Abstract
The canonical Wnt signalling pathway induces the β-catenin/LEF transcription factors. It is activated in various cancers, most characteristically carcinomas, in which it promotes metastatic spread by increasing migration and/or invasion. The Wnt/β-catenin signalling pathway is frequently activated in melanoma, but the presence of β-catenin in the nucleus does not seem to be a sign of aggressiveness in these tumours. We found that, unlike its positive role in stimulating migration and invasion of carcinoma cells, β-catenin signalling decreased the migration of melanocytes and melanoma cell lines. In vivo, β-catenin signalling in melanoblasts reduced the migration of these cells, causing a white belly-spot phenotype. The inhibition, by β-catenin of migration was dependent on MITF-M, a key transcription factor of the melanocyte lineage, and CSK, a Src-inhibitor. Despite reducing migration, β-catenin signalling promoted lung metastasis in the Nras-driven melanoma murine model. Thus, β-catenin may play conflicting roles in the metastatic spread of melanoma, repressing migration while promoting metastasis. These results highlight that metastasis formation requires a series of successful cellular processes, any one of which may not be optimally efficient.
Keywords: Wnt, melanoblast, melanoma, MITF, CSK, mouse
Introduction
The canonical Wnt/β-catenin signalling pathway integrates cell-cell and cell-matrix cues into a well-defined transcriptional response. In particular, β-catenin is a multifunctional protein regulating cadherin-associated cell-cell adhesion and transcription mediated by DNA-binding proteins such as TCF/LEF. The Wnt/β-catenin pathway is activated in many cancers, including carcinoma and melanoma. However, the downstream outcomes are significantly different. In colorectal cancer, high rates of Wnt signalling have been shown to favour both initiation and progression, including metastatic spread, resulting in a poor prognosis (1, 2). By contrast, for melanoma, an increasingly common highly metastatic disease that is often fatal, patients with high nuclear β-catenin levels have a better prognosis (3, 4).
The different biology of β-catenin activation may be related to the opposing features of epithelial cells and melanocytes. In the classical, carcinoma-centric model, incipient cancer cells, initially locked within the epithelial architecture, first become locally invasive, then reach blood/lymph vessels for metastasis. However, unlike epithelial cells, cells of the melanocytic lineage have intrinsic “horizontal” migratory properties, a reflection of their colonization of embryonic tissues during development. Thus, for melanomas, the functional effects of β-catenin signaling need to be evaluated not only on invasion and metastasis, but also on migration. In fact, it is even possible that migration needs to be reduced in incipient melanoma cells for them to acquire “vertical” invasion and metastasis.
Beta-catenin appears to play a variety of roles in melanomagenesis, not only during initiation (increased proliferation and bypassing of senescence) but also during progression (5).. β-catenin has been shown to inhibit proliferation in murine melanoblasts and human melanoma (4, 6). However, this protein also promotes melanocyte immortalization by overcoming senescence (7). Thus, β-catenin modulates the initiation of melanoma through antagonistic effects: inhibiting proliferation, but bypassing senescence.
In vivo, β-catenin is essential for the cell-cell adhesion mediated by cadherins and is required for cell-matrix adhesion (8). In vitro, the function of β-catenin in invasion has been evaluated, which does not reflect the whole invasion in vivo process, in various human melanoma cell lines. Depending on the melanoma cell line and protocol used, β-catenin seems to be able either to induce or to repress invasion in vitro (9–11). At the molecular level, these discrepancies may best be understood by considering that MITF-M, a target of β-catenin-LEF/TCF, has several different functions, depending on its activity (12). MITF affects the actin cytoskeleton through mDia1 and Rho-GTPases, and the MT1-MMP collagenase (also known as MMP14), thereby affecting invasion (9, 12).
In vivo, the effects of β-catenin signalling on the migration of cells of the melanocyte lineage remain largely unknown (13). In vitro, the inhibition of GSK3β by LiCl has been shown to prevent the migration of neural crest cells from explanted chicken neural tubes onto fibronectin-coated dishes (14), which suggests an involvement of β-catenin; however, other cellular targets may be affected by LiCl. Finally, high levels of β-catenin/LEF1 were correlated with faster cell migration in a panel of six melanoma cell lines, although β-catenin transcriptional activity was not measured (15), which does not allow to conclude on the role of β-catenin in migration.
Little is known about the role of β-catenin in late stages of metastatic spread. These late stages require a combination of proteins responsible for ensuring survival, adhesion and extravasation at the distant site of spread, together with proliferation and angiogenesis (16). Recently, it has been shown that β-catenin on a BRAF-PTEN context was inducing metastasis formation, but the mechanism remains fully unknown (17). A homogeneous genetic background, from the mice to the cells in culture, should improve definition of the complex role of β-catenin during melanomagenesis.
We show here that high β-catenin levels inhibit the migration of cells of the melanocyte lineage (including melanoblasts, melanocytes and melanoma) both in vivo and in vitro. The inhibition of migration is associated with the induction by β-catenin of MITF-M and CSK. β-catenin signalling, despite its inhibitory effects on migration, increases metastatic potential in NRAS-driven melanoma.
Results
LiCl treatment and bcat* expression inhibits the migration of melanocytes in vitro
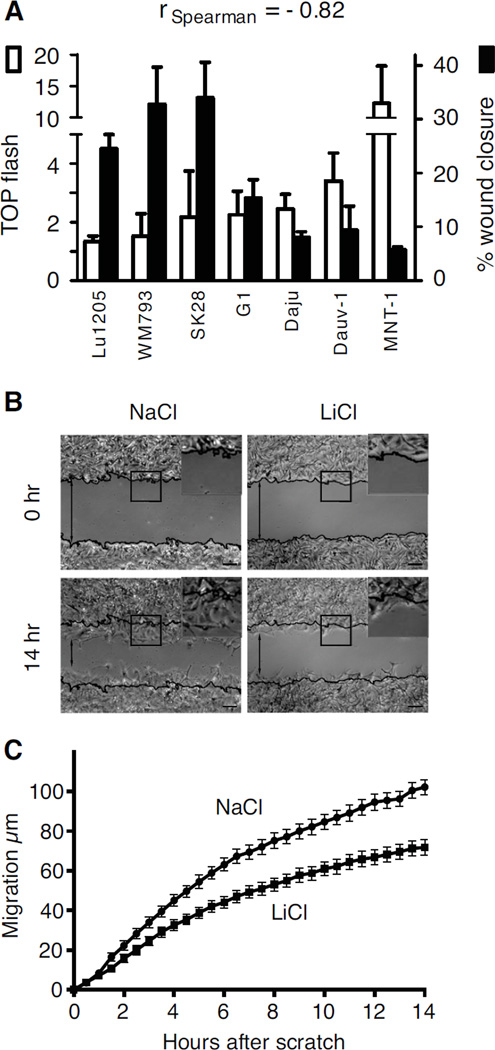
We first evaluated the strength of β-catenin signalling in a panel of human melanoma cells lines, using the TOP-flash assay. In parallel, we assessed the same panel of human melanoma cell lines for their ability to fill a wound scratch. We observed that the strength of the β-catenin signalling was inversely correlated (Spearman) with the migration of the melanoma cell lines (Fig. 1A). This correlative analysis was corroborated by pharmacological and genetic approaches. Melan-a murine melanocyte cells were treated with lithium chloride (LiCl), an inhibitor of GSK-3β, and their migration was measured in a scratch wound assay. Fourteen hours after the scratch, LiCl-treated cells had not migrated as far into the scratch wound as cells treated with sodium chloride (NaCl), used as controls (Fig. 1B). LiCl-treated cells migrated more slowly during most of the experiment and they had migrated approximately 30% less far than control cells after 14 hours (p-value <10−4) (Fig. 1C). The numbers of cells at the end of the experiment were similar for NaCl- and LiCl-treated cells (Fig. S1A), so the difference in migration was not due to a difference in cell proliferation. The inhibitory effect of LiCl increased with its concentration (Fig. S1B), but we used a low concentration of LiCl (10 mM) to ensure that cell viability was not affected. Indeed, the cell death rate was low and similar to that for NaCl-treated controls (Fig. S1C). As a control, we verified that LiCl was able to induce the Top Flash activity, revealing an induction of the Wnt signaling in melan-a cells (Fig. S1D).
Figure 1. Wnt/β-catenin signalling pathway and migration of melanocytes.
(A) The canonical β-catenin activity of a panel of human melanoma cell lines was measured in the TOP flash assay, according to (31), with each result standardised so that the FOP flash was equal to one. TOP flash activity was compared with cell migration speed in a scratch wound assay. Standard deviations are included. We calculated the Spearman correlation coefficient of −0.82 showing an inverse tendency between TOP flash activity and cell migration. The results were considered as significant since r < −0.5. All cell lines were transfected with TOP flash vector and with the pPGKB-geobpA construct as a loading control (32).
(B) Melan-a murine melanocytes were treated with 10 mM NaCl (control) or LiCl and their migration was measured in a scratch wound assay. Representative pictures of the size of the scratch wound 0 and 14 h after wounding are shown. Black lines indicate the starting positions of the cells. Inset: magnification of the outlined area. Scale bars: 50 µm.
(C) The percentage of closure for each wound was evaluated from microscopy images at 30-minute intervals. Experiments were performed three times, with six replicates in each experiment. Error bars: 95% confidence interval of the mean. At 14 hours, p-value <10−4.
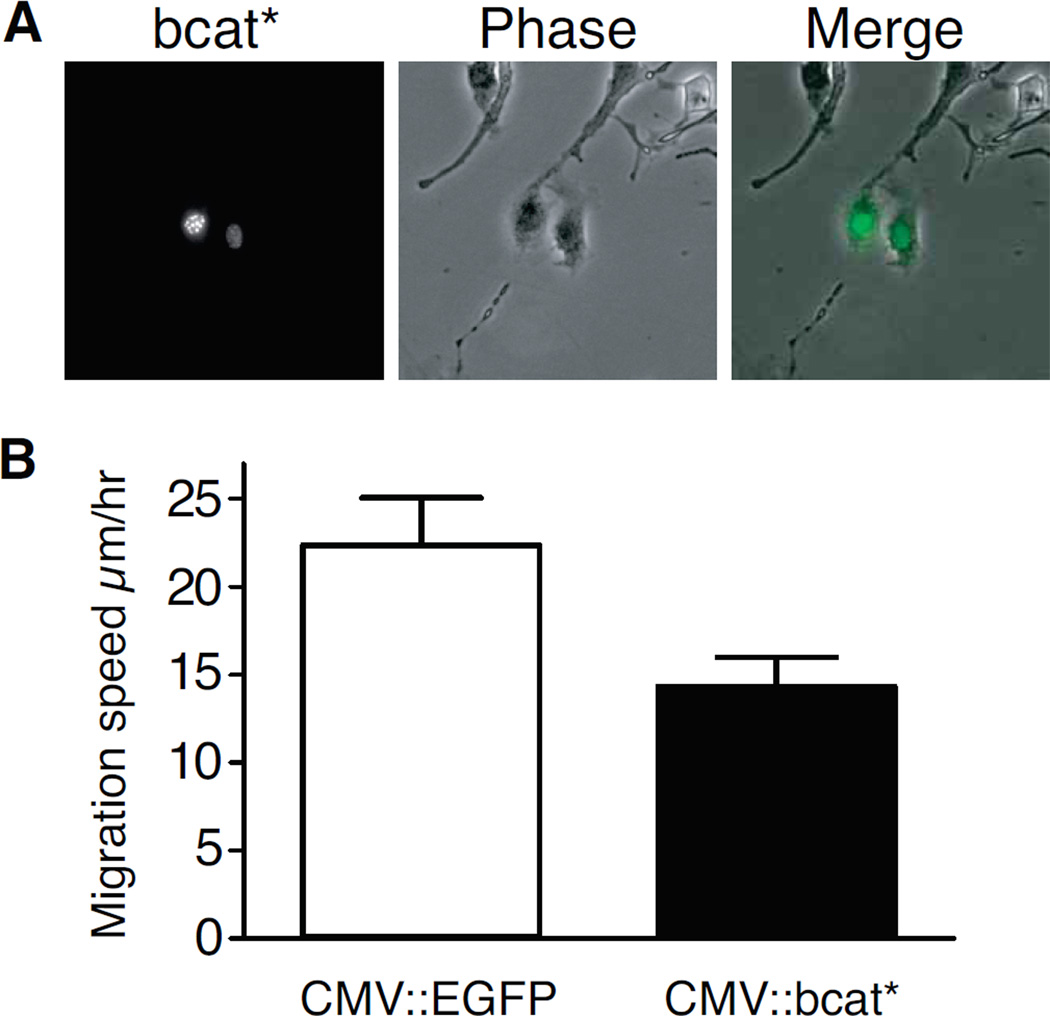
As LiCl treatment may have effects independent of canonical β-catenin signalling, we then investigated the effect of canonical β-catenin signalling specifically. We analysed the migration of melanocytes transiently transfected with CMV::bcat* or CMV::EGFP (control vector). CMV::bcat* is an expression vector encoding a β-catenin protein tagged with EGFP and stabilized by four serine/threonine (S/T) to alanine (A) mutations in its N-terminal region. These mutations prevent the phosphorylation of these residues by CK1/GSK-3β, thereby also preventing the subsequent degradation of the protein (7). This protein also has a nuclear localization signal (nls), restricting it to the nucleus. Transfected melan-a melanocytes were used to seed a six-well plate at low density, and the migration of the fluorescent, transfected cells was monitored at four-minute intervals for 12 hours. Cells transiently transfected with CMV::bcat* migrated at a rate of 14.3±1.6 µm/h, substantially slower than the 22.4±2.7 µm/h recorded for control EGFP-transfected cells (Fig. 2). We also calculated the maximum distance between any two positions occupied by each cell during the tracking time. This measurement made it possible to distinguish between cells showing persistent migratory behaviour and motile cells moving within a confined area. The transfection of melan-a cells with CMV::bcat* decreased the mean maximum distance between any two points from 65.8±8.2 µm to 41.3±6.1 µm (p-value <10−4). These findings clearly demonstrate that nuclear β-catenin signalling reduces the migration of melanocytes in vitro.
Figure 2. Nuclear β-catenin represses the migration of melan-a murine melanocytes.
(A) Live-cell microscopy of melan-a murine melanocytes transfected with a vector expressing for bcat*, an EGFP-tagged β-catenin molecule targeted to the nucleus by an NLS and resistant to CK1/GSK-3β phosphorylation due to four mutations of its N terminal serine/threonine residues (at positions 33, 37, 41, and 45).
(B) bcat* transfection reduced the random cell migration of melan-a cells plated at low density. Error bars: 95% confidence interval of the mean, p-value =1×10−3.
bcat* inhibits the migration of melanoblasts in vivo
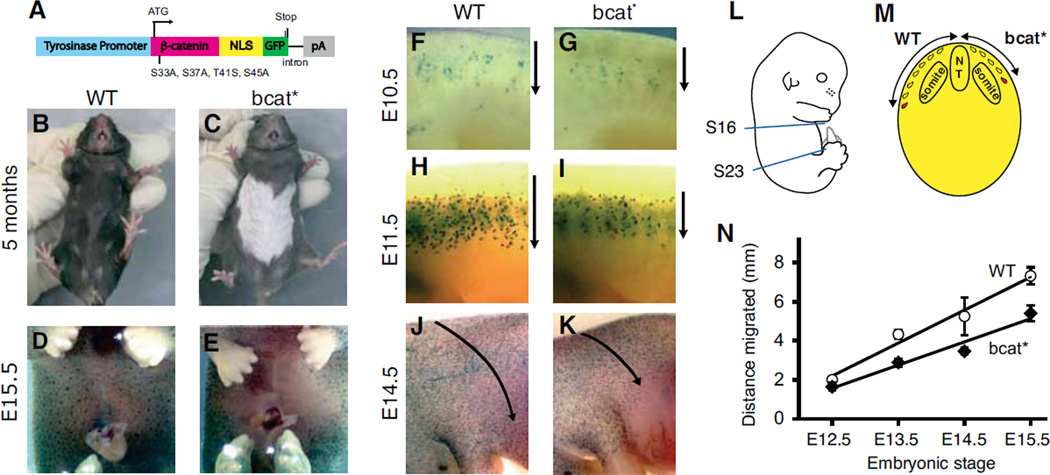
We then assessed the effect of canonical β-catenin signalling on the migration of melanoblasts in vivo. Dermal melanoblasts migrate long distances after emerging from the neural tube at around embryonic day (E) 9.0, allowing them to colonise most areas of the skin. In the trunk region of embryos, melanoblasts migrate dorsolaterally from the neural tube to reach the most ventral part. Consequently, mutations reducing melanoblast migration often result in a “white belly-spot” phenotype. We investigated bcat* mice, which produce the stabilized, nuclear β-catenin described above under the control of a tyrosinase promoter, restricting the production of this protein to the melanocyte lineage (Fig. 3A) (7). bcat* mice had a white belly spot (Fig. 3B and C) that covered approximately 28±8% of the belly area between the four limbs. The white belly spot phenotype was fully penetrant and not due to a mouse founder effect: all bcat* mouse lines developed from different founders had a white belly spot, as did a mouse line developed with a different bcat* construct that did not contain a NLS (Fig. S2A–F).
Figure 3. Activated β-catenin expression in the melanocyte lineage in vivo impairs the dorsolateral migration of melanoblasts and results in a white belly phenotype.
(A) Map of the Tyr:: β-cat-mut-nls-egfp (bcat*) transgene.
(B, C) White belly patch in a 5-month old bcat* mouse and a WT mouse.
(D, E) X-gal staining revealing the absence of ventral melanoblasts at embryonic stage E15.5 in bcat* mice. The embryos contain the Dct-LacZ transgene restricting lacZ expression to the melanoblasts.
(F–K) Whole-mount photographs of X-gal-stained melanoblasts in the flank region of WT-LacZ and bcat*-LacZ mouse embryos at stages E10.5, E11.5 and E14.5. Arrows indicate the direction of melanoblast migration.
(L) Embryos between embryonic stages E12.5 and E15.5 were transversely sectioned through the trunk region, between somites 16 and 23, and the distance migrated by the most advanced melanoblast from the dorsal tip was measured (M) and plotted against time (N). Error bars: standard deviation. Linear regression, difference in slope p-value: 0.037
In order to confirm that the white belly spot was caused by a lack of melanocytes in the affected area (as opposed to a lack of tyrosinase or other essential melanogenesis proteins, or a failure of melanin transport in melanocytes, or melanin transfer from melanocytes to surrounding keratinocytes), we tested for the presence of melanoblasts/melanocytes in the ventral belly region of bcat* embryos and mice. We used Dct::LacZ mice, in which X-gal staining can be used to identify the melanocyte lineage (18). bcat*/° mice were crossed with Dct::LacZ homozygous mice to produce bcat*/°; Dct::LacZ/° and WT; Dct::LacZ/° littermates, henceforth referred to as bcat*-LacZ and WT-LacZ, respectively. bcat*-LacZ embryos at stage E15.5 had no melanoblasts in the ventral belly region, whereas this area was already colonised by melanoblasts at this stage in WT-LacZ embryos (Fig. 3D,E). Histological analyses also showed that melanocytes were absent from the white belly region of bcat* mice at postnatal day (p) 8 (data not shown), further confirming that the white belly spot is caused by a lack of melanocytes, rather than an inability to produce or transfer melanin. Although fully penetrant, the white belly spot in bcat* mice varied in size and shape (Fig. S2A–C). This variation could be attributed to natural variation between mice, with the extent of melanoblast colonization of the ventral belly area at E16.5 varying between individual bcat*-LacZ embryos (Fig. S2G–J). Such variation was also observed in WT-LacZ embryos (Fig. S2K–N).
We then investigated the effect of bcat* on the distance melanoblasts had migrated at different stages of embryogenesis. The migration of melanoblasts from the neural tube commences around E9.0 and is completed at around E16.0. At E10.5, melanoblasts have only recently exited the migration staging area and the bcat* transgene has just started to be expressed from the tyrosinase promoter (commencing around E10, (19)). At E10.5, the advancing front of melanoblasts in bcat*-LacZ embryos was only slightly behind that of WT-LacZ embryos (Fig. 3F,G). At E13.5, melanoblasts in WT-LacZ embryos had migrated further along the dorsolateral pathway than in bcat*-LacZ embryos, and this difference was even larger at E14.5: in embryos expressing bcat* the melanoblasts had not colonised the belly region, by contrast to what was observed for WT-LacZ embryos (Fig. 3F–N). We measured the distance covered by migrating melanoblasts in bcat* and WT embryos, on transverse sections cut from X-gal-stained WT-LacZ and bcat*-LacZ embryos between stages E12.5 and E15.5. The distance from the dorsal tip of the embryo to the most advanced melanoblast was measured in the region between somites 16 and 23 (Fig. 3L,M). The distance migrated by the most advanced melanoblast increased roughly linearly with time in both mouse types. At E15.5, the most advanced melanoblast was 7.3 mm from the dorsal tip of the embryo in WT-LacZ embryos, but only 5.4 mm (26% less) from the dorsal tip in bcat*-LacZ embryos (Fig. 3N). This corresponds to an apparent average migration speed of 1.2 mm/day for bcat* mice and 1.7 mm/day in WT C57BL/6 mice for melanoblasts between E12.5 and E15.5 (p-value = 0.037). These data strongly suggest that canonical β-catenin signalling reduces the migration of melanoblasts in vivo, causing a decrease in migration soon after the initiation of signalling that is maintained during embryogenesis.
Melanocyte cell lines established from bcat* mice migrate more slowly in vitro
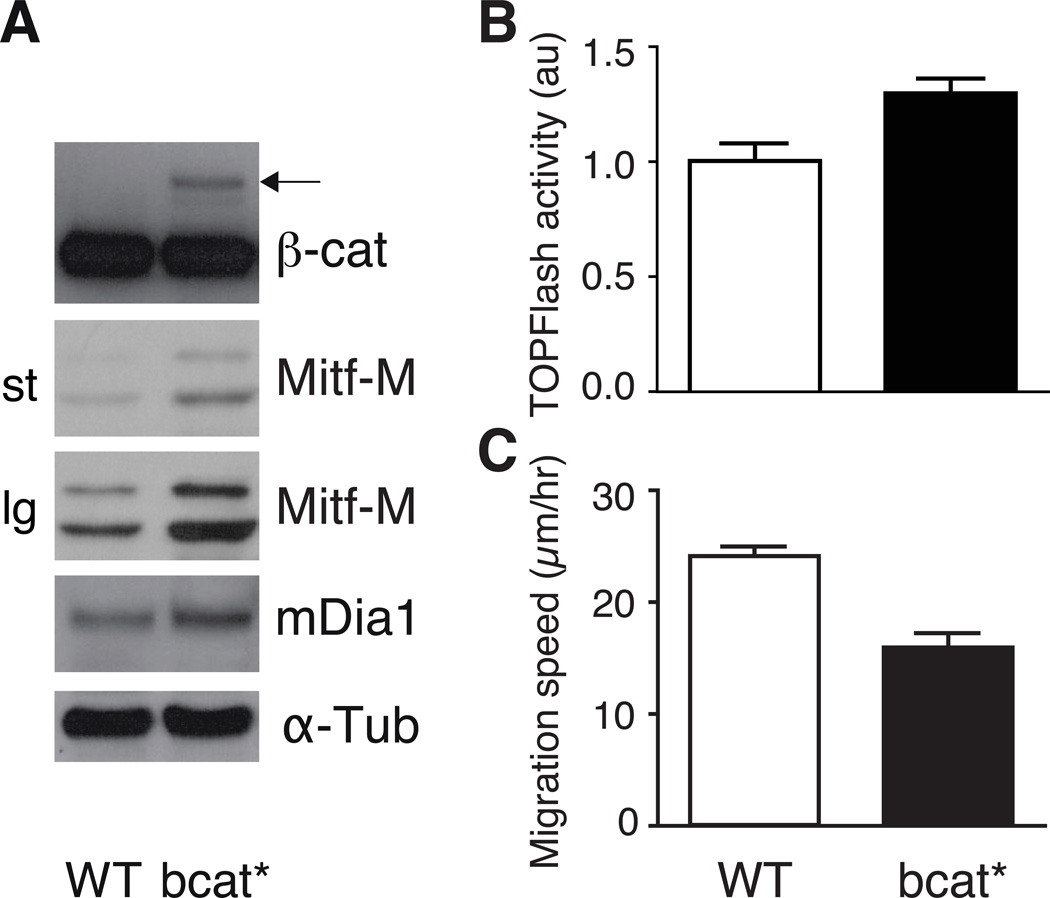
Melanoblasts migrate slower in bcat* mice than in wild-type controls. We then investigated the possible impairment of migration when the melanoblasts had differentiated into melanocytes. As melanocytes in vivo display limited migration that is difficult to detect, we investigated immortalized melanocyte cell lines established in our laboratory from neonates of WT and bcat* littermates. This also made it possible to carry out a preliminary investigation of the potential targets interacting with β-catenin in the reduction of migration. For example, β-catenin signals through MITF-M, which alters the cell cytoskeleton by activating mDia1 expression, stimulating peripheral actin assembly and decreasing cell movement (12). The bcat* and WT melanocyte cell lines established from mutant and WT littermate neonates have been described before (7). As expected, both mRNAs and proteins for MITF-M and mDia1 were less abundant in WT than in bcat* melanocyte cell lines (Fig. 4A, Fig. S3). The TOP flash activity in WT melanocytes indicates that beta-catenin is transcriptionally active. Such TOP flash activity is slightly but significantly induced in bcat* melanocytes (Fig. 4B). As seen in vivo, WT melanocytes migrated faster than bcat* melanocytes in a random migration assay (Fig. 4C).
Figure 4. Immortalised melanocyte cell lines established from bcat* neonates contain higher levels of MITF-M and mDia1 and migrate more slowly than WT melanocytes.
(A) Western blot analysis of β-catenin, MITF-M and mDia1 proteins in WT and bcat* melanocyte cell lines. st and lg indicate that the exposure time are short and long, respectively. The arrow indicates bcat* protein, which is larger than the endogenous β-catenin (darker bands lower down the blot) due to the NLS and EGFP tags. •-tubulin: loading control.
(B) WT and bcat* melanocyte cell lines were transfected with TOP flash vector and with the pPGKB-geobpA construct as a loading control (32). p-value = 0.002.
(C) The mean migration velocity of the WT and bcat* cell lines was calculated from cells grown at low-density and tracked by live imaging, at 4-minute intervals, over 12 hours. Experiments were performed 3 times, with at least 30 cells tracked per experiment. Error bars: 95% confidence interval of the mean. p-value <10−4.
β-catenin signals through MITF-M to reduce migration
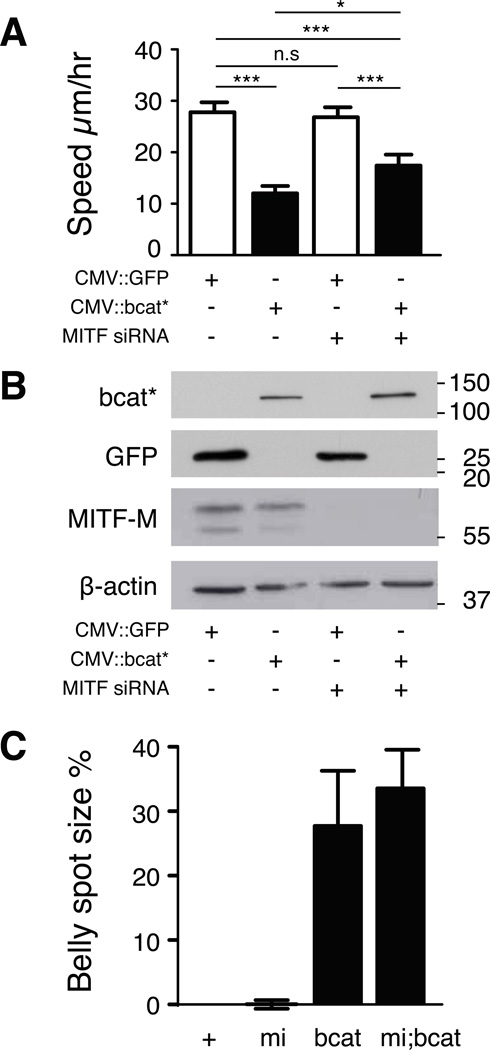
We then investigated the contribution of MITF-M to reducing migration in response to bcat* signalling in human melanoma cells. For these experiments, we used the human Mull melanoma cell line, which expresses β-catenin weakly and MITF-M moderately. As expected, transient bcat* expression reduced the random migration of these cells from 27.8±1.9 to 12.0±1.4 µm/h (Fig. 5A). When MITF-M levels were decreased by an siRNA, the cells migrated at about the same speed as control siRNA-treated cells (26.8±1.9 µm/h). The expression of bcat* reduced the migration speed of Mull cells in which MITF-M was eliminated by siRNA to 17.4±2.2 µm/h, a smaller decrease (−35%) than that for cells with normal levels of MITF-M (−57%). Western blotting confirmed that the siRNA treatment eliminated MITF-M protein and that bcat* was produced (Fig. 5B). As only 5 to 10% of Mull cells transfected with CMV::bcat* produced the protein, we did not expect the effect of bcat* on MITF-M level to be detectable on western blotting. As expected, we revealed by immunofluorescence that bcat* induces MITF (Fig. S4). From these experiments we concluded that although β-catenin signals through MITF-M to reduce the migration of melanoma cells, there must be other targets independent of MITF-M. We investigated this possibility in vivo, by crossing bcat* mice with mice of the MITFvga9/+ genotype. MITFvga9/+ mice have one defective MITF allele, and have either no belly spot, or in rare cases, a small white belly spot. Crossing bcat* mice onto a MITFvga9/+ background resulted in mice with white belly spots of the same size as those in bcat* mice (Fig. 5C). This result suggests that the reduction of melanoblast migration by β-catenin during embryogenesis does not result solely from an increase in MITF-M levels and that there are, therefore, other, MITF-M-independent targets.
Figure 5. bcat* has a partial requirement for MITF-M to inhibit migration.
(A) The random migration speed of Mull human melanoma cells transfected with MITF-M or control siRNA, and a vector expressing the EGFP-tagged bcat* or EGFP, was measured by manually tracking transfected cells plated at low density. Asterisks above lines indicate a significant difference between treatments (Tukey test). Experiments were performed three times, with at least 80 cells tracked per treatment.
(B) The abundance of MITF-M and bcat* proteins in the cells tracked in panel A was assessed by western blotting. bcat* and GFP was detected with an antibody against GFP. We did not expect the effects of bcat* expression on MITF-M levels to be detectable on western blots due to the low percentage of transfected cells expressing the bcat* vector.
(C) The size of the white belly spot in WT (+), MITFvga9/+ (=mi), bcat* (=bcat), and MITFvga9/+; bcat* (=mi;bcat) mice was measured and is expressed as a percentage of the belly area between the four limbs. Error bars: standard deviation.
β-catenin signals through CSK to reduce migration
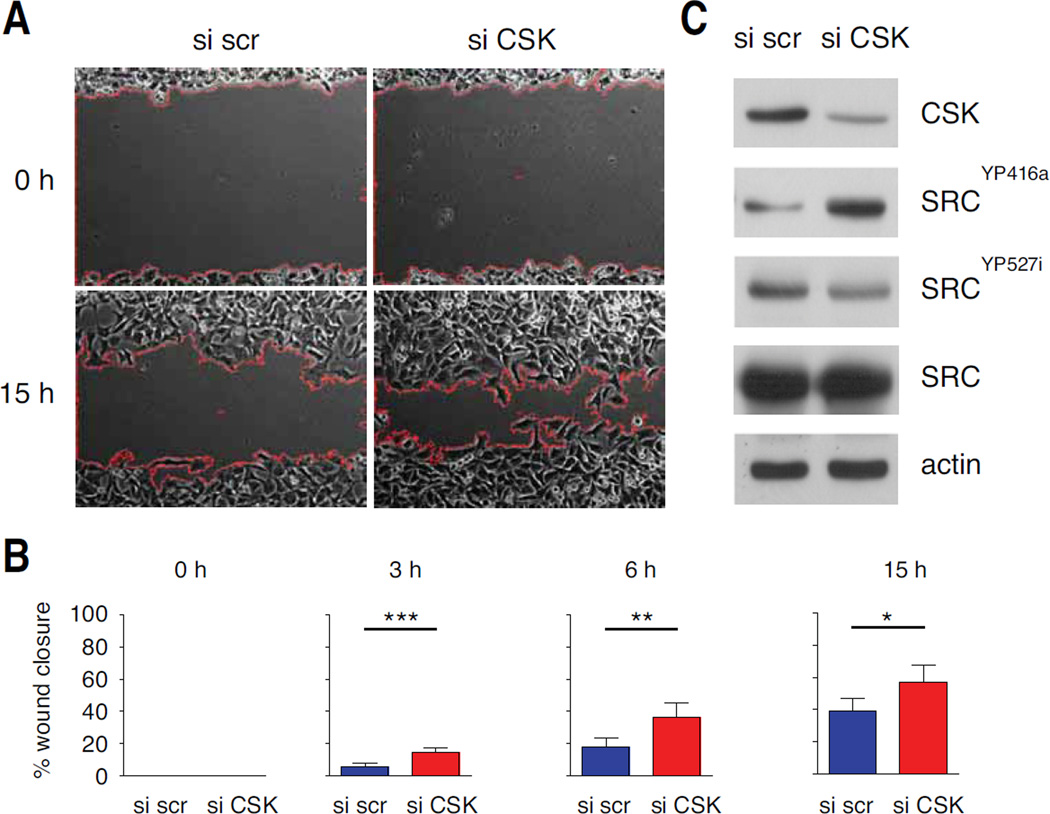
An in silico approach was performed to generate a list of potential β-catenin targets involved in the migration of the melanocyte lineage. First, analysis of a published genome-wide analysis of β-catenin promoter occupancy in human HCT116 colon cancer cells revealed 988 regulated target genes (20). From these 988 target genes an Ingenuity Pathway Analysis (IPA) was performed. Functional IPA terms for "cell movement", "cell-to-cell interaction" and "angiogenesis" were chosen to generate a list of 211 β-catenin target genes. Second, we searched for genes differentially regulated between melanoma cell lines identified as having “proliferative" and "invasive” signatures (21). Expression data from six laboratories were analysed with the online tool developed by Hoek (http://www.jurmo.ch/php/genehunter.html). Six genes (BCL2, SHC1, NF1, CSK, ADAM9 and NRG2) were differentially expressed between the “proliferative” and “invasive” groups in at least two data sets from the six laboratories. These six genes are β-catenin targets, known to be involved in cell movement, cell-to-cell interaction or angiogenesis, and associated with “proliferative" and "invasive” signatures in melanoma cell lines (Fig. S5). We correlated the level of expression of the six genes, estimated by q-RT-PCR, in a panel of seven human melanoma cells lines, with the TOP flash activity of the cells (Fig. S6) and with their potential to close a wound (Fig. S7). With a correlation cut-off of r > +0.5 or r < −0.5, it appears that the expression of CSK, SHC1, and NF1 are correlated/anti-correlated with the activity of β-catenin. With a correlation cut-off of r > +0.5 or r < −0.5, it appears that the expression of CSK, NF1 and ADAM9 are correlated/anti-correlated with the potential of the cells to close the wound. We focused on CSK, a negative regulator of SRC, a key kinase regulating cell migration (22). As expected, β-catenin was able to induce the level of CSK protein (data not shown). We evaluated the ability of CSK to affect migration of human melanoma cells. Wound scratch analysis showed that in the presence of siRNA directed against CSK (siCSK), melanoma cells were able to close the wound faster than in the presence of control siRNA (Fig. 6A,B). In the presence of siCSK, the level of SRC phosphorylated at the level of tyrosine 527 is less abundant (Fig. 6C). Given the role of SRC in migration, the reduction of this phosphorylated form may explain the induction of migration.
Figure 6. Reduction of CSK in human melanoma cell lines induces wound closure.
(A) Dauv-1 human melanoma cells were transfected with 100 nM siRNA scramble (si scr) or 100 nM siRNA directed against CSK (si CSK) and their migration was measured in a scratch wound assay. Representative pictures of the size of the scratch wound 0 and 15 h after wounding are shown. Red lines indicate the front of migration of the cells.
(B) The percentage of closure for each wound was evaluated from microscopy images at 30-minute intervals. Experiments were performed three times, with six replicates in each experiment. Error bars: 95% confidence interval of the mean. p-values were estimated at different times: p-value at 3 hours = 10−4, p-value at 6 hours = 2 10−3 and p-value at 15 hours = 10−2.
(C) The abundance of CSK, SRC and its phosphorylated forms in the cells tracked in panel A was assessed by western blotting. The phosphorylation of tyrosine 416 of Src (SRCYP416a) upregulates its kinase activity whereas the phosphorylation of tyrosine 527 of Src (SRCYP527i) renders the enzyme less active. Actin was as loading control.
β-catenin signalling may increase the metastatic potential of melanoma
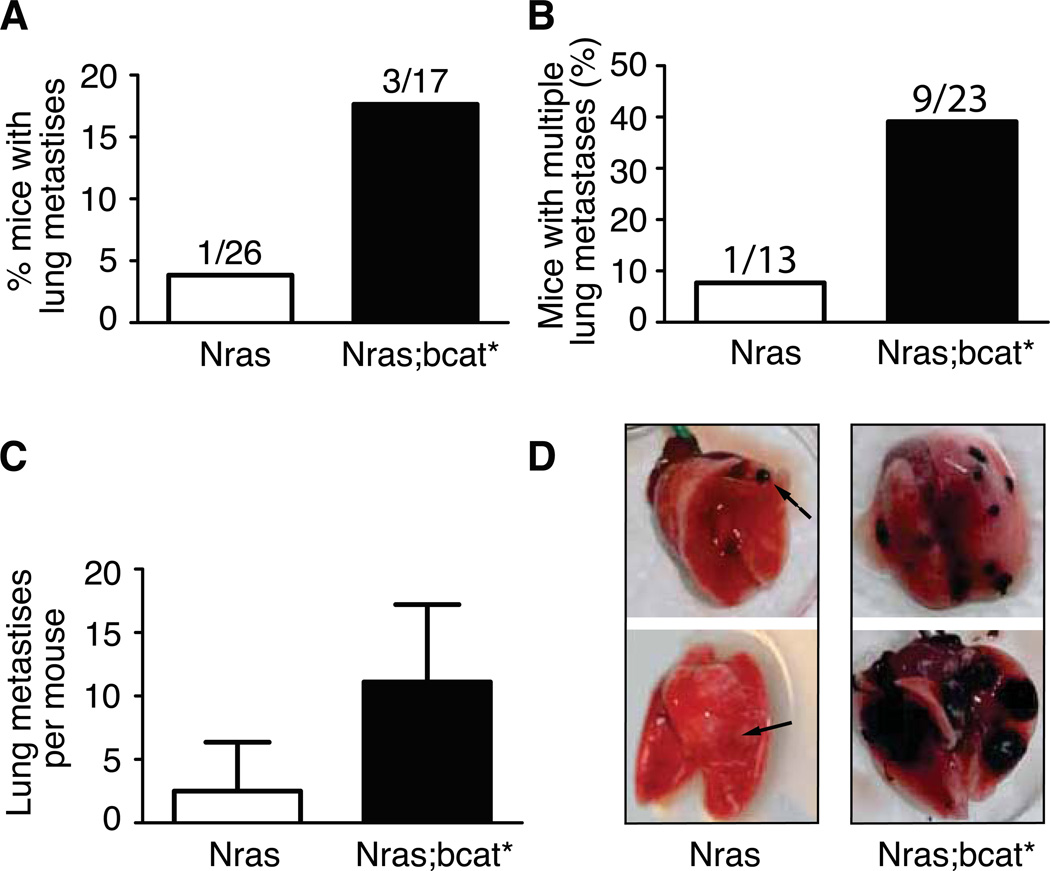
We also investigated the impact of canonical β-catenin signalling on the metastatic potential of murine melanoma in vivo, by crossing bcat* mice onto a Tyr::NrasQ61K background. Mice containing the Tyr::NrasQ61K transgene produce oncogenic, activated NRAS in the melanocyte lineage, which is known to induce proliferation, and develop melanoma after a long lag period, with moderate penetrance (23). We crossed bcat* mice onto a Tyr::NrasQ61K genotype and the resulting Tyr::NrasQ61K/° ; bcat*/° (=Nras;bcat*) mice developed melanoma with a higher rate of penetrance and shorter latency than Tyr::NrasQ61K/° (=Nras) mice (7). However, these mice had a white belly spot, suggesting that the migration of melanoblasts is still impaired, even on an Nras background (Fig. S8). Primary melanomas in both Nras and Nras;bcat* mice were allowed to grow until the mouse was moribund. An autopsy was then performed, searching for metastases. Against expectation, a higher proportion of Nras;bcat* mice (3/17; 18%) than Nras mice (1/26; 4%) developed distant metastases (Fig. 7A), suggesting that bcat* promotes lung metastasis in Nras mice.
Figure 7. bcat* increases the metastatic potential of murine melanoma.
(A) The lungs of the Nras and Nras;bcat* mice that developed primary melanoma were investigated for distant metastases. (p-value = 0.28, Fisher’s exact test).
(B) The number of nude mice with multiple lung tumours was scored after the injection of 2.5 × 105 cells from Nras or Nras;bcat* tumours into the tail vein. χ2 = 0.043.
(C) Mean number of lung metastases per nude mouse presenting with lung tumours after the injection of Nras or Nras;bcat* melanoma cells into the tail vein (p-value = 0.024).
(D) Lungs from nude mice, following the injection of cells from Nras or Nras;bcat* tumours into the tail vein. Arrows show melanoma tumours. The lungs shown were removed from mice about 100 days after the injection of Nras or Nras;bcat* cells. OCT was injected into the lungs to facilitate the visualisation and preservation of lung tissue.
Thus, it appears that β-catenin signalling inhibits the initial stages of metastatic spread by reducing cell migration, but facilitates later stages of metastatic spread by promoting survival/proliferation, implantation and/or angiogenesis. We investigated late melanoma progression, by injecting cells derived from Nras or Nras-bcat* primary melanoma tumours into the tail-vein of immuno-deficient mice and evaluating the ability of these cells to form multiple lung metastases. As the melanoma cells were injected directly into the bloodstream, the initial migration and invasion steps were bypassed, and the lung was the primary site at which the injected cells formed tumours. Multiple lung metastases were observed in 8% (1/13) of mice injected with Nras cells and 39% (9/23) of mice injected with Nras-bcat* cells (Fig. 7B). The mean number of tumours in mice developing lung metastases was also higher in mice injected with Nras-bcat* than in mice receiving Nras tumour cells (12 vs. 3, p-value = 0.024) (Fig. 7C,D). These findings suggest that bcat* signalling favours certain aspects of the process of metastatic spread.
Discussion
In colorectal, liver, lung and breast cancers, β-catenin promotes migration, invasion and proliferation. It was therefore not unreasonable to expect β-catenin to act similarly in the melanocyte lineage. However, it has now been clearly demonstrated that β-catenin signalling does not fully mimic all these processes in the melanocyte lineage. Instead, in this lineage β-catenin has been shown to alter invasion in vitro (9–11) and proliferation (4, 7, 9); here we show, additionally, that β-catenin also inhibits migration.
The negative effect of beta-catenin on migration is important when seen in the context of the melanocytic lineage because it highlights critical differences in the biology of epithelial cells and melanocytes. In fact, epithelial cells are normally locked-in in the organized architecture of the epithelium and cell-cell and cell-matrix adhesion are mediated by β-catenin’s structural role; thus, incipient carcinoma cells in addition to increased proliferation/reduced apoptosis must acquire properties of local invasiveness and increased migration in order to reach lymphatic and blood vessels for distant metastasis; β-catenin activation stimulates both properties in carcinoma cells. To the contrary, cells of the melanocytic lineage are not organized in a multicellular architecture requiring the structural function of β-catenin and in fact have intrinsic migratory properties from early development during colonization of the skin; here we show that β-catenin activation remarkably reduces migration in cells of the melanocytic lineage (from melanoblasts to melanocytes to melanomas). Despite the ambiguous behaviour of β-catenin in migration and invasion of melanocytic lineages, we found it surprizing that activation of β-catenin seems to result in increased metastatic potential in both epithelial and melanocytic lineages. In our homogeneous NRAS;bcat* model, a possible explanation for increased metastatic potential compared with NRAS model would depend on a combination of various cellular mechanisms including migration, matrix degradation and proliferation, but also survival, adhesion (cell-cell and cell-matrix) and angiogenesis.
Since melanomas may have an intrinsic metastatic potential, it is possible that the environmental context of the primary melanoma may determine whether β-catenin signalling drives or suppresses metastatic spread. The spread of tumours surrounded by extensive matrix and cellular barriers may be impeded by β-catenin signalling, through a decrease in migration and invasion. Conversely, the pro-survival signals, cell adhesion and angiogenesis signals induced by β-catenin may favour tumour spread for tumours located close to lymphatic or vascular systems, which do not require such high levels of invasiveness for the initiation of metastatic spread. This is certainly consistent with our data showing that Nras;bcat* tumours are more aggressive than controls when injected into the tail-vein of Nude mice. In this experimental model, the initial requirements for migration and invasion are bypassed and cell survival, attachment and growth at distant sites are the primary determinants of aggressive behaviour. In the future, β-catenin specific targets involved in these three processes will have to be identified and validated with appropriate biological tests, including mouse models.
Differences in the biology of β-catenin in epithelial cells and melanocytes and, in particular, the dual nature of β-catenin signalling in melanoma, may eventually reflect in the different significance of β-catenin function and activation in the natural history and progression of carcinomas and melanomas. While β-catenin activation in carcinomas is invariably associated with poor prognosis (1, 2), the relationship among β-catenin signalling, disease progression and patient survival is more complex in melanoma. Several studies have shown that nuclear β-catenin levels decrease as melanoma progresses, this decrease being associated with a better prognosis (3, 24–26). Conversely, other studies have reported that β-catenin increases migration, proliferation and survival and that nuclear β-catenin is found in one third of metastatic melanomas (10, 15, 26, 27). This work highlights the pro- and anti-metastatic potential of β-catenin. Here, using the same and homogeneous genetic context, we showed that β-catenin can inhibit proliferation and migration, but that this protein also induce immortalization and metastasis during melanomagenesis. β-catenin thus appears to be an essential modulator during melanomagenesis.
The opposite patterns of behaviour observed for β-catenin signalling in the melanocyte lineage and in carcinoma may be accounted for in part by MITF-M signalling, which is absent from other cell lineages. MITF-M has been shown to play a critical role in the reduction of melanoma invasiveness by β-catenin and in colon cancer cell lines in which MITF-M is produced ectopically (9). We show here that the inhibition of migration by β-catenin signalling displays only partial dependence on MITF-M. The mechanism by which MITF-M inhibits melanoma cell migration and invasiveness has not been fully elucidated, but certainly involves some MITF-M target-genes (9, 12). Moreover, it has been shown that β-catenin and MITF-M interact and that the expression of MITF-M and β-catenin target genes can be affected by this protein-protein interaction (6, 28).
We showed that CSK, as a β-catenin target and an inhibitor of Src, (known to induce migration), can induce melanoma cell migration when it is repressed. This finding is certainly of importance, it shows that the induction of the Wnt pathway may repress migration through at least two proteins, which are M-Mitf and CSK. M-Mitf is certainly not a simple protein to target, because, depending on its activity its function is capricious (12). Being a kinase, CSK is certainly targetable and theoretically can be repressed or induced. Using “Scratch wound assay automatic analysis" macros that we developed (see materials and methods), it would be very easy to screen a large amount of chemicals for their ability to affect migration. Such potential activator/repressor of CSK would affect cell migration and in consequence melanoma progression.
In summary, we show here that, opposite to its role in epithelial lineages and carcinomas, β-catenin signalling may inhibit the migration of all cells of the melanocyte lineage: melanoblasts, melanocytes and melanoma cells. However, much like carcinomas, we clearly show that β-catenin activation promotes metastasis of melanomas in vivo. Targeting the β-catenin pathway is an attractive approach for the treatment of melanoma, and chemical inhibitors and activators of this pathway have already been identified. However, β-catenin signalling may have both positive and negative effects on melanomas, depending on cell context, so careful consideration is required when contemplating the use of such treatments.
Materials and Methods
Cell lines and migration assays
WT (9v and melan-a) and bcat* (10d) melanocyte cells lines were previously described (7, 29).
Confluent cells were wounded by scratching with a 200 µL yellow pipette tip and medium replaced. Cells were imaged every 30 minutes for 14 hours, and the distance migrated was evaluated by measuring the size of the wound with ImageJ software, using the "Scratch wound assay automatic analysis" macros from http://imagejdocu.tudor.lu/doku.php?id=plugin:analysis:scratch_wound_assay_automatic_analysis_macro:start. Cell viability and numbers were assessed by trypan blue staining and counting in a haemocytometer.
Exponentially growing cells were seeded at a density of 3.5×104 in a six-well plate. After 20 hours of incubation, the medium was replaced by TPA-free medium. The cells were incubated for a further five hours and then imaged every 4 minutes for 12 hours. The nucleus of each cell was manually tracked with the Manual Tracking plugin for ImageJ. Experiments were performed three times, with at least 80 cells tracked for each treatment.
About 3×105 cells were transfected with 2 µg CMV::bcat*, CMV::EGFP, 40 nM siRNA against MITF (100 nM siRNA against CSK) or non-targeting siRNA pool from Dharmacon. The following day, cells were released from the plates by trypsin digestion and used to seed fresh six-well plates, at a density of 1×105 cells per well. The cells were allowed to adhere for 16–20 hours and imaged. Experiments were performed three times, with at least 80 cells tracked for each treatment.
Transgenic mice and tail-vein injections
Mice were previously described (7, 23). All animals were housed in specific pathogen-free conditions at Institut Curie, in accordance with French and European Union law.
Tail-vein injection experiments were performed on nu/nu mice obtained as described elsewhere (30). Small pieces (8 mm3) of primary tumours from Nras or (Nras;bcat*) mice were initially implanted subcutaneously into the neck-pad of a nu/nu mouse. When the implanted tumour had attained a volume of about 1 cm3, it was removed and cells were dissociated. We then injected 2.5×105 viable (trypan blue-negative) into the tail-vein of nu/nu mice and followed the mice for 80–150 days. The mice were then killed humanely and an autopsy was carried out, with investigation of the lungs for tumours.
Staining of embryonic melanoblasts
Melanoblasts were visualized and located as previously described (6, 18). At least 20 sections, evenly spaced between somites 16 and 23, were viewed for each embryo, and the distance migrated by the most advanced melanoblast was measured from the dorsal tip of the embryo. Several embryos (from 3 to 5) were analysed for each genotype and embryonic stage from E12.5 to E15.5. The area of the white belly spot of adult mice was calculated as a ratio of spot area to total belly area between the four limbs, and is expressed as a percentage. ImageJ software was used to measure the sizes of the belly and white belly spot.
Real-time RT-PCR, western blotting and antibodies
Total RNA was extracted and real-time RT-PCR carried out as previously described (30). The primer sequences are given in Sup. Table 1.
Western blots were performed as previously described (7). The antibodies used were specific for β-actin (AC-15, Sigma), β-catenin (ab6302, Abcam), mDia-1 (clone 51, BD Transduction Labs), MITF-M (C5, Abcam), GFP (ab290, Abcam), •-tubulin (DM1A, Sigma), phospho-Src (Tyr527) (2105, Cell Signaling), phosphor-Src (Tyr416) (2101, Cell Signaling), Src (2101, Cell Signaling) and CSK (HPA028425, Sigma)
Supplementary Material
Acknowledgements
We would like to thank D. Bennett, M. Herlyn and F. Beermann for kindly providing materials. We thank staff from the animal colony and imaging facilities of Curie, including Y. Bourgeois, F. Cordelières and H. Harmange in particular. SG was supported by LNCC and Curie. This work was supported by the Ligue Nationale Contre le Cancer (Equipe labellisée) and INCa.
Footnotes
Conflict of interest
We do not have any conflict of interest to declare
References
- 1.Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, et al. Prognostic significance of the wnt signalling pathway molecules APCbeta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. [Research Support, Non-U.S. Gov't] 2007 Mar;50(4):453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 2.Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. [Research Support, Non-U.S. Gov't] 2004 Apr 15;10(8):2790–2796. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 4.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jan 27;106(4):1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larue L, Beermann F. Cutaneous melanoma in genetically modified animals Pigment. Cell Res. 2007;20:485–497. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 6.Luciani F, Champeval D, Herbette A, Denat L, Aylaj B, Martinozzi S, et al. Biological and mathematical modeling of melanocyte development. Development. 2011 Sep;138(18):3943–3954. doi: 10.1242/dev.067447. [DOI] [PubMed] [Google Scholar]
- 7.Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes & development. 2007 Nov 15;21(22):2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. [Research Support, Non-U.S. Gov't] 1995 Nov;121(11):3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 9.Arozarena I, Bischof H, Gilby D, Belloni B, Dummer R, Wellbrock C. In melanoma, beta-catenin is a suppressor of invasion. Oncogene. 2011 May 16; doi: 10.1038/onc.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, et al. beta-Catenin Signaling Increases during Melanoma Progression and Promotes Tumor Cell Survival and Chemoresistance. PLoS One. 2011;6(8):e23429. doi: 10.1371/journal.pone.0023429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichhoff OM, Weeraratna A, Zipser MC, Denat L, Widmer DS, Xu M, et al. Differential LEF1 and TCF4 expression is involved in melanoma cell phenotype switching. Pigment Cell Melanoma Res. 2011 May 20; doi: 10.1111/j.1755-148X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes & development. 2006 Dec 15;20(24):3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 14.de Melker AA, Desban N, Duband JL. Cellular localization and signaling activity of beta-catenin in migrating neural crest cells. Dev Dyn. 2004 Aug;230(4):708–726. doi: 10.1002/dvdy.20091. [DOI] [PubMed] [Google Scholar]
- 15.Murakami T, Toda S, Fujimoto M, Ohtsuki M, Byers HR, Etoh T, et al. Constitutive activation of Wnt/beta-catenin signaling pathway in migration-active melanoma cells: role of LEF-1 in melanoma with increased metastatic potential. Biochem Biophys Res Commun. 2001 Oct 19;288(1):8–15. doi: 10.1006/bbrc.2001.5719. [DOI] [PubMed] [Google Scholar]
- 16.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta histochemica. [Review] 2010;112(1):3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, et al. beta-Catenin Signaling Controls Metastasis in Braf-Activated Pten-Deficient Melanomas. Cancer Cell. 2011 Dec 13;20(6):741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Developmental biology. [Research Support, Non-U.S. Gov't] 1997 Dec 1;192(1):99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 19.Delmas V, Martinozzi S, Bourgeois Y, Holzenberger M, Larue L. Cre-mediated recombination in the skin melanocyte lineage. Genesis. 2003 Jun;36(2):73–80. doi: 10.1002/gene.10197. [DOI] [PubMed] [Google Scholar]
- 20.Bottomly D, Kyler SL, McWeeney SK, Yochum GS. Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic acids research. [Research Support, N.I.H., Extramural] 2010 Sep 1;38(17):5735–5745. doi: 10.1093/nar/gkq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006 Aug;19(4):290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 22.Guarino M. Src signaling in cancer invasion. J Cell Physiol. [Review] 2010 Apr;223(1):14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. [Research Support, Non-U.S. Gov't] 2005 May 15;65(10):4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 24.Kielhorn E, Provost E, Olsen D, D'Aquila TG, Smith BL, Camp RL, et al. Tissue microarray-based analysis shows phospho-beta-catenin expression in malignant melanoma is associated with poor outcome. Int J Cancer. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't P.H.S.] 2003 Feb 20;103(5):652–656. doi: 10.1002/ijc.10893. [DOI] [PubMed] [Google Scholar]
- 25.Kageshita T, Hamby CV, Ishihara T, Matsumoto K, Saida T, Ono T. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001 Aug;145(2):210–216. doi: 10.1046/j.1365-2133.2001.04336.x. [DOI] [PubMed] [Google Scholar]
- 26.Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of beta-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer. [Research Support, Non-U.S. Gov't] 2001 Jun 15;92(6):839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- 27.Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, et al. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. Epub 2002 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, et al. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol Cell Biol. 2006 Dec;26(23):8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989 Feb;105(2):379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher SJ, Luciani F, Berlin I, Rambow F, Gros G, Champeval D, et al. General strategy to analyse melanoma in mice. Pigment Cell Melanoma Res. 2011 Sep 29; doi: 10.1111/j.1755-148X.2011.00907.x. [DOI] [PubMed] [Google Scholar]
- 31.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 1997 Mar 21;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 32.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, et al. A role for cadherins in tissue formation. Development. 1996;122(10):3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.