrulation. When used at a high concentration, it primarily induces endoderm formation [30]. Kubo et al. reported that activin A can induce ESCs to differentiate into definitive endoderm cells [31]. In addition, activin can improve insulin secretion in cultured human pancreatic islets and regulate differentiation of pancreatic beta-cells during development and regeneration of beta-cells in diabetic neonatal rats [32,33]. On the initiation of the induction, cells started to express definitive endoderm markers Sox17 and Foxa2 with the addition of activin A, suggesting that the cells were efficiently induced to transdifferentiate into definitive endoderm cells.
To promote further pancreatic lineage differentiation, the cells were treated with RA. Following the treatment with RA, the cells started to express Pdx1, Pax6, insulin1, glucagon, as well as Glut-2. RA is a well-characterized signaling molecule that acts in the anteroposterior patterning of neuroectoderm and mesoderm in vertebrates [34]. Recent evidence indicates that RA is also involved in the regulation of the embryonic endoderm differentiation pattern, especially the early pancreas bud formation, and it is able to improve insulin expression in pancreatic beta-cells and the INS-1 cell line [35]. It has been demonstrated that the combination of activin A and RA was able to induce the Xenopus presumptive ectoderm region of the blastula to differentiate into pancreatic insulin-positive cells [36].
A high-glucose environment played an indispensable role in pancreatic islet differentiation [26]. It is well known that glucose is a growth factor for beta-cells [37]. At a 20 to 30 mmol/l concentration, it promotes beta-cell replication in vitro and in vivo, and at a 5 mmol/l concentration, it increases insulin content in cell lines derived from embryonic stem cells [8,38]. In a previous study, we demonstrated that long-term culture of MSCs in high concentrations of glucose could generate insulin-positive cells, whereas, low concentrations of glucose (negative control) showed no significant changes (data not shown). Zalzman et al. indicated that culture of immortalized human fetal Pdx1-expressing hepatocytes in media containing 25 mmol/l glucose activated multiple beta-cell genes, produced and stored considerable amounts of insulin, and released insulin in a regulated manner [39].
Matrigel also has a pivotal role in the differentiation process. Its major components, including laminin and growth factors such as fibroblast growth factor and TGF-β, are essential for pancreatic progenitor cell migration, the three-dimensional cystic structure formation, and protrusion of the islet bud [40]. Bonner-Weir et al. showed that human pancreatic ductal epithelial cells, which are cultured on the Matrigel, can form insulin-positive islet-like clusters [10]. In the current study, we found that Matrigel could increase the efficiency of IPCs and the size of cell clusters similar to pancreatic islets. In addition, a combination of nicotinamide and exendin4 might promote differentiated cell maturation [25].
In summary, we adopted a gene-free, nonintegrated protocol which could efficiently reprogram MSCs into IPCs in a short time period through a series of endodermal intermediates resembling those that occur during pancreatic development in vivo. The efficiency of IPCs was markedly enhanced with the use of Matrigel. However, due to the co-expression of glucagon and insulin in the final induced cells, the MSC-derived cells may not be fully mature compared with normal pancreatic beta-cells. Further differentiation and maturation may be required through either in vitro culture with some other beta-cell-promoting factors or transplantation of the cells into diabetic animals. One concern which we should keep in mind is the safety of the method for generating beta-cells. Here we adopted a gene-free, non-integrated method for generating beta-cells from MSCs. This makes it possible to apply MSC-derived beta-cells for future diabetic cell replacement therapy. Taken together, the current study provides direct evidence of reprogramming of MSCs into IPCs and it may be a potential way to solve the shortage of cells for islet transplantation. MSCs can be easily accessible and expanded to meet clinical application without the ethical concern inherent to fetal embryonic tissues. In addition, MSCs and MSC-derived cells do not elicit alloreactive lymphocyte proliferative responses and can modulate immune responses [41]. They can also be transplantable between HLA-incompatible individuals. Therefore, MSC-based therapy may be a promising therapy for diabetes mellitus.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30300191).
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Knight RJ, Lawless A, Patel SJ, Gaber AO. Simultaneous kidney-pancreas transplantation for end-stage renal disease patients with insulin-dependen t diabetes and detectable C-peptide. Transplant Proc. 2010;42:4195–4196. doi: 10.1016/j.transproceed.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Valdes-Gonzalez R, Rodriguez-Ventura AL, White DJ, Bracho-Blanchet E, Castillo A, Ramirez-Gonzalez B, Lopez-Santos MG, Leon-Mancilla BH, Dorantes LM. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010;162:537–542. doi: 10.1111/j.1365-2249.2010.04273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig B, Ludwig S, Steffen A, Saeger HD, Bornstein SR. Islet versus pancreas transplantation in type 1 diabetes: competitive or complementary? Curr Diab Rep. 2010;10:506–511. doi: 10.1007/s11892-010-0146-y. [DOI] [PubMed] [Google Scholar]
- 5.Han DJ, Sutherland DE. Pancreas transplantation. Gut Liver. 2011;4:450–465. doi: 10.5009/gnl.2010.4.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 7.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorechi KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 8.Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 9.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro transdifferentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci USA. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida S, Ishikawa F, Kawano N, Shimoda K, Nagafuchi S, Shimoda S, Yasukawa M, Kanemaru T, Ishibashi H, Shultz LD, Harada M. Human cord blood-derived cells generate insulin-producing cells in vivo. Stem Cells. 2005;23:1409–1416. doi: 10.1634/stemcells.2005-0079. [DOI] [PubMed] [Google Scholar]
- 13.Hori Y, Gu X, Xie X, Kim SK. Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med. 2005;2:347–356. doi: 10.1371/journal.pmed.0020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells transdifferentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 15.Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas JD, Cheng J, Qu T. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. 2011;33:1083–1093. doi: 10.1179/1743132811Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Wang QW, Chen ZL, Piao YJ. Mesenchymal Stem Cells Differentiate into Tenocytes by Bone Morphogenetic Protein (BMP) 12 Gene Transfer. J Biosci Bioeng. 2005;100:418–422. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 20.Wan SY, Zhang TF, Ding Y. Galectin-3 enhances proliferation and angiogenesis of endothelial cells differentiated from bone marrow mesenchymal stem cells. Transplant Proc. 2011;43:3933–3938. doi: 10.1016/j.transproceed.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 21.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Hou L, Tang F, Jiang W, Wang P, Ding M, Deng H. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656–662. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]
- 23.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:5034–5039. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 27.Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 28.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 29.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- 31.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 32.Florio P, Luisi S, Marchetti P, Lupi R, Cobellis L, Falaschi C, Sugino H, Navalesi R, Genazzani AR, Petraglia F. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest. 2000;23:231–234. doi: 10.1007/BF03343713. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Yi Z, Seno M, Kojima I. Activin A and betacellulin: effect on regeneration of pancreatic β-cells in neonatal streptozotocin-treated rats. Diabetes. 2004;53:608–615. doi: 10.2337/diabetes.53.3.608. [DOI] [PubMed] [Google Scholar]
- 34.Maden M. Role and distribution of retinoic acid during CNS development. Int Rev Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- 35.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in Zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 36.Moriya N, Komazaki S, Takahashi S, Yokota C, Asashima M. In vitro pancreas formation from Xenopus ectoderm treated with activin and retinoic acid. Dev Growth Differ. 2000;42:593–602. doi: 10.1046/j.1440-169x.2000.00542.x. [DOI] [PubMed] [Google Scholar]
- 37.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 38.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci USA. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrineprogenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 41.Gotherstrom C, Ringden O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]


