Background: The corazonin system is of importance for insects to control multiple physiological processes.
Results: Bombyx BNGR-A21 was activated in signaling and internalization by direct interaction with corazonin.
Conclusion: Bombyx corazonin was a specific ligand for dual Gq- and Gs-coupled BmCrzR.
Significance: Our findings will lead to a better understanding of the corazonin system in regulation of fundamental physiological processes.
Keywords: Arrestin, G Protein-coupled Receptors (GPCR), MAP Kinases (MAPKs), Neuropeptide, Signaling
Abstract
Corazonin, an undecapeptide neurohormone sharing a highly conserved amino acid sequence across Insecta, plays different physiological roles in the regulation of heart contraction rates, silk spinning rates, the induction of dark color and morphometric phase changes, and ecdysis. Corazonin receptors have been identified in Drosophila melanogaster, Manduca sexta, and Musca domestica. However, detailed information on the signaling and major physiological functions of corazonin and its receptor is largely unknown. In the current study, using both the mammalian cell line HEK293 and insect cell lines BmN and Sf21, we paired the Bombyx corazonin neuropeptide as a specific endogenous ligand for the Bombyx neuropeptide G protein-coupled receptor A21 (BNGR-A21), and we therefore designated this receptor as BmCrzR. Further characterization indicated that synthetic BmCrz demonstrated a high affinity for and activated BmCrzR, resulting in intracellular cAMP accumulation, Ca2+ mobilization, and ERK1/2 phosphorylation via the Gq- and Gs-coupled signaling pathways. The direct interaction of BmCrzR with BmCrz was confirmed by a rhodamine-labeled BmCrz peptide. Moreover, experiments with double-stranded RNA and synthetic peptide injection suggested a possible role of BmCrz/BmCrzR in the regulation of larval growth and spinning rate. Our present results provide the first in-depth information on BmCrzR-mediated signaling for further elucidation of the BmCrz/BmCrzR system in the regulation of fundamental physiological processes.
Introduction
Corazonin (Crz)3 is an undecapeptide and is a member of the neurohormone family that is ubiquitously present in arthropods with the exception of beetles, an aphid, and a spider mite (1, 2). Crz was initially purified from the corpora cardiaca of the cockroach Periplaneta americana with a functional ability to accelerate the heartbeat (3). The structure of corazonin is conserved and occurs as a single isoform in all insects studied thus far. Crz possesses a pyroglutamated N terminus and an amidated C terminus. To date, six modifications of corazonin have been identified. [Arg7]Corazonin (with Arg as the seventh residue from the N terminus) is the most universal in insect lineages (4). Bombyx [Arg7]corazonin has been isolated from the larval brain of the silkworm, Bombyx mori (5), and it was found to colocalize with the circadian clock marker proteins Doubletime and Period (6). Studies have also demonstrated that all residues are required for the interaction of the peptide with its receptor, which is consistent with its conserved structure during evolution (7–9).
Corazonin executes diverse functions in various insects. Besides its initial assignment as an accelerator of heartbeat in cockroaches, additional functions ranging from pigment migration in the eye of locusts, melanization of the locust cuticle to initiating the ecdysis, and clock functions in the moth Manduca sexta (10, 11). In Drosophila corazonin is supposed to regulate various stress responses, metabolism, female fecundity, and probably the downstream principal clock neuron (12–16). It had been reported that injected corazonin reduced the spinning rate, cocoon size, and hemolymph ecdysteroid level in the silkworm, meanwhile prolonging the pupal period (17). However, recent data indicated that RNA interference failed to detect a significant role for this neuropeptide in modulating mosquito heart physiology (18). Besides, [His7]corazonin has been shown to play an important role in the regulation of the nutritional and physiological stress response in other insects (14, 15, 19, 20). However, the function of corazonin neuropeptides in insects remains obscure.
The physiological function of corazonin peptides is mediated by the corazonin receptor. The first corazonin receptor (CrzR) was identified from the fruit fly Drosophila melanogaster in 2002 (21), followed by the identification of homologous receptors in M. sexta (11), Anopheles gambiae (22), Apis mellifera (23), and Musca domestica (24). These receptors all possess the typical features of a seven transmembrane G protein-coupled receptor (GPCR). The transmembrane regions of these receptors are conserved, whereas the N terminus is highly divergent. In Drosophila, corazonin has been identified as the endogenous ligand for the G protein-coupled receptor CG10698, and this CrzR is expressed in embryos, larvae, pupae, and adult flies. In M. sexta, the corazonin receptor exhibited high sensitivity and selectivity for corazonin in Xenopus oocytes and was used as a biosensor to verify the initiation of the ecdysis behavioral sequence in Inka cells by corazonin signaling (11). The CrzR in A. gambiae was found to only be activated by its endogenous corazonin, with an EC50 value of 4 × 10−9 m, resulting in cAMP accumulation (22). In M. domestica, tissue-specific RT-PCR revealed a high level of M. domestica corazonin receptor expression in the larval salivary glands and a moderate level in the CNS, whereas in adults, the receptor was expressed in both the head and body, suggesting multifunctionality of the Crz signaling system (24).
Recent studies have highlighted the physiological functions of corazonin, such as rhythm control, metabolism, ecdysis, and stress response. However, the detailed information on Crz/CrzR signaling remains to be further elucidated. By genomic data mining and phylogenetic analysis, the Bombyx neuropeptide G protein-coupled receptor BNGR-A21 has been identified as a corazonin-like receptor in B. mori (25–27). Although adipokinetic hormone (AKH), which mobilize lipids and carbohydrates from the insect fat body, and corazonin receptors are closely related to the gonadotropin-releasing hormone (GnRH) receptors (28–30), our previous data suggested that BNGR-A21 is independent of other members of the AKHR family by comparing the signaling and internalization of Bombyx AKHR, AKHR2a, and AKHR2b in the silkworm (31, 32). Therefore, in this study, we report on the cloning of the cDNA encoding the putative BNGR-A21 from the silkworm B. mori and its functional expression in the mammalian cell line HEK293 and insect cell lines BmN and Sf21. Our characterization provides an outline for Crz/CrzR signaling and perhaps aids in the interpretation of the signaling in ecdysis, spinning, development, and stress response in B. mori.
EXPERIMENTAL PROCEDURES
Materials
Cell culture media and fetal bovine serum (FBS) were purchased from HyClone (Beijing, China). G418, Lipofectamine 2000, and Opti-MEM® I reduced serum medium were purchased from Invitrogen. SuperFectinTM II was purchased from Pufei Biotech (Shanghai, China). The pEGFP-N1 and pCMV-FLAG vectors were purchased from Clontech Laboratories, Inc. and Sigma, respectively. Monoclonal anti-FLAG® M2 antibody, monoclonal anti-FLAG M2-FITC antibody, horseradish peroxidase (HRP)-conjugated anti-mouse IgG, and Nifedipine were purchased from Sigma. Anti-phospho-ERK1/2 (Thr202/Tyr204) and anti-ERK1/2 antibodies were purchased from Cell Signaling Technology (Danvers, MA). H89, Go6983, U73122, U0126, cholera toxin (CTX), and pertussis toxin (PTX) were purchased from Tocris Bioscience. The membrane probe DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), nuclear dye, RIPA lysis buffer, and horseradish peroxidase-conjugated secondary antibody were purchased from Beyotime (Haimen, China). [Arg7]Corazonin was synthesized by GL Biochem Ltd. (Shanghai, China).
Cloning of the BNGR-A21 cDNA and Construction of the Mammalian and Insect Expression Vectors
To construct the BNGR-A21 plasmid, RT-PCR was performed as described previously (32). To amplify the full-length sequence encoding BNGR-A21, two pairs of primers for the receptor were designed based on the sequence from the GenBankTM AB330442 as described below. The coding sequence of A21 was amplified for the pCMV-FLAG vector using the sense primer 5′-AAGCTTATGGACAACGAAGGCAACAGTAC-3′ and the antisense primer 5′-GGATCCTTATAACAAACTAATGTTCTGTCCAT-3′; an additional pair of primers was also designed for the pEGFP-N1 vector: the sense primer 5′-AAGCTTGCCACCATGGACAACGAAGGCAACAGTAC-3′ and the antisense primer 5′-GGATCCCGTAACAAACTAATGTTCTGTCCATTCG-3′. The corresponding PCR products were cloned into the pCMV-FLAG, pEGFP-N1, pBmIE1-FLAG, and pBmIE1-EGFP expression vectors using the Rapid DNA Ligation Kit (Beyotime, China) and we named these vectors Flag-A21, A21-EGFP, BmFlag-A21, and BmA21-EGFP, respectively. All constructs were sequenced to verify the correct sequences, orientations, and reading frames. Arrestin3-EGFP was generated as previously described (33).
Cell Culture and Transfection
The human embryonic kidney cell line (HEK293) was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (HyClone) and 4 mm l-glutamine (Invitrogen). The BNGR-A21 cDNA plasmid constructs were transfected or cotransfected into HEK293, BmN, and Sf21 cells using Lipofectamine 2000 (Invitrogen) and SuperFectinTM II (Pufei, China) according to the manufacturer's instructions. Selection for stable expression was initiated by the addition of G418 (800 μg/ml) 1–2 days after transfection.
Flow Cytometry Analysis
Approximately 2 × 105 cells were washed with phosphate-buffered saline (PBS) containing 0.5% BSA (FACS buffer) and incubated with 10 μg/ml of FITC-labeled anti-FLAG M2 monoclonal antibody (Sigma) in a total volume of 100 μl. After incubation for 60 min at 4 °C, the cells were pelleted and washed three times in FACS buffer. The cells were then fixed with 4% paraformaldehyde in FACS buffer and subjected to flow cytometry analysis on a FACScan flow cytometer (Cytomics FC 500 MCL, Beckman Coulter).
cAMP Assay and Accumulation Measurement
After seeding in a 48-well plate overnight, HEK293 or Sf21 cells transiently or stably cotransfected with BNGR-A21 and pCRE-Luc were grown to 90–95% confluence, stimulated with the corazonin peptides in DMEM without FBS, and incubated for 4–6 h at 37 °C. Luciferase activity was detected by a firefly luciferase assay kit (KenReal, Shanghai, China). The cAMP concentration was assessed using a commercially available cAMP detection kit (R&D Systems).
Intracellular Calcium Measurement
The stable FLAG-A21- and BmFlag-A21-expressing HEK293 and Sf21 cells were washed twice with phosphate-buffered saline and resuspended at 5 × 106 cells/ml in Hanks' balanced salt solution. The cells were detached by a Nonenzymatic Cell Dissociation Solution (M&C Gene Technology, China) or 0.02% EGTA and then loaded with 3 μm Fura 2-AM (Dojindo Laboratories, Japan) for 30 min at 37 °C. Cells were washed twice in Hanks' solution and then resuspended in Hanks' solution at a concentration of 3 × 107 cells/ml. Calcium flux was measured using excitation wavelengths of 340 and 380 nm in a fluorescence spectrometer (Tecan Infinite 200 PRO, Switzerland).
ERK1/2 Activation Assay
The HEK293 and Sf21 cells transiently or stably expressing BNGR-A21 were seeded in 24-well plates and starved for 60 min in serum-free medium to reduce background ERK1/2 activation and to eliminate the effects of the change of medium. After stimulation with the agonist, the cells were lysed by RIPA buffer (Beyotime, China). Equal amounts of total cell lysate were size-fractionated by SDS-PAGE (10–12%) and transferred to a PVDF membrane (Millipore). Membranes were blocked in blocking buffer (TBS containing 0.05–0.1% Tween 20 and 5% nonfat dry milk) for 1 h at room temperature and then incubated with rabbit monoclonal anti-phospho-ERK1/2 antibody (Cell Signaling) and anti-rabbit HRP-conjugated secondary antibody (Beyotime, China) according to the manufacturers' protocols. Total ERK1/2 was assessed as a loading control after p-ERK1/2 chemiluminescence detection using an HRP substrate purchased from Cell Signaling (Danvers, MA).
Measurement of Cell Surface Receptors by ELISA
HEK293 cells stably transfected with the FLAG-A21 construct were seeded in 24-well dishes coated with poly-l-lysine. The cells were stimulated with the indicated concentrations of the agonist at the indicated times the following day. The medium was aspirated, and the cells were washed once with Tris-buffered saline (TBS). After fixing the cells for 5 min at room temperature with 3.7% formaldehyde in TBS, the cells were washed three times with TBS and then blocked for 60 min with 1% bovine serum albumin/TBS followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (1:5000 in 1% BSA/TBS) for 60 min. To each well, 200 μl of HRP substrate (Sigma) was added, and the samples were incubated at 37 °C for 20–30 min. The reactions were stopped by adding an equal volume of 1% SDS, and plates were read at 405 nm in a microplate reader (Tecan, Switzerland).
Confocal Microscopy
For receptor surface expression analysis and internalization assays, HEK293 and BmN cells transiently or stably expressing receptor-EGFP were seeded onto glass coverslips coated with 0.1 mg/ml of poly-l-lysine and allowed to attach overnight under normal growth conditions. After 24 h, cells were stained with the membrane probe DiI (Beyotime, China) at 37 °C for 5–10 min, fixed with 4% paraformaldehyde for 15 min, and finally incubated with DAPI for 10 min. For the internalization assay, cells expressing A21-EGFP were treated with different concentrations of corazonin for different incubation times at 37 °C. For the β-arrestin and kurtz translocation assays, cells were cotransfected with the corresponding FLAG-A21 receptor constructs and β-arrestin1-EGFP, β-arrestin2-EGFP, or Bmkurtz-EGFP. After transfection, the cells were seeded onto glass coverslips, allowed to recover for 24–36 h in a 6-well plate, and incubated with 1 ml of DMEM without FBS, and if necessary, the corresponding ligands were added to stimulate the receptors for 5–15 min. The cells were visualized by fluorescence microscopy on a Zeiss LSM 510 laser scanning confocal microscope attached to a Zeiss Axiovert 200 microscope using a Zeiss Plan-Apochromat ×63, 1.40 NA oil immersion lens.
Ligand Competition Binding Assay
HEK293 cells stably expressing the FLAG-A21 construct were plated onto poly-d-lysine-coated 96-well plates 1 day before the experiments. Cells were washed once with PBS, and 50 μl of PBS with 0.2% BSA was added to each well. Samples of labeled and unlabeled peptides were made in ligand buffer at a concentration four times the final concentration specified. An amount of 25 μl of labeled ligand and 25 μl of buffer or unlabeled ligand was added to each well. Cells were incubated at room temperature for 90 min and transferred onto ice. Cells were then washed three times with 100 μl of ice-cold PBS with 0.1% BSA as previously described (34). Binding was determined by measuring the fluorescence intensity with a Tecan microplate reader. Binding is presented as the percentage of total binding. The binding displacement curves were analyzed by GraphPad Prism.
Quantitative Real-time PCR
Quantitative RT-PCR was performed as described previously with slight modifications (21). Total RNA was extracted from tissue samples as detailed in the manufacturer's protocol (Qiagen). Reverse transcription was completed using the PrimeScript First Strand cDNA Synthesis Kit (TaKaRa, China). The cDNA from the samples was quantified on a real-time PCR machine (CFX-Touch, Bio-Rad) using SYBR Premix Ex Taq (TaKaRa, China). BNGR-A21 used the sense primer 5′-TTCTGAATCCAGACAAAAACCAA-3′ and the antisense primer 5′-ATGATGTGTTGTTATCACCACGA-3′; actin used the sense primer 5′-CGTTCGTGATATCAAGGAGAAGCT-3′ and the antisense primer 5′-TCCATACCCAAGAACGAGGGTTG-3′. The possibility of genomic DNA contamination was excluded by DNase treatment. Differential expression of the cell lines was compared using the ΔΔCT method.
Peptide Synthesis
The corazonin peptides were prepared by solid-phase synthesis using the N-(9-fluorenyl)methoxycarbonyl strategy on a 430A peptide synthesizer (Applied Biosystems, Foster City, CA) and a 9050 Pepsynthesizer Plus (Perceptive Biosystems, Cambridge, MA). Crude peptides were purified by preparative reverse-phase high-performance liquid chromatography using a Dynamax-300 Å C18 25 cm × 21.4-mm inner diameter column with a flow rate of 9 ml/min and two solvent systems of 0.1% TFA/H2O and 0.1% TFA/acetonitrile. Fractions containing the appropriate peptide were pooled together and lyophilized. The purity of the final product was assessed by analytical reverse-phase high-performance liquid chromatography, capillary electrophoresis, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Silkworm Rearing and Bioassay
B. mori P50 were reared at 27 ± 3 °C and with a light:dark regime of 12 h:12 h on fresh mulberry leaves. Fourth instar larvae of similar size were divided into different treatment groups in the experiment. These larvae began molting on day 5 of the fourth instar and began spinning on day 7 of the fifth instar. Larvae were immobilized on ice before injection. B. mori CrzR dsRNA was generated in vitro by the MEGAscript® T7 Kit from Invitrogen according to the manufacturer's instructions using the sense primer 5′-TAATACGACTCACTATAGGGCTTCATTGGCAATGTGGCA-3′ and the antisense primer 5′-TAATACGACTCACTATAGGGGATTCTCGGTCACTATGCTGGT-3′. Corazonin and CrzR dsRNA were dissolved in PBS and diethyl pyrocarbonate/H2O, respectively, and injected into fourth and fifth instar larvae using a 10-μl microsyringe through the abdominal segments without injury of the silk glands. PBS without corazonin and pXef dsRNA (from MEGAscript® T7 Kit) were injected as the control. The weight of the larvae was recorded at an identical time each day. Bombycis and remnant mulberry leaves were weighed when completely dry. The cocoon layer and cocoon with pupa were weighed at the end of spinning.
Data Analysis
All results are expressed as the mean ± S.E. Data were analyzed using nonlinear curve fitting (GraphPad Prism version 5.0) to obtain EC50 values. Statistical significance was determined using Student's t test. Probability values that were less than or equal to 0.05 were considered significant.
RESULTS
Cloning and Functional Expression of the BNGR-A21 Receptor in HEK293 and BmN Cell
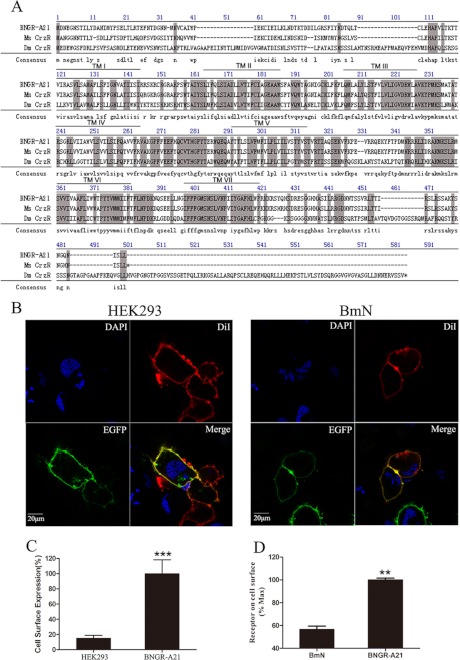
The Bombyx neuropeptide receptor BNGR-A21 has been proposed to have a close neighbor-joining phylogenetic tree with the Drosophila corazonin receptor by genomic and phylogenetic analysis (26). The full-length cDNA sequence encoding BNGR-A21 (GenBank accession number AB330442.1) was obtained by RT-PCR from the silk gland of silkworm larvae. As shown in Fig. 1A, the predicted open reading frame of BNGR-A21 encoding a protein with 437 amino acids contains a typical structure of seven putative transmembrane domains and shows 37.2, 36.7, 46.1, and 81.3% identity to D. melanogaster, A. gambiae, A. mellifera, and M. sexta corazonin receptors, respectively. To further assess its functional activity as a transmembrane receptor, BNGR-A21 with an N-terminal FLAG tag and BNGR-A21 with the enhanced green fluorescent protein (EGFP) fused to the C terminus were constructed and stably expressed in human embryonic kidney 293 (HEK293) cells and insect Spodoptera frugiperda (Sf21) or B. mori (BmN) cells. Significant cell surface expression was detected by FACS and ELISA analysis (Fig. 1C) and observed under fluorescence microscopy (Fig. 1B), suggesting that the N-terminal FLAG tag and the C-terminal GFP tag did not affect BNGR-A21 expression and orientation in the cell membrane in HEK293 and Sf21 cells.
FIGURE 1.
Protein sequence alignment and expression of BNGR-A21. A, protein sequence alignment of BNGR-A21 with the corazonin receptor from M. sexta (MsCrzR accession number AY369029); D. melanogaster (DmCrzR accession number AF373862). The seven transmembrane α-helices are indicated by TMI-TMVII. Amino acid residues that are common to these three receptors are highlighted. B, HEK293 and BmN cells expressing A21-EGFP fusion protein were stained with a membrane plasma probe DiI and a nuclei probe (DAPI). The cell surface expression of FLAG-A21 in HEK293 cells was analyzed by FACS (C) and in BmN cells by ELISA (D). Receptor cell surface expression was calculated by the value of mean fluorescence intensity (MFI), all data are shown as the MFI ratio calculated by dividing the MFI value of cells stained with anti-FLAG mAb by the MFI value for the same cells stained with isotype-matched control Ig. Data are expressed as the mean ± S.D. Data were analyzed by using the Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Activation of the BNGR-A21 Receptor by the BmCrz Peptide Dually Couples to the Gq and Gs Signaling Pathways
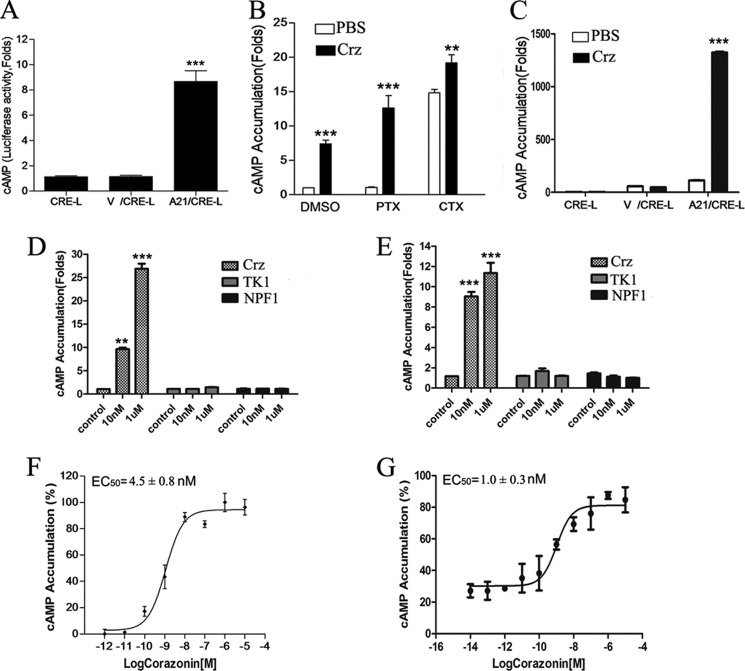
Previous studies have shown that the corazonin receptor was only activated by its endogenous corazonin, leading to cAMP accumulation, in A. gambiae (22), however, the detailed signaling pathways remain to be elucidated. To examine BNGR-A21-mediated G protein coupling and signaling, HEK293 and Sf21 cells were stably cotransfected with BNGR-A21 and a reporter gene system consisting of the firefly luciferase coding region under control of a minimal promoter containing cAMP-response elements. As shown in Fig. 2, F and G, upon stimulation with corazonin, BNGR-A21 was activated to induce significant accumulation of intracellular cAMP in both HEK293 and Sf21 cells with EC50 values of 4.5 ± 0.8 and 1 ± 0.3 nm, respectively, whereas Bombyx tachykinin-1 (TK1) and neuropeptide F-1 (NPF1) were unable to activate BNGR-A21 (Fig. 2, D and E). As a control, no change in the CRE-driven luciferase activity was detected in parental HEK293 and Sf21 cells. Pretreatment with 100 ng/ml of PTX, an inhibitor of Gαi protein, exhibited no effect on cAMP generation in BNGR-A21-expressing cells stimulated by corazonin, whereas stimulation with CTX, which constitutively activates Gαs subunits, led to a remarkable increase in the cellular levels of cAMP (Fig. 2, A–C), suggesting that Gs was likely involved in the BNGR-A21-mediated signaling in HEK293 and Sf21 cells.
FIGURE 2.
Corazonin-mediated cAMP formation in BNGR-A21-expressing cells. A, cAMP accumulation in HEK293 cells transiently co-transfected CRE-Luc (CRE-L) and BNGR-A21 with or without vehicle vector (V) was determined in response to corazonin treatment (1 μm). B, effects of PTX or CTX on corazonin-mediated stimulation of cAMP accumulation. HEK293 cells expressing BNGR-A21 were pre-treated with PTX (100 ng/ml) or CTX (300 ng/ml) overnight prior to incubation with coraoznin (1 μm) for 4 h. C, cAMP accumulation in Sf21 cells transiently co-transfected with CRE-Luc and FLAG-A21 was determined in response to DMEM (control) and corazonin treatments (1 μm). Effects of tachykinin-1 and neuropeptide F-1 on BNGR-A21-mediated stimulation of cAMP accumulation in HEK293 (D) and Sf21 (E) cells. cAMP accumulation was assayed in response to different doses of corazonin in HEK293 (F) and Sf21 (G) cells transiently co-transfected FLAG-A21/CRE-Luc. All data are shown as mean ± S.E. from at least three independent experiments. Data are expressed as the mean ± S.E. Data were analyzed by using the Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
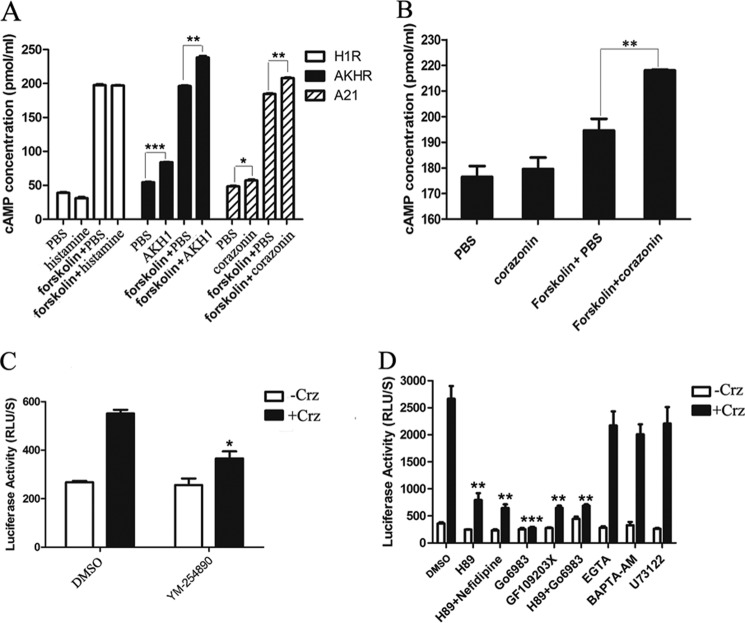
To confirm whether corazonin can bind to BNGR-A21 and trigger a second messenger cAMP signaling pathway, a direct cAMP assay by ELISA was used in this study. As shown in Fig. 3, A and B, BmCrz-activated BNGR-A21 triggered a significant increase in intracellular cAMP formation compared with the Gq-coupled human histamine H1 receptor (H1R), and cotreatment of BmCrz with forskolin induced a significant cAMP accumulation compared with treatment with only forskolin in both HEK293 and Sf21 cells. Moreover, BmCrz-induced luciferase activity was significantly inhibited by treatment with the Gq inhibitor YM-254890, and PKA inhibitor H89, PKC inhibitor Go6983, calcium chelators (EGTA and BAPTA-AM), and PLC inhibitor U73122 (Fig. 3, C and D), indicating that both Gq and Gs are likely involved in BNGR-A21-mediated through PKA, PKC, Ca2+, and phospholipase C activation of CRE-driven reporter transcription.
FIGURE 3.
Corazonin-mediated activation of adenylate cyclase through BNGR-A21. cAMP accumulation in HEK293 (A) and Sf21 (B) cells stimulated with DMEM, corazonin (1 μm), DMEM with forskolin (10 μm), and corazonin with forskolin (10 μm) for 10 min was detected by cAMP detection using the ELISA kit. HIR and AKHR were used as controls for Gq and Gs receptors, respectively. C and D, effects of different inhibitors on CRE-driven luciferase activity. HEK293 cells were transiently co-transfected with FLAG-A21 and CRE-Luc, and pre-treated with Gq inhibitor (YM-254890, 1 μm) (C) and PKA (H89, 10 μm), PKC (Go6983,10 μm, and GF109203X, 10 μm), l-calcium channel (nefidipine, 10 μm), calcium chelators (EGTA, 5 mm, and BAPTA-AM, 50 μm), and phospholipase C (PLC) (U73122, 10 μm) inhibitors (D) for 2 h prior to incubation with coraoznin (1 μm) for 4 h. The cAMP concentration was assessed by using a commercially available kit (R&D Systems). Data were analyzed by using the Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). All pictures and data shown are representative of at least three independent experiments.
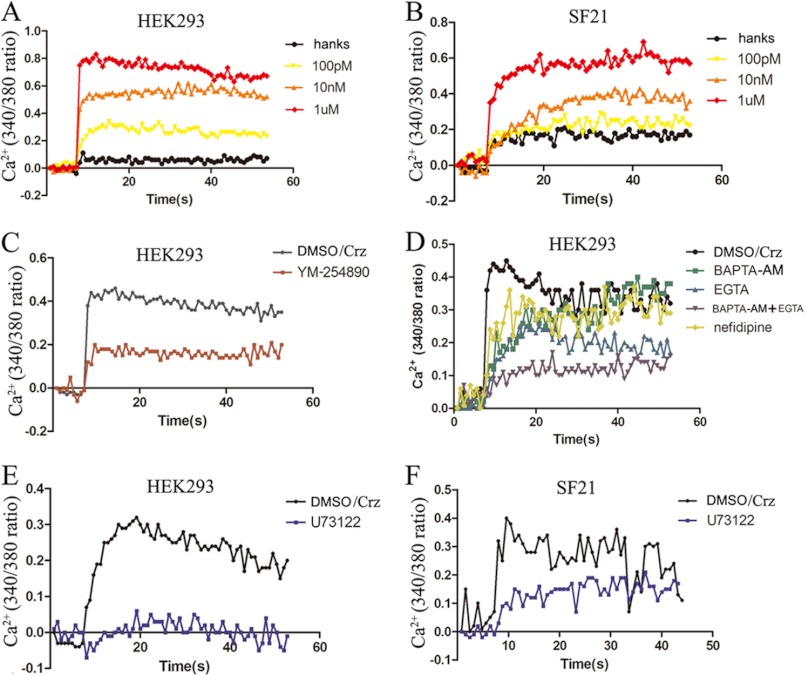
We then examined the effects of corazonin peptides on the intracellular Ca2+ change in the A21-expressing cells using the calcium probe fura-2. As indicated in Fig. 4, A and B, corazonin peptides elicited a rapid increase of Ca2+ in the FLAG-A21-expressing cells in a dose-dependent manner in both HEK293 and Sf21 cells. Preincubation with 1 μm Gq inhibitor YM-254890 led to a significant decrease in the intracellular Ca2+ mobilization (Fig. 4C). BNGR-A21-mediated intracellular Ca2+ mobilization was found to be sensitive to the PLC inhibitor U73122, calcium chelators EGTA and BAPTA-AM, and l-type calcium channel inhibitor nifedipine (Fig. 4, D--F). Taken together, these results suggest that the BNGR-A21 receptor was specifically activated by the BmCrz peptide by primarily coupling to the Gq signaling pathway and also dually coupling to the Gs signaling cascade, leading to intracellular Ca2+ mobilization and cAMP accumulation. Therefore, we suggest designating BNGR-A21 as the Bombyx corazonin receptor (BmCrzR).
FIGURE 4.
Intracellular Ca2+ influx. HEK293 (A) and Sf21 (B) cells, respectively, transfected with FLAG-A21 and BmFlag-A21 were measured in response to different concentrations of corazonin peptide using the fluorescent Ca2+ indicator fura-2. C, effect of pretreatment of the Gq inhibitor on corazonin (1 μm)-mediated Ca2+ influx in HEK293 cells. D, effects of pretreatment of calcium chelators (EGTA, 5 mm, and BAPTA-AM, 50 μm) and l-calcium channel (nefidipine, 10 μm) inhibitors on corazonin-mediated Ca2+ influx in HEK293 cells. Effect of pretreatment of PLC inhibitor (U73122, 10 μm) on corazonin-mediated Ca2+ influx in HEK293 (E) and Sf21 (F) cells. The figures are representative of more than three independent experiments.
A Rhodamine Red-labeled BmCrz Directly Binds to and Activates BNGR-A21
To confirm the direct interaction of BmCrz peptides with BNGR-A21 receptors, we designed and synthesized a fluorescent BmCrz analog conjugated with rhodamine red. Although corazonin has been shown to be highly conserved in structure, sequence alignment demonstrates the existence of a few mutants including Q1E, Q4T, and R7H. Therefore, we synthesized the Q4K peptide and conjugated it with rhodamine red at the Lys residue (Rho-[Lys4]BmCrz). Functional assays indicated that the Rho-[Lys4]BmCrz peptide could activate BmCrzR with an EC50 value of 86.1 nm (Fig. 5A). Using a displacement analysis method, Rho-[Lys4]BmCrz was found to compete with unlabeled BmCrz, with an IC50 value of 0.49 nm (Fig. 5B), suggesting that BmCrz directly binds and activates BmCrzR.
FIGURE 5.
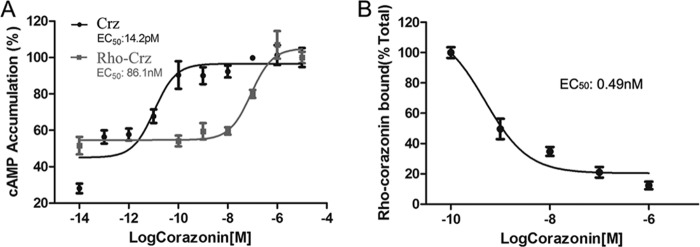
Direct interaction of BNGR-A21 with corazonin. A, rhodamine red-labeled [Lys4]corazonin activity was assayed using the CRE-driven luciferase system. B, binding of Rho-[Lys4]BmCrz to BNGR-A21 expressed in HEK293 cells was measured in the presence of different concentrations of unlabeled peptides or in the absence of unlabeled peptides (total binding). The extent of binding was determined by fluorescence intensity and is presented as a percentage of the total binding. The results represent the mean ± S.D. (n = 3). All images and data are representative of at least three independent experiments.
Corazonin Activates the ERK1/2 Signaling Pathway via BmCrzR
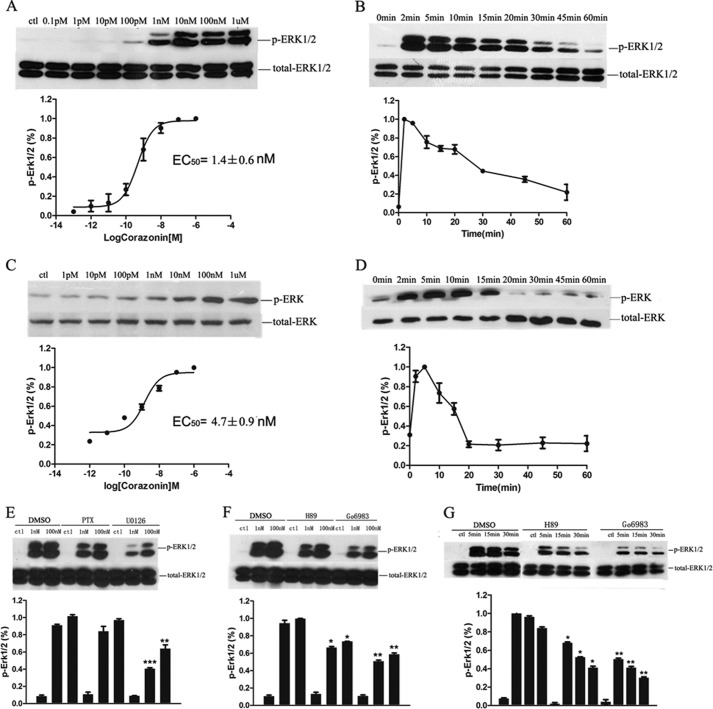
It has been well established that the activated GPCRs signal the ERK1/2 signaling pathway, which functions in transcriptional regulation that is involved in control of diverse processes ranging from proliferation and differentiation to apoptosis and can be used to assess the functional outcome of receptor stimulation (35, 36). We next sought to assess corazonin-mediated activation of ERK1/2 in both HEK293 and Sf21 cell lines stably expressing BmCrzR using a phosphospecific antibody known to bind only to the phosphorylated (Thr202 and Tyr204 of ERK1 and Thr185 and Tyr187 of ERK2) forms of these kinases (37). As indicated in Fig. 6, A–D, corazonin treatment did not provoke any appreciable effects on ERK1/2 in the parental or transiently mock-transfected HEK293 and Sf21 cells, whereas in the BmCrzR-stably transfected HEK293 and Sf21 cells, stimulation with corazonin elicited transient activation kinetics of ERK1/2 with maximal phosphorylation evident at 2 min in HEK293 cells and 5 min in Sf21 cells, which returned to nearly basal levels by 15 min. A dose-dependent curve indicated that the response to corazonin had an EC50 value of 1.4 ± 0.6 nm in HEK293 cells and 4.7 ± 0.9 nm in Sf21 cells for BmCrzR (Fig. 6). In addition, different inhibitors were used to elucidate the signaling pathways involved in the activation of ERK1/2 in HEK293 cells stably transfected with BmCrzR. We demonstrated that the BmCrzR-mediated activation of ERK1/2 was significantly blocked by the PKA inhibitor H89, PKC inhibitor Go6983, and MEK inhibitor U0126 in a dose- and time-dependent manner (Fig. 6, E-G) but not by the EGFR tyrosine kinase inhibitor AG1478, PI3K inhibitor wortmannin, and Src inhibitor PP2, confirming that BmCrz is the major agonist for BmCrzR.
FIGURE 6.
Effects of inhibitors PKA, PKC, and MEK on corazonin-mediated ERK1/2 activation in BNGR-A21-expressing cells. Concentration dependence (A) and time course (B) of corazonin-stimulated phosphorylation of ERK1/2 in stable BNGR-A21-expressing HEK293 cells. Concentration dependence (C) and time course (D) of corazonin-stimulated phosphorylation of ERK1/2 in Sf21 cells transfected with BNGR-A21. E, dose-dependent effects of pertussis toxin (PTX, 100 ng/ml) and MEK inhibitor U0126 (1 μm) on BNGR-A21 mediated activation of ERK1/2. Dose-dependent (F) and time course (G) of PKA inhibitor H89 (10 μm) and PKC inhibitor Go6983 (10 μm) on BNGR-A21-mediated activation of ERK1/2. The cells were pretreated with or without (control) inhibitors for 1 h and then stimulated with corazonin (1 nm, 5 min). The p-ERK was normalized to a loading control (total-ERK). The data shown are representative of at least three independent experiments. Statistical analysis was performed by a two-tailed Student's t test (**, p < 0.01; ***, p < 0.001, versus counterpart control).
Corazonin-activated BmCrzR Undergoes Rapid Internalization
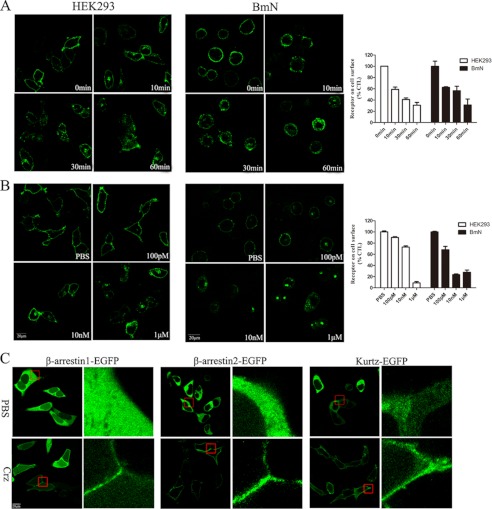
Agonist-induced internalization is a well characterized phenomenon for most GPCRs that is believed to contribute to the regulation of the strength and duration of receptor-mediated cell signaling (38). To visualize the internalization and trafficking of BmCrzR, we constructed vectors to express a fusion protein of BmCrzR with EGFP at the C terminus, and functional assays demonstrated that BmCrzR-EGFP functioned normally as wild-type BmCrzR in intracellular Ca2+ mobilization and CRE-driven luciferase activity (data not shown). Observation with confocal microscopy revealed that the fluorescence of BmCrzR-EGFP was primarily localized in the plasma membrane and was dramatically and rapidly internalized into the cytoplasm in response to the BmCrz peptide in HEK293 and BmN cells (Fig. 7, A-D). The internalization of BmCrzR-EGFP was detectable 5 min after agonist stimulation and reached a maximum within 60 min. The internalized BmCrzRs were largely clustered in the perinuclear region at 30 min (Fig. 7, B-D). The quantitative ELISA data are highly consistent with our observation by confocal microscopy. Moreover, we cloned kurtz, a novel nonvisual arrestin in insects, from B. mori and constructed vectors to express kurtz and arrestins fused with EGFP at the C terminus. HEK293 cells were cotransfected with BmCrzR and arrestin-EGFP or kurtz-EGFP and were examined by confocal microscopy after exposure to the agonist. As shown in Fig. 7, E and F, in the presence of the BmCrz peptide, β-arrestin1-EGFP, β-arrestin2-EGFP, and kurtz-EGFP showed the same ability to be recruited to the plasma membrane, although we observed that β-arrestin2-EGFP and kurtz-EGFP, but not β-arrestin1-EGFP, remained associated with receptor throughout internalization (data not shown). Our data on receptor internalization confirm that BmCrz is the major agonist for BmCrzR.
FIGURE 7.
Internalization of BNGR-A21-expressing cells. A, time course of A21-EGFP internalization induced by corazonin in HEK293 and BmN cells were determined by confocal microscopy and ELISA. B, HEK293 cells and BmN cells transfected with A21-EGFP were activated by the indicated concentrations of corazonin for 60 min and detected by confocal microscopy and ELISA. C, HEK293 cells transiently co-transfected with arrestin2-EGFP, arrestin3-EGFP, and kurtz-EGPF with FLAG-A21, respectively. β-arrestin1, β-arrestin2, and kurtz resided in the cytosol prior to corazonin stimulation (PBS) and was translocated to bind receptors in the membrane in response to treatment with 1 μm corazonin for 5 min. Error bars represent S.E. for three replicates. All of experiments were examined by fluorescence microscopy as described under “Experimental Procedure.” All panels are representative of at least three independent experiments.
Examination of in Vivo Expression and Physiological Roles of BmCrzR
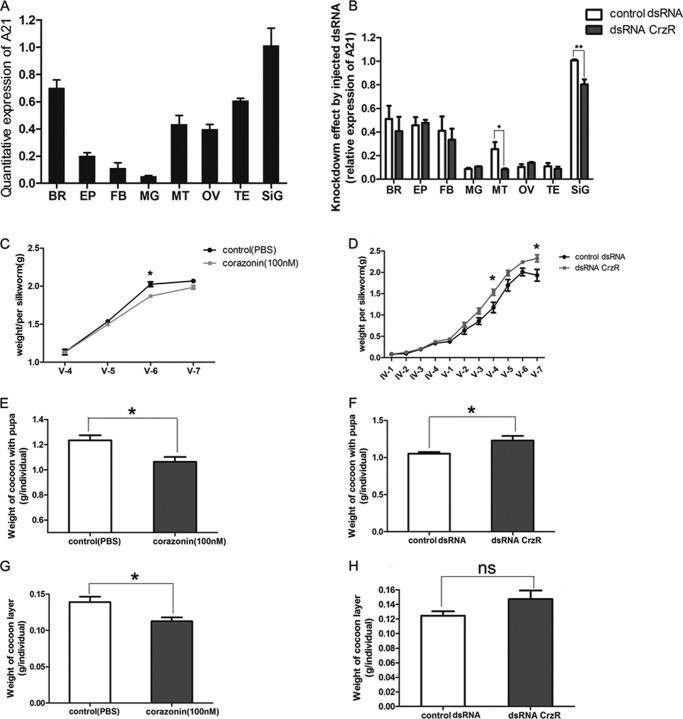
To examine the tissue-specific expression of BmCrzR, RT-PCR was used to analyze the expression of BmCrzR in silkworm larvae. As shown in Fig. 8A, in fifth instar larvae, BmCrzR expression was detectable in most of the tissues of which the silk gland was found as a major expression site, whereas other tissues, including brain, fat body, epidermis, midgut, Malpighian tubule, testis, and ovary, express BmCrzR at a lower level. This finding is in agreement with the observations made by Yamanaka et al. (26). To assess the physiological role of BmCrzR in silkworm larvae in vivo, RNAi-mediated knockdown of BmCrzR was performed by the injection of dsRNA at the beginning of the fourth and fifth instar. pXef dsRNA that was provided in the MEGAscript® Kit was injected as a control. As shown in Fig. 8B, the mRNA level of BmCrzR in the posterior silk gland and Malpighian tubule was significantly suppressed compared with controls. Although all the larvae that were injected with dsRNA pupated and enclosed normally, the body weight of the larvae and the weight of the cocoon layer markedly increased. In contrast, the larvae receiving the injection of the corazonin peptide demonstrated a significant reduction in body weight and cocoon layer (Fig. 8, C-H). These results suggest the possible role of BmCrZ and BmCrzR in the negative regulation of silkworm growth and silk production.
FIGURE 8.
BNGR-A21 expression in tissues and effects of exogenous corazonin and dsRNA-mediated expression knock-down on silk spinning and growth. A, BNGR-A21 expression in tissues of fifth larval instar. BR, brain; EP, epidermis; FB, fat body; MG, midgut; MT, Malpighian tubule; OV, ovary; TE, testis; SiG, Poster silk gland. Transcript levels of the BNGR-A21 mRNA were normalized to that of β-actin within each sample. B, effect of dsRNA injection on BNGR-A21 expression in different tissues. BNGR-A21 dsRNA (10 μg) was injected on the second day of fifth instar larvae. BNGR-A21 gene expression was determined by RT-PCR 36 h after injection. Each cDNA was analyzed in triplicate and bars represent the S.D. C, effects of [Arg7]corazonin injection on silkworm body weight. D, effects of dsRNA treatment on silkworm body weight and cocoon layer. Effects of [Arg7]corazonin injection on cocoon with pupa (E) and cocoon layer (G). Effects of dsRNA treatment on cocoon with pupa (F) and (H) cocoon layer. All panels are representative of at least three independent experiments (ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
DISCUSSION
The insect neuropeptide corazonin was initially isolated from cockroaches in 1989 and the first CrzR was identified by functional expression from the fruit fly D. melanogaster 10 years ago, however, detailed information on the signaling and physiological roles for corazonin and the corazonin receptor remains largely unknown. Recently, the Bombyx neuropeptide GPCR A21 (BNGR-A21) was identified as a corazonin-like receptor in B. mori by genomic data mining and phylogenetic analysis (25–27). In the present study, we functionally characterized this putative corazonin receptor with synthetic corazonin peptides using the mammalian cell line HEK293 and insect cell lines BmN and Sf21 stably or transiently transfected with BNGR-A21. Our data demonstrated that this corazonin-like receptor was specifically activated by synthetic Bombyx corazonin peptides, leading to intracellular Ca2+ mobilization, cAMP accumulation, ERK1/2 phosphorylation, and receptor internalization, but not by Bombyx tachykinin, NPF1 or AKH (31). We, therefore, suggest that the BNGR-A21 receptor is better designated as the B. mori corazonin receptor (BmCrzR).
To date, five insect corazonin receptors have been cloned and characterized (11, 21–24). In M. sexta, the corazonin receptor exhibits high sensitivity and selectivity for corazonin in Xenopus oocytes (11). The A. gambiae corazonin receptor was found to only be activated by its endogenous corazonin, which had an EC50 of 4 × 10−9 m, resulting in cAMP accumulation (22). In this study, we first established a cAMP-response element-driven reporter gene assay using both HEK293 and Sf21 cells to functionally analyze the activation of BmCrzR. Our data demonstrated that BmCrzR was activated in response to BmCrz peptides, leading to a significant increase in CRE-driven luciferase activity in a PTX-insensitive but PKA inhibitor H89- and Gq inhibitor YM-254890-sensitive manner. This result is also confirmed by direct quantitative analysis of intracellular cAMP using ELISA. These results suggested that Gs was likely involved in the BmCrzR-mediated signaling cascades in HEK293 and Sf21 cells, although the Gq/PLC/PKC signaling pathway was shown to partially contribute to the BmCrzR-induced CRE-driven luciferase activity. Previous studies have shown that Gq-coupled receptors H1R and prostaglandin EP3 receptor activate adenylyl cyclase through the release of the Gβγ subunits from G proteins (39) or via the Gq/PLC/Ca2+ pathway (40, 41), thereby elevating intracellular cAMP levels. In BmCrzR, more experiments are required to further elucidate the exact mechanism of signaling cross-talk between the Gq-coupled signaling pathway and the PKA/cAMP-response element-binding protein cascade.
We next used a Ca2+ probe to examine the intracellular Ca2+ mobilization in both mammalian and insect cells. The BmCrz peptide could elicit a rapid increase of intracellular Ca2+ in a dose-dependent manner in both HEK293 and Sf21 cells expressing BmCrzR. BmCrz-triggered Ca2+ mobilization was significantly blocked by the Gq inhibitor YM-254890 and PLC inhibitor U73122 and was also partially impaired by the calcium chelators EGTA and BAPTA-AM, and the l-type calcium channel inhibitor nifedipine. It is likely that BmCrzR predominantly couples to Gq to activate PLC, leading to Ca2+ mobilization from the endoplasmic reticulum Ca2+ pool to the cytoplasm, but BmCrzR can also modulate PLC or the l-type calcium channel by the βγ subunits released from various G-proteins, resulting in Ca2+ influx from the extracellular pool (42–44). Moreover, activated BmCrzR was found to effectively signal to the ERK1/2 transduction pathway in both HEK293 and Sf21 cells, and ERK1/2 activation was significantly inhibited by the PKA inhibitor H89, PKC inhibitors Go6983, and MEK inhibitor U0126 in a dose- and time-dependent manner, which is in high agreement with the observation of intracellular cAMP accumulation and Ca2+ mobilization. There are many well documented examples of a GPCR activating different signaling cascades by directly interacting with two different G-proteins. For example, the adrenergic α1B receptor preferentially couples to Gαq/PLC to mobilize Ca2+ from the ER to the cytoplasm but can also interact with Gαs/AC to stimulate intracellular cAMP production (45), and conversely, the histamine H2 receptor predominantly couples to the Gαs/AC/cAMP/PKA signaling cascade but also stimulates the Gαq/PLC/Ca2+/PKA signaling pathway (46). Taken together, these results strongly demonstrate that agonist-activated BmCrzR preferentially couples to Gq but also dually couples to Gs, resulting in the activation of the PLC/Ca2+/PKC and AC/cAMP/PKA signaling pathways, respectively.
Internalization is one of the predominant mechanisms that control GPCR signaling for ensuring appropriate cellular responses to stimuli. In this study, we constructed a chimeric protein in which EGFP was fused to the C terminus of BmCrzR (BmCrzR-EGFP). BmCrzR-EGFP that was stably expressed in both HEK293 and Sf21 cells exhibited functional activities in intracellular cAMP accumulation and Ca2+ mobilization comparable with wild-type BmCrzR. Using confocal microscopy combined with cell surface ELISA, we found that the BmCrz peptide promoted rapid BmCrzR internalization in a dose- and time-dependent manner. G protein-coupled receptor kinase-mediated phosphorylation and arrestin binding are involved in the regulation of GPCR internalization (47). To confirm the internalization of BmCrzR, we cloned kurtz, a novel nonvisual arrestin in insects (48), from B. mori and compared the activity of Bombyx kurtz with human β-arrestin1 and β-arrestin2 in the regulation of BmCrzR internalization. GPCR internalization has been characterized by employing an arrestin-GFP fusion protein to monitor the translocation of the arrestin from the cytoplasm to the cell membrane (49). The arrestin-GFP recruitment assay was first successfully used to identify endogenous ligands for Drosophila orphan GPCRs (50). The kurtz gene encoding the only nonvisual arrestin in Drosophila contains both amino- and carboxyl-terminal arrestin domains and shares 72% similarity to the mammalian β-arrestin2 and 74% similarity to β-arrestin1 (48). No differences were found between the receptor-kurtz interactions and the receptor-β-arrestin2 associations (51). Our data strongly suggest that Bombyx kurtz behaves more similarly to the mammalian β-arrestin2 in the regulation of BmCrzR internalization than to the mammalian β-arrestin1, suggesting that Bombyx kurtz can serve as a useful tool for the characterization of insect GPCR internalization and signaling.
Phylogenetic studies have suggested that not only the AKH and corazonin neuropeptides but also the AKH and corazonin receptors are closely related, apparently originating from one ancestral receptor having one ancestral ligand (26, 28, 52). AKH and the Gs-coupled AKHR system is well known to mobilize carbohydrates and lipids during energy-expensive activities such as long-distance flight (53), whereas no universal function for corazonin and the corazonin receptor has been recognized, although this neuropeptide shows high conservation in structure and the spatial expression pattern across most insect orders and has been shown to regulate seemingly unrelated functions, including the cardioacceleratory activity in P. americana (3), induction of the dark color and morphometric phase changes in locusts (54), reduction of the spinning rates of silk in silkworms (17), initiation of the ecdysis behavioral sequence (11), and regulation of the stress response (15). In this study, quantitative RT-PCR demonstrated that BmCrzR expression was detectable in most of the tissues of which the silk gland was found as a major expression site, whereas other tissues, including brain and fat body, demonstrated lower expression levels, suggesting the possible role of BmCrzR in the regulation of silk gland growth and silk production. Our results from exogenous corazonin injection and dsRNA-mediated knockdown of BmCrzR suggested the possible physiological role of Bombyx corazonin and its receptor in the negative regulation of growth and silk production. Therefore, further efforts should be focused on the molecular and functional dissection of the physiological roles played by Bombyx corazonin and its receptor.
In summary, in the present study, we have identified the Bombyx orphan receptor BNGR-A21 as a receptor for the neuropeptide Bombyx corazonin. BmCrzR was activated by direct interaction with the corazonin peptide, leading to intracellular Ca2+ mobilization via a Gq-dependent signaling cascade, cAMP accumulation, and ERK1/2 phosphorylation through possibly Gs-and Gq-dependent signaling pathways. In response to the corazonin peptide, BmCrzR underwent a rapid internalization via an arrestin-dependent pathway (Fig. 9). Furthermore, functional analysis using exogenous corazonin injection and dsRNA-mediated knockdown of BmCrzR demonstrated a likely role of corazonin and its receptor in the regulation of silkworm growth and silk production. These results will lead to a better understanding of the BmCrz/BmCrzR system in the regulation of fundamental physiological processes.
FIGURE 9.
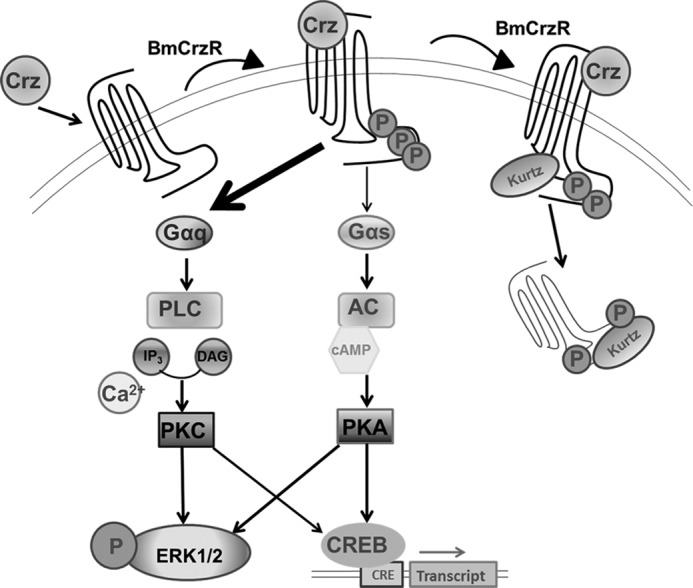
Schematic diagram of agonist-induced BmCrzR ERK1/2 activation and internalization. Corazonin binding to BmCrzR activates both Gs and Gq families of heterotrimeric G proteins, leading to dissociation of G protein subunits. Gβγ, enhanced adenylate cyclase and phospholipase C activity, leading to intracellular cAMP and Ca2+ accumulation, which, respectively, activated PKA and PKC, and stimulated phosphorylation of ERK1/2. The binding of corazonin also promoted the recruitment of arrestin or kurtz proteins followed by rapid BmCrzR internalization. DAG, diacylglycerol; CREB, cAMP-response element-binding protein.
Acknowledgments
We thank Aiping Shao, Ming Ding, and Hanmin Chen for technical assistance and equipment usage. We thank Dr. M Taniguchi of ASTELLAS for the generous gift of YM-254890.
This work was supported by Ministry of Science and Technology of China Grants 2012CB910402 and the National Natural Science Foundation of China Grants 31072090 and 31272375.
- Crz
- corazonin
- CrzR
- corazonin receptor
- BmCrz
- B. mori corazonin
- GPCR
- G-protein-coupled receptor
- BNGR
- B. mori neuropeptide G-protein-coupled receptor
- AKHR
- adipokinetic hormones receptor
- H1R
- human histamine H1 receptor
- EGFP
- enhanced green fluorescent protein
- CRE
- cAMP-response element
- BmCrzR
- B. mori corazonin receptor
- CTX
- cholera toxin
- PTX
- pertussis toxin
- dsRNA
- double-stranded RNA
- BAPTA
- 1,2-bis(2-aminophenoxyl)ethane-N,N,N′,N′-tetraacetic acid
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate.
REFERENCES
- 1. Hauser F., Cazzamali G., Williamson M., Park Y., Li B., Tanaka Y., Predel R., Neupert S., Schachtner J., Verleyen P., Grimmelikhuijzen C. J. (2008) A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front. Neuroendocrinol. 29, 142–165 [DOI] [PubMed] [Google Scholar]
- 2. Veenstra J. A., Rombauts S., Grbić M. (2012) In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem. Mol. Biol. 42, 277–295 [DOI] [PubMed] [Google Scholar]
- 3. Veenstra J. A. (1989) Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 250, 231–234 [DOI] [PubMed] [Google Scholar]
- 4. Predel R., Neupert S., Russell W. K., Scheibner O., Nachman R. J. (2007) Corazonin in insects. Peptides 28, 3–10 [DOI] [PubMed] [Google Scholar]
- 5. Hua Y. J., Ishibashi J., Saito H., Tawfik A. I., Sakakibara M., Tanaka Y., Derua R., Waelkens E., Baggerman G., De Loof A., Schoofs L., Tanaka S. (2000) Identification of [Arg7] corazonin in the silkworm, Bombyx mori and the cricket, Gryllus bimaculatus, as a factor inducing dark color in an albino strain of the locust, Locusta migratoria. J. Insect Physiol. 46, 853–859 [DOI] [PubMed] [Google Scholar]
- 6. Qi-Miao S., Tanaka S., Takeda M. (2003)Immunohistochemical localization of clock proteins (DBT and PER), and [His7]- and [Arg7]corazonins in the cerebral ganglia of Bombyx mori. Are corazonins downstream regulators of circadian clocks? Eur. J. Entomol. 100, 283–286 [Google Scholar]
- 7. Yerushalmi Y., Pener M. P. (2002) The dark-colour-inducing neurohormone of locusts in relation to an albino mutant of Schistocerca gregaria. Physiol. Entomol. 25, 127–132 [Google Scholar]
- 8. Grach C., Yerushalmi Y., Inlow M., Pener M. P. (2004) The dark-colour-inducing neurohormone of locusts. Strain-dependent and phase-independent effects on adults of the migratory locust, Locusta migratoria. Physiol. Entomol. 29, 97–107 [Google Scholar]
- 9. Tanaka Y., Ishibashi J., Tanaka S. (2003) Comparison of structure-activity relations of corazonin using two different bioassay systems. Peptides 24, 837–844 [DOI] [PubMed] [Google Scholar]
- 10. Wise S., Davis N. T., Tyndale E., Noveral J., Folwell M. G., Bedian V., Emery I. F., Siwicki K. K. (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J. Comp. Neurol. 447, 366–380 [DOI] [PubMed] [Google Scholar]
- 11. Kim Y. J., Spalovská-Valachová I., Cho K. H., Zitnanova I., Park Y., Adams M. E., Zitnan D. (2004) Corazonin receptor signaling in ecdysis initiation. Proc. Natl. Acad. Sci. U.S.A. 101, 6704–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergland A. O., Chae H. S., Kim Y. J., Tatar M. (2012) Fine-scale mapping of natural variation in fly fecundity identifies neuronal domain of expression and function of an aquaporin. PLoS Genet. 8, e1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi S. H., Lee G., Monahan P., Park J. H. (2008) Spatial regulation of Corazonin neuropeptide expression requires multiple cis-acting elements in Drosophila melanogaster. J. Comp. Neurol. 507, 1184–1195 [DOI] [PubMed] [Google Scholar]
- 14. Kapan N., Lushchak O. V., Luo J., Nassel D. R. (2012) Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol. Life Sci. 69, 4051–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y., Bretz C. A., Hawksworth S. A., Hirsh J., Johnson E. C. (2010) Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS One 5, e9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee G., Kim K. M., Kikuno K., Wang Z., Choi Y. J., Park J. H. (2008) Developmental regulation and functions of the expression of the neuropeptide corazonin in Drosophila melanogaster. Cell Tissue Res. 331, 659–673 [DOI] [PubMed] [Google Scholar]
- 17. Tanaka Y., Hua Y., Roller L., Tanaka S. (2002) Corazonin reduces the spinning rate in the silkworm, Bombyx mori. J. Insect Physiol. 48, 707–714 [DOI] [PubMed] [Google Scholar]
- 18. Hillyer J. F., Estévez-Lao T. Y., Funkhouser L. J., Aluoch V. A. (2012) Anopheles gambiae corazonin. Gene structure, expression and effect on mosquito heart physiology. Insect Mol. Biol. 21, 343–355 [DOI] [PubMed] [Google Scholar]
- 19. Veenstra J. A. (2009) Does corazonin signal nutritional stress in insects? Insect Biochem. Mol. Biol. 39, 755–762 [DOI] [PubMed] [Google Scholar]
- 20. Boerjan B., Verleyen P., Huybrechts J., Schoofs L., De Loof A. (2010) In search for a common denominator for the diverse functions of arthropod corazonin. A role in the physiology of stress? Gen. Comp. Endocrinol. 166, 222–233 [DOI] [PubMed] [Google Scholar]
- 21. Cazzamali G., Saxild N., Grimmelikhuijzen C. (2002) Molecular cloning and functional expression of a Drosophila corazonin receptor. Biochem. Biophys. Res. Commun. 298, 31–36 [DOI] [PubMed] [Google Scholar]
- 22. Belmont M., Cazzamali G., Williamson M., Hauser F., Grimmelikhuijzen C. J. (2006) Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem. Biophys. Res. Commun. 344, 160–165 [DOI] [PubMed] [Google Scholar]
- 23. Honeybee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443, 931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sha K., Conner W. C., Choi D. Y., Park J. H. (2012) Characterization, expression, and evolutionary aspects of corazonin neuropeptide and its receptor from the house fly, Musca domestica (Diptera: Muscidae). Gene 497, 191–199 [DOI] [PubMed] [Google Scholar]
- 25. Roller L., Yamanaka N., Watanabe K., Daubnerová I., Zitnan D., Kataoka H., Tanaka Y. (2008) The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 38, 1147–1157 [DOI] [PubMed] [Google Scholar]
- 26. Yamanaka N., Yamamoto S., Zitnan D., Watanabe K., Kawada T., Satake H., Kaneko Y., Hiruma K., Tanaka Y., Shinoda T., Kataoka H. (2008) Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS One 3, e3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan Y., Sun P., Wang Y., He X., Deng X., Chen X., Zhang G., Chen X., Zhou N. (2010) The G protein-coupled receptors in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 40, 581–591 [DOI] [PubMed] [Google Scholar]
- 28. Hansen K. K., Stafflinger E., Schneider M., Hauser F., Cazzamali G., Williamson M., Kollmann M., Schachtner J., Grimmelikhuijzen C. J. (2010) Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 285, 10736–10747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park Y., Kim Y. J., Adams M. E. (2002) Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc. Natl. Acad. Sci. U.S.A. 99, 11423–11428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roch G. J., Busby E. R., Sherwood N. M. (2011) Evolution of GnRH. Diving deeper. Gen. Comp. Endocrinol. 171, 1–16 [DOI] [PubMed] [Google Scholar]
- 31. Shi Y., Huang H., Deng X., He X., Yang J., Yang H., Shi L., Mei L., Gao J., Zhou N. (2011) Identification and functional characterization of two orphan G-protein-coupled receptors for adipokinetic hormones from silkworm Bombyx mori. J. Biol. Chem. 286, 42390–42402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu C., Huang H., Hua R., Li G., Yang D., Luo J., Zhang C., Shi L., Benovic J. L., Zhou N. (2009) Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett. 583, 1463–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li G., Deng X., Wu C., Zhou Q., Chen L., Shi Y., Huang H., Zhou N. (2011) Distinct kinetic and spatial patterns of protein kinase C (PKC)- and epidermal growth factor receptor (EGFR)-dependent activation of extracellular signal-regulated kinases 1 and 2 by human nicotinic acid receptor GPR109A. J. Biol. Chem. 286, 31199–31212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cvejic S., Zhu Z., Felice S. J., Berman Y., Huang X. Y. (2004) The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat. Cell Biol. 6, 540–546 [DOI] [PubMed] [Google Scholar]
- 35. Werry T. D., Sexton P. M., Christopoulos A. (2005) “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol. Metab. 16, 26–33 [DOI] [PubMed] [Google Scholar]
- 36. Pierce K. L., Luttrell L. M., Lefkowitz R. J. (2001) New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene 20, 1532–1539 [DOI] [PubMed] [Google Scholar]
- 37. Chen Z., Gibson T. B., Robinson F., Silvestro L., Pearson G., Xu B., Wright A., Vanderbilt C., Cobb M. H. (2001) MAP kinases. Chem. Rev. 101, 2449–2476 [DOI] [PubMed] [Google Scholar]
- 38. Shenoy S. K., Lefkowitz R. J. (2003) Trafficking patterns of β-arrestin and G protein-coupled receptors determined by the kinetics of β-arrestin deubiquitination. J. Biol. Chem. 278, 14498–14506 [DOI] [PubMed] [Google Scholar]
- 39. Maruko T., Nakahara T., Sakamoto K., Saito M., Sugimoto N., Takuwa Y., Ishii K. (2005) Involvement of the βγ subunits of G proteins in the cAMP response induced by stimulation of the histamine H1 receptor. Naunyn-Schmiedebergs Arch. Pharmacol. 372, 153–159 [DOI] [PubMed] [Google Scholar]
- 40. Yamaoka K., Yano A., Kuroiwa K., Morimoto K., Inazumi T., Hatae N., Tabata H., Segi-Nishida E., Tanaka S., Ichikawa A., Sugimoto Y. (2009) Prostaglandin EP3 receptor superactivates adenylyl cyclase via the Gq/PLC/Ca2+ pathway in a lipid raft-dependent manner. Biochem. Biophys. Res. Commun. 389, 678–682 [DOI] [PubMed] [Google Scholar]
- 41. Rubovitch V., Gafni M., Sarne Y. (2003) The μ opioid agonist DAMGO stimulates cAMP production in SK-N-SH cells through a PLC-PKC-Ca2+ pathway. Brain Res. Mol. Brain Res. 110, 261–266 [DOI] [PubMed] [Google Scholar]
- 42. Smrcka A. V. (2008) G protein βγ subunits. Central mediators of G protein-coupled receptor signaling. Cell Mol. Life Sci. 65, 2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Callaghan B., Zhong J., Keef K. D. (2006) Signaling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gβγ subunits. Am. J. Physiol. Heart Circ. Physiol. 291, H2541–2546 [DOI] [PubMed] [Google Scholar]
- 44. Viard P., Macrez N., Mironneau C., Mironneau J. (2001) Involvement of both G protein αs and βγ subunits in β-adrenergic stimulation of vascular L-type Ca2+ channels. Br. J. Pharmacol. 132, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horie K., Itoh H., Tsujimoto G. (1995) Hamster α1B-adrenergic receptor directly activates Gs in the transfected Chinese hamster ovary cells. Mol. Pharmacol. 48, 392–400 [PubMed] [Google Scholar]
- 46. Kühn B., Schmid A., Harteneck C., Gudermann T., Schultz G. (1996) G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol. Endocrinol. 10, 1697–1707 [DOI] [PubMed] [Google Scholar]
- 47. Roy S. J., Parent A., Gallant M. A., de Brum-Fernandes A. J., Stanková J., Parent J. L. (2010) Characterization of C-terminal tail determinants involved in CRTH2 receptor trafficking. Identification of a recycling motif. Eur. J. Pharmacol. 630, 10–18 [DOI] [PubMed] [Google Scholar]
- 48. Roman G., He J., Davis R. L. (2000) Kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155, 1281–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barak L. S., Ferguson S. S., Zhang J., Caron M. G. (1997) A β-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J. Biol. Chem. 272, 27497–27500 [DOI] [PubMed] [Google Scholar]
- 50. Johnson E. C., Bohn L. M., Barak L. S., Birse R. T., Nässel D. R., Caron M. G., Taghert P. H. (2003) Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-β-arrestin2 interactions. J. Biol. Chem. 278, 52172–52178 [DOI] [PubMed] [Google Scholar]
- 51. Johnson E. C., Tift F. W., McCauley A., Liu L., Roman G. (2008) Functional characterization of kurtz, a Drosophila non-visual arrestin, reveals conservation of GPCR desensitization mechanisms. Insect Biochem. Mol. Biol. 38, 1016–1022 [DOI] [PubMed] [Google Scholar]
- 52. Kaufmann C., Merzendorfer H., Gäde G. (2009) The adipokinetic hormone system in Culicinae (Diptera: Culicidae). Molecular identification and characterization of two adipokinetic hormone (AKH) precursors from Aedes aegypti and Culex pipiens and two putative AKH receptor variants from A. aegypti. Insect Biochem. Mol. Biol. 39, 770–781 [DOI] [PubMed] [Google Scholar]
- 53. Gäde G., Hoffmann K. H., Spring J. H. (1997) Hormonal regulation in insects. Facts, gaps, and future directions. Physiol. Rev. 77, 963–1032 [DOI] [PubMed] [Google Scholar]
- 54. Tawfik A. I., Tanaka S., De Loof A., Schoofs L., Baggerman G., Waelkens E., Derua R., Milner Y., Yerushalmi Y., Pener M. P. (1999) Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc. Natl. Acad. Sci. U.S.A. 96, 7083–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]