Significance
Genetic mutations in mice and humans have dramatic effects on the overall ability of the organism to regulate body weight and maintain a homeostatic balance between energy expenditure and calorie intake. We are studying mice with a null mutation in the RIIβ subunit of the cAMP-dependent protein kinase system that renders them lean and resistant to diet-induced obesity. These mice also exhibit greatly increased physical activity. Our studies show that the brain regulates all of these phenotypes, but, surprisingly, the specific neurons that determine the phenotypes are distinct and the increased physical activity is not required for the lean phenotype.
Keywords: mouse genetics, obesity, exercise, cAMP
Abstract
Targeted disruption of RIIβ-protein kinase A (PKA) in mice leads to a lean phenotype, increased nocturnal locomotor activity, and activation of brown adipose tissue. Because RIIβ is abundantly expressed in both white and brown adipose tissue as well as the brain, the contribution of neuronal vs. peripheral PKA to these phenotypes was investigated. We used a Cre-Lox strategy to reexpress RIIβ in a tissue-specific manner in either adipocytes or neurons. Mice with adipocyte-specific RIIβ reexpression remained hyperactive and lean, but pan-neuronal RIIβ reexpression reversed both phenotypes. Selective RIIβ reexpression in all striatal medium spiny neurons with Darpp32-Cre corrected the hyperlocomotor phenotype, but the mice remained lean. Further analysis revealed that RIIβ reexpression in D2 dopamine receptor-expressing medium spiny neurons corrected the hyperlocomotor phenotype, which demonstrated that the lean phenotype in RIIβ-PKA–deficient mice does not develop because of increased locomotor activity. To identify the neurons responsible for the lean phenotype, we used specific Cre-driver mice to reexpress RIIβ in agouti-related peptide (AgRP)-, proopiomelanocortin (POMC)-, single-minded 1 (Sim1)-, or steroidogenic factor 1 (SF1)-expressing neurons in the hypothalamus, but observed no rescue of the lean phenotype. However, when RIIβ was reexpressed in multiple regions of the hypothalamus and striatum driven by Rip2-Cre, or specifically in GABAergic neurons driven by Vgat-ires-Cre, both the hyperactive and lean phenotypes were completely corrected. Bilateral injection of adeno-associated virus1 (AAV1)-Cre directly into the hypothalamus caused reexpression of RIIβ and partially reversed the lean phenotype. These data demonstrate that RIIβ-PKA deficiency in a subset of hypothalamic GABAergic neurons leads to the lean phenotype.
We reported previously that mice lacking the protein kinase A (PKA) regulatory subunit RΙΙβ (RΙΙβ KO) exhibit a 50% reduction in white adipose tissue (WAT) and are resistant to diet-induced obesity (DIO) and diabetes (1, 2). These mice have an extended lifespan and show diminished age-related metabolic dysfunctions such as fatty liver and insulin resistance (3). Compared with their WT littermates, RIIβ KO mice have normal to slightly increased food intake, a twofold increase in nocturnal physical activity, and a basal metabolic rate (VO2 consumption) that is higher than WT if calculated based on total body weight (4–6). RIIβ is highly expressed in the mouse CNS, brown adipose tissue (BAT), and WAT with limited expression elsewhere (1, 7–9). At the molecular level, RIIβ deficiency is accompanied by a compensatory increase in the PKA regulatory subunit RIα, which results in increased basal PKA activity and decreased total C subunit activity in brain, BAT, and WAT (1). These changes in PKA subunit composition may also alter PKA holoenzyme localization to specific signaling complexes because RIIβ has a much higher affinity for anchoring proteins (AKAPs) than RI (10). RIIβ KO mice have elevated uncoupling protein 1 (UCP-1) in BAT and exhibit enhanced thermogenesis (6). In the WAT, an increased basal lipolysis in RIIβ KO mice was observed, and it was suggested that changes in both BAT and WAT could contribute to the lean phenotype (9). RIIβ deficiency in brain regions where RIIβ is the major PKA isoform, such as the striatum, is accompanied by defective gene expression and related behavioral changes (7, 11). Additional studies from our laboratory have demonstrated that RIIβ deficiency can partially rescue the obese phenotypes of the agouti yellow mice (4) and stimulates locomotor activity and cold-induced thermogenesis in the obese mouse (ob/ob) (5). These studies have suggested that both CNS and adipose RIIβ deficiency may contribute to the overall lean phenotype of RIIβ KO mice; however, the relative contribution of adipose vs. brain RIIβ deficiency to the KO phenotype has not been directly addressed.
The most prominent phenotypes of RΙΙβ KO mice are their hyperlocomotor activity, reduced fat mass, and remarkably low serum leptin. It was unknown whether these phenotypes were caused by RΙΙβ deficiency in the same tissue/brain region, and whether the increased physical activity and energy expenditure contributed to the leanness. Reduced physical activity has been intuitively blamed as a culprit in the epidemic obesity in modern society. Although the health benefits of moderate exercise are clear, there is a lack of evidence supporting a direct correlation between physical activity and body fat content in humans (12). In animal models with small body size, such as mouse and rat, some studies indicate that spontaneous locomotor activity accounts for only a small fraction of total energy expenditure and is not related to diet-induced obesity (13, 14). However, other researchers have suggested that reduced locomotor activity can be a major contributor to DIO in mice (15). We speculated that RΙΙβ KO mice could be an ideal model to study this controversy. We therefore examined the physiological effects of tissue-specific reexpression of RIIβ using cell type-specific Cre drivers. Our results indicate that the increased locomotor activity and leanness of RIIβ KO mice are independent phenotypes that can be differentially traced to RIIβ deficiency in striatum and hypothalamus, respectively. The increased physical activity seen in RIIβ KO mice does not make a significant contribution to their lean phenotype.
Results
Metabolic Analysis of Mice Engineered to Reexpress RIIβ in Specific Tissues.
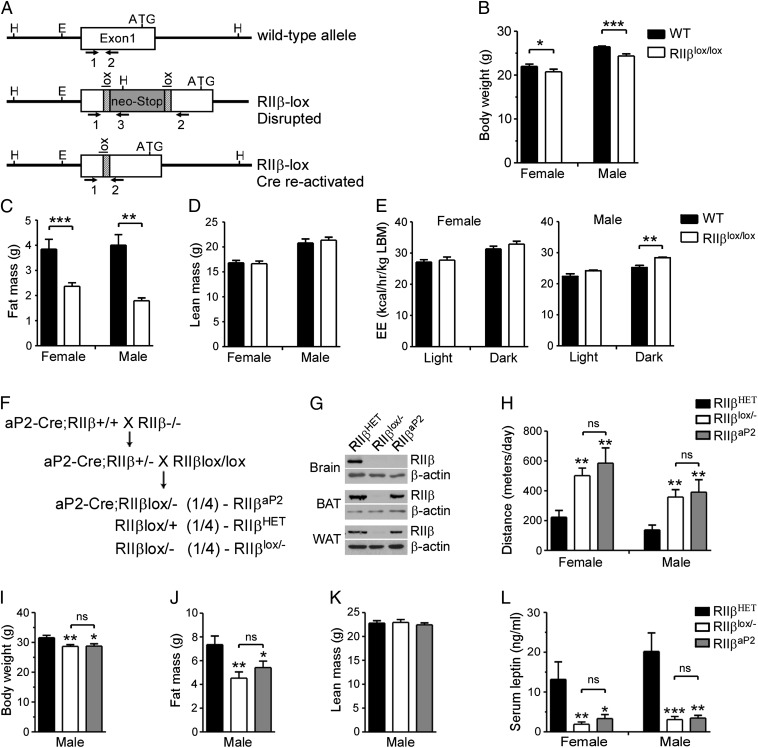
Mice were engineered to reexpress RIIβ in response to Cre recombinase activity. A loxP-flanked cassette containing the neomycin resistance gene was inserted between the transcription start site and the ATG codon of the RIIβ gene (Fig. 1A). Mice homozygous for the inactive allele, designated RIIβlox/lox, do not express RIIβ protein (Fig. 1G) and are phenotypically identical to the previously generated RIIβ−/− mice (1, 4). Thus, both RΙΙβlox/lox and RΙΙβlox/− mice are referred to as RIIβ KO in this report, unless otherwise designated. When Cre-recombinase is expressed in RΙΙβlox/lox mice, the neo-STOP sequence is deleted, leaving one loxP sequence in the 5′-untranslated region of the RΙΙβ gene (Fig. 1A).
Fig. 1.
Generation and characterization of RIIβlox/lox mice and mice with adipocyte-specific RIIβ expression. (A) Strategy for generation of RIIβlox/lox mice. (B) Body weight and (C) QMR analysis of fat mass and (D) lean mass of RIIβlox/lox and WT control mice at 12 wk of age. (E) EE of WT and RIIβlox/lox mice was measured over 2 d and averaged and normalized to LBM. For both sexes, n = 8 for each genotype; values represent mean ± SEM. **P < 0.01. (F) Breeding strategy for generation of aP2-Cre mice with adipocyte-specific RIIβ reexpression (RIIβaP2). (G) Western blots for RIIβ in lysates from BAT, WAT, and brain of RIIβaP2 mice. β-actin was used as loading control. (H) The 72-h total locomotor activity in HET, RIIβlox/−, and RIIβaP2 mice (n = 5–8 for each group). (I–K) Body weight (I), QMR measurements of fat mass (J), and lean mass (K) of male HET (n = 16), RIIβlox/− (n = 14), and RIIβaP2 (n = 10) mice. (L) Serum leptin levels of male and female HET, RIIβlox/−, and RIIβaP2 mice at 20–22 wk of age (n = 6–14 for each group). Values represent mean ± SEM. ns, not significant. **P < 0.01; ***P < 0.001 compared with HET.
We previously reported that RΙΙβ KO mice had increased resting oxygen consumption (VO2/total body weight), which we hypothesized was related to their elevated basal metabolism and lean phenotype (1, 6). To further assess the physiological impact of RIIβ deficiency on metabolism, RIIβlox/lox animals were subjected to metabolic monitoring for two consecutive days with free access to food and water. Adiposity and lean body mass were also assessed in these mice at the beginning of the study using quantitative magnetic resonance (QMR; Fig. 1 B–D). We then calculated total energy expenditure (EE) based on both oxygen consumption and CO2 production. In agreement with our previous results, the RIIβlox/lox mice had increased EE based on total body mass. However, when calculated based on lean body mass (LBM), RIIβlox/lox and WT mice have the same EE per LBM during the light phase when the mice are at rest (Fig. 1E). During the active dark phase, the EE of male RIIβlox/lox mice was significantly higher than WT controls (Fig. 1E); a similar trend was seen in female RIIβlox/lox mice. Thus, RIIβlox/lox mice have similar resting EE compared with WT controls when normalized to LBM, and the slightly increased EE during the dark phase is likely due to increased nocturnal locomotor activity. The respiratory exchange ratio (RER) is an indicator of relative utilization of carbohydrate (high RER), protein (moderate RER), or fat (lower RER) as a fuel source. The RER for male RIIβlox/lox mice was higher than WT in both the light and dark phases, consistent with the reduced adiposity of the RIIβlox/lox. The RERs for both WT and RIIβlox/lox females were the same in both light and dark phases (Fig. S1A).
RIIβ Reexpression in Adipocytes Does Not Rescue the Adiposity of RIIβ KO Mice.
We previously observed that RIIβ KO mice had elevated basal lipolysis in WAT but a reduced lipolytic response to β-adrenergic stimulation (9). The KO mice also exhibited enhanced thermogenesis in the BAT due to increased expression of the mitochondrial UCP-1 (1, 9). Both phenotypes were possible contributors to the lean phenotype of RIIβ KO mice. To test this hypothesis, RIIβ expression was selectively reactivated in BAT and WAT by crossing the RIIβlox/lox mice with an adipocyte-specific aP2 promoter-driven Cre recombinase transgenic line (aP2-Cre) (16). Because many Cre recombinase transgenes become active during passage through the germ line, including the aP2-Cre, we bred the aP2-Cre transgene onto the RIIβ−/− background and then mated them to RIIβlox/lox mice to obtain aP2-Cre/RIIβlox/− mice (Fig. 1F). Because aP2-Cre/RIIβlox/− mice carry only one functional loxP-modified allele, heterozygote (HET) RIIβlox/+ littermates were used as controls in the subsequent studies. A mild lean phenotype was previously documented in RIIβ HET mice, but they have no hyperlocomotor activity phenotype (4).
RΙΙβ was expressed in both BAT and WAT in aP2-Cre/RΙΙβlox/− mice at a level similar to HET controls, but not in the brain (Fig. 1G). The aP2-Cre/RΙΙβlox/− mice exhibited the same hyperlocomotor activity as RΙΙβlox/− mice (Fig. 1H), demonstrating that RIIβ deficiency in BAT and WAT is not the cause of elevated physical activity in RIIβ KO mice. Both RΙΙβlox/− and aP2-Cre/RΙΙβlox/− male mice weighed significantly less and had lower body fat content (Fig. 1 I–K) compared with their HET littermates. The reproductive fat pads, which in RIIβ KO mice are the most severely reduced (1), weighed significantly less in both male and female RΙΙβlox/− and aP2-Cre/RΙΙβlox/− mice compared with HET controls (Fig. S1B). Finally, serum leptin levels are also very low in RΙΙβlox/− mice, and they remained low in the aP2-Cre/RΙΙβlox/− mice compared with HET controls (Fig. 1L). These data demonstrate that RΙΙβ deficiency in either WAT or BAT is not a major contributor to the leanness or hyperactivity of RIIβ KO mice.
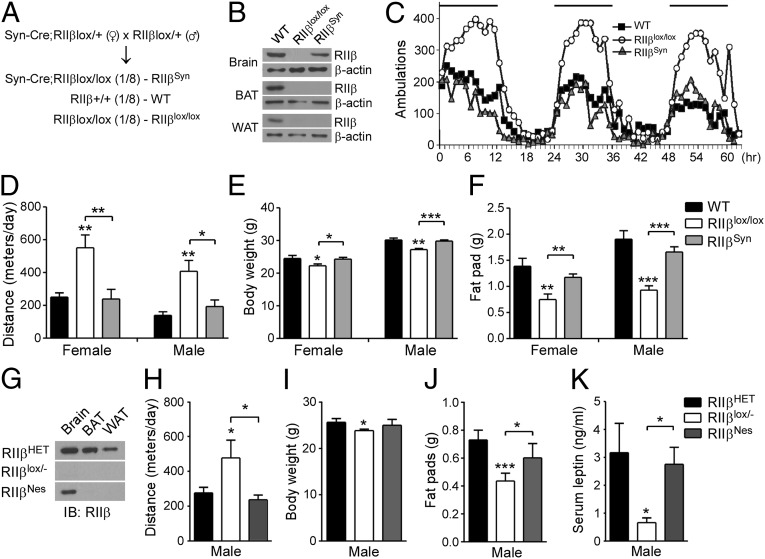
Neuronal Reexpression of RIIβ Reverses the Hyperactive and Lean Phenotypes.
A neuron-specific synapsin-Cre transgenic line (Syn-Cre) was used to reexpress RIIβ in neurons (17). Syn-Cre/RΙΙβlox/lox mice were obtained by crossing male RΙΙβlox/+ mice to Syn-Cre/RΙΙβlox/+ females to minimize the frequency of germ-line recombination (Fig. 2A) (18). In Syn-Cre/RΙΙβlox/lox mice, RΙΙβ was reexpressed in brain but not in adipose tissues, and the levels of RIIβ in brain were restored to ∼50% of WT levels (Fig. 2B). The locomotor activity of Syn-Cre/RΙΙβlox/lox mice was rescued to WT level (Fig. 2 C and D), the body weight increased to WT levels (Fig. 2E), and the major fat pads were comparable to WT mice and significantly larger than those in RIIβ KO mice (Fig. 2F). To confirm these observations, we also created mice with brain-specific RIIβ expression using a nestin-Cre transgenic line (Nes-Cre) (19). RIIβ was specifically expressed in the brain and not in BAT or WAT of Nes-Cre/RIIβlox/− mice (Fig. 2G). Similar to the Syn-Cre/RΙΙβlox/lox mice, reexpression of RIIβ is only partial, but sufficient to rescue the lean phenotypes, including hyperactivity (Fig. 2H), body weight (Fig. 2I), reproductive fat pads (Fig. 2J), and circulating leptin (Fig. 2K). These results demonstrate that neuronal deficiency in RIIβ-PKA is the major cause of the elevated physical activity and overall lean phenotype of RIIβ KO mice.
Fig. 2.
Neuronal RIIβ expression rescues the lean and hyperlocomotor phenotypes of RIIβ KO mice. (A) Breeding strategy for generation of RIIβSyn mice with neuron-specific RIIβ reexpression. (B) Western blot analysis showed that RIIβ was expressed in brain but not in BAT or WAT of RIIβSyn mice. (C) Average locomotor activity traces of WT, RIIβlox/lox, and RIIβSyn mice. Black bars depict dark cycles. (D) Locomotor activity, (E) body weight, and (F) total fat pads of WT, RIIβlox/lox, and RIIβSyn mice (n = 5–8 for each genotype and each sex). Values represent mean ± SEM. *P < 0.05; **P < 0.01 compared with WT or as indicated. (G) Immunoblots of RIIβ in HET, RIIβlox/−, and RIIβNes mice. (H) Locomotor activity, (I) body weight, (J) reproductive fat pads, and (K) serum leptin concentrations of male HET (n = 8), RIIβlox/− (n = 6), and RIIβNes mice (n = 6) at 12 wk of age. Error bars are shown as SEM. *P < 0.05 compared with HET or as indicated.
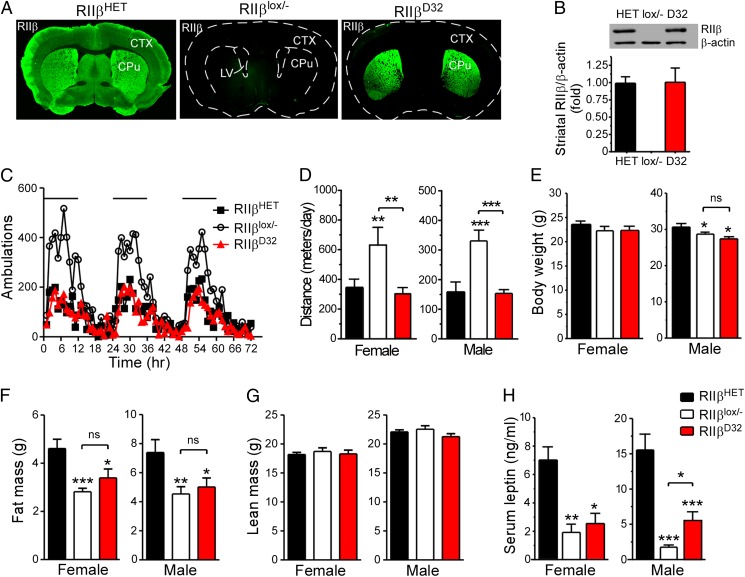
Specific RIIβ Reexpression in Striatum Reverses the Hyperactivity but Not the Leanness.
The striatum has the highest RIIβ expression of mouse brain regions and RIIβ KO mice exhibit altered striatum-dependent behaviors (7, 11). We speculated that the increased nocturnal activity of RIIβ KO mice was caused by RIIβ deficiency in the striatum (5). To examine this hypothesis we generated mice with selective RIIβ reexpression in striatal medium spiny neurons (MSNs) by using Darpp32-Cre (D32-Cre) mice (20). In D32-Cre/RΙΙβlox/− mice, RΙΙβ was exclusively reexpressed in the striatum and remained undetectable in any of the peripheral tissues examined, including WAT, BAT, pituitary, thyroid, and adrenal gland. (Fig. 3A; Fig. S2). The level of RΙΙβ expression in the striatum was comparable between HET and D32-Cre/RΙΙβlox/− mice (Fig. 3B), indicating efficient Cre-induced recombination in MSNs. RΙΙβ deficiency leads to greatly reduced total PKA activity in the striatum due to decreased catalytic subunits (7). This deficiency of PKA activity in the striatum was significantly restored in D32-Cre/RΙΙβlox/− mice (Fig. S2D).
Fig. 3.
Selective RIIβ expression in the striatum rescues locomotion but not adiposity. (A) Immunostaining of RIIβ in the brain of HET, RIIβlox/−, and D32-Cre/RIIβlox/− (RIIβD32) mice. (B) Immunoblots and quantification of RIIβ expression in the striatum of RIIβD32 mice compared with HET and RIIβlox/− mice. (C and D) Locomotor activity Black bar in C indicates dark cycle., (E) body weight, (F) fat mass, and (G) lean mass as determined by QMR scan, and (H) serum leptin level of HET, RIIβlox/−, and RIIβD32 mice at 20 wk of age (n = 7–16 for each group). Data are expressed as mean ± SEM. ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t test compared with HET control or as indicated.
The locomotor activity of D32-Cre/RΙΙβlox/− mice was comparable to HET control mice and significantly lower than RΙΙβ KO mice (Fig. 3 C and D). However, QMR measurements showed that D32-Cre/RΙΙβlox/− mice remained as lean as RΙΙβ KO control mice, as determined by their body weight, fat, and LBM (Fig. 3 E–G). Consistent with their reduced fat content, D32-Cre/RΙΙβlox/− and RΙΙβ KO mice had significantly lower serum leptin concentrations than the HET controls, although male D32-Cre/RΙΙβlox/− mice showed slightly higher leptin levels compared with RΙΙβ HET control mice (Fig. 3H). These data indicate that the increased physical activity in RΙΙβ KO mice is not the major cause of their leanness.
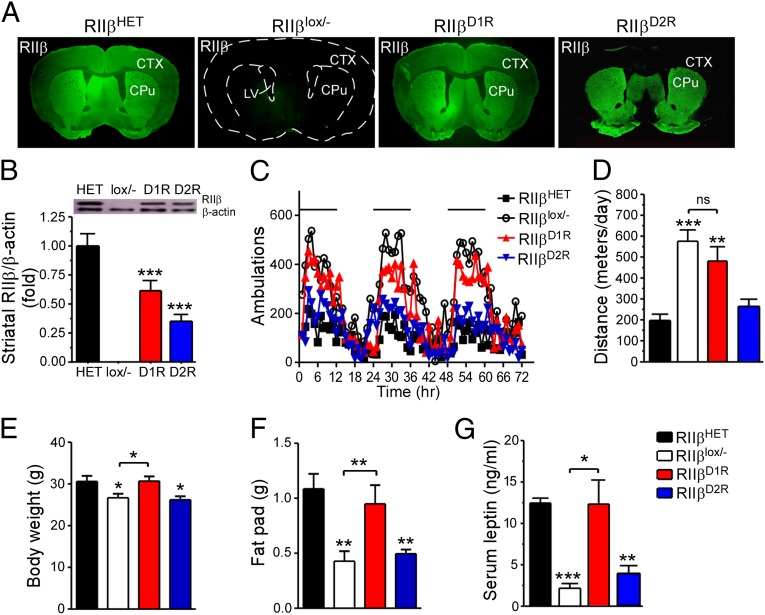
RIIβ-PKA in D2 Receptor Neurons Rescues the Hyperlocomotor Phenotype.
To determine the effect of RΙΙβ in D1 (D1R-expressing) and D2 (D2R-expressing) subtypes of MSNs on locomotion, we generated D1R-Cre/RΙΙβlox/− and D2R-Cre/RΙΙβlox/− mice. In D1R-Cre/RΙΙβlox/− mice, RΙΙβ is expressed in all of the brain regions examined, including striatum, cortex, hypothalamus, hippocampus, and midbrain (Fig. 4A; Fig. S3A). This extensive expression of RΙΙβ is consistent with previous reports that D1R-Cre is expressed in multiple brain regions known to express the D1 receptor (21). In D2R-Cre/RΙΙβlox/− mice, RΙΙβ is predominately expressed in the striatum (Fig. 4A; Fig. S3A). Immunohistochemistry showed that RΙΙβ is expressed mostly in dynorphin-negative MSNs in the striatum of D2R-Cre/RΙΙβlox/− mice (Fig. S3D), indicating that Cre-induced recombination occurred in D2 neurons but not in D1 neurons. A small number of cells showed costaining of dynorphin and RΙΙβ as shown by the arrowheads in Fig. S3D, suggesting that they might express both D1R and D2R. Both D1R-Cre/RΙΙβlox/− and D2R-Cre/RΙΙβlox/− mice had no detectable RΙΙβ expression in peripheral tissues, including BAT, WAT, pituitary, thyroid, and adrenal glands (Fig. S3 B and C). Quantification of striatal RΙΙβ expression showed that D1R-Cre,RΙΙβlox/− and D2R-Cre,RΙΙβlox/− mice had about 60% and 40%, respectively, of HET level of RΙΙβ expression (Fig. 4B).
Fig. 4.
Effects of selective RIIβ expression in D1R or D2R neurons on locomotion and adiposity. (A) Immunostaining of RIIβ in brain sections of HET, RIIβlox/−, D1R-Cre/RIIβlox/− (RIIβD1R), and D2R-Cre/RIIβlox/− (RIIβD2R) mice. (B) Immunoblots and quantification of RIIβ level in the striatum of HET, RIIβlox/−, RIIβD1R, and RIIβD2R mice. ***P < 0.001, unpaired t test compared with HET controls (n = 3 for each genotype). (C and D) Locomotor activity (black bar in C indicates dark cycle), (E) body weight, (F) reproductive fat-pad weight, and (G) serum leptin level of HET, RIIβlox/−, RIIβD1R, and RIIβD2R mice at 16–20 wk of age (n = 8–12 for each group). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t test compared with HET control or as indicated.
Despite the extensive RΙΙβ expression in the brain of D1R-Cre/RΙΙβlox/− mice, they continued to display hyperactivity similar to RΙΙβlox/− mice (Fig. 4 C and D). However, these mice had the same body weight, adiposity, and serum leptin as HET controls compared with RΙΙβlox/− mice (Fig. 4 E–G). By comparison, D2R-Cre/RΙΙβlox/− mice had lost the hyperactivity phenotype (Fig. 4 C and D) but remained lean and displayed the same body weight, adiposity, and serum leptin levels as the RΙΙβlox/− mice (Fig. 4 E–G). Thus, RΙΙβ expression in D2 neurons reversed the hyperactivity but not the leanness of RΙΙβ KO mice, as observed in mice with D32-Cre–mediated RΙΙβ expression in the whole striatum (Fig. 3). These data demonstrate that the increased locomotion of RΙΙβ KO mice requires RΙΙβ deficiency in D2 neurons, whereas the reduced adiposity is due to RΙΙβ deficiency in extrastriatal brain regions expressing the D1R-Cre. It is critical to note that the D1R-Cre caused extensive reexpression of RIIβ in the hypothalamus as well other brain regions (Fig. S3A).
Hyperactive and Lean Phenotypes Are Rescued by Rip2-Cre and Vgat-ires-Cre–Driven Reexpression of RIIβ.
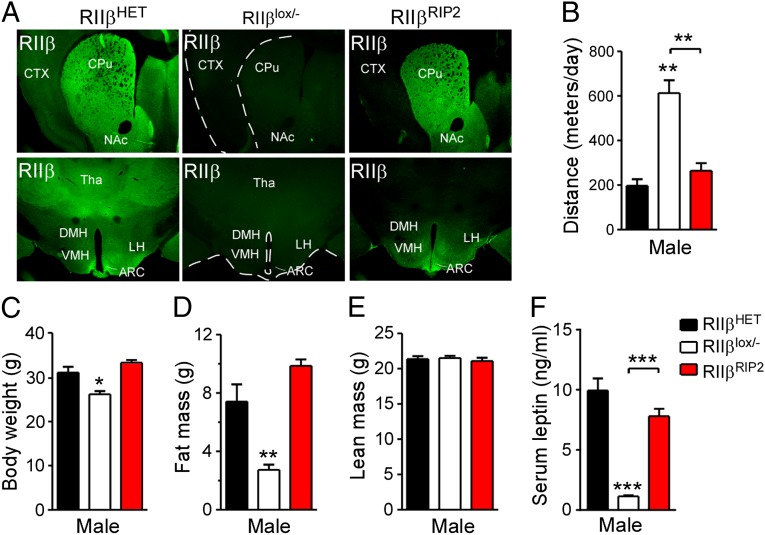
The hypothalamus plays a pivotal role in energy homeostasis regulation, but we were unable to find a specific Cre transgenic that would drive reexpression of RIIβ only in hypothalamus. The rat insulin promoter-driven Cre recombinase (Rip2-Cre) has been reported to drive expression in hypothalamus as well as in β-cells of the pancreas, but it also shows activity in other brain regions (22). We generated Rip2-Cre/RΙΙβlox/− mice and found that RΙΙβ expression was primarily restricted to the hypothalamus and the striatum. Other brain regions, including the cortex, hippocampus, thalamus, and midbrain were either devoid of or had minimal RΙΙβ expression (Fig. 5A). In the hypothalamus, Rip2-Cre–induced RΙΙβ expression was observed in the arcuate (ARC), ventromedial hypothalamic (VMH), dorsomedial hypothalamic (DMH), and lateral hypothalamus (LH) nucleus. The selective expression of Rip2-Cre in striatum and hypothalamus has been previously reported (22).
Fig. 5.
Rip2-Cre–induced RIIβ expression rescued both locomotion and adiposity. (A) Immunohistochemistry for RIIβ expression in HET, RIIβlox/−, and Rip2-Cre/RIIβlox/− (RIIβRIP2) mice. ARC, arcuate; CPu, caudate putamen; CTX, cortex; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; NAc, nucleus accumbens; Tha, thalamus; VMH, ventromedial hypothalamus. (B) Locomotor activity, (C) body weight, (D) fat mass, and (E) lean mass determined by QMR and (F) serum leptin level of HET, RIIβlox/−, and RIIβRIP2 male mice at 16 wk of age (n = 8–10 for each group). Error bars are shown as SEM. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t test compared with HET control or as indicated.
As expected, the locomotor activity of Rip2-Cre/RΙΙβlox/− mice was returned to HET levels, which is significantly lower than RΙΙβlox/− mice (Fig. 5B). In addition, the body weight, adiposity, LBM, and serum leptin of Rip2-Cre/RΙΙβlox/− mice were all indistinguishable from their HET controls, though significantly different from the RΙΙβlox/− mice (Fig. 5 C–F). Because the D32-Cre/RΙΙβlox/− mice that express RIIβ only in the striatum are lean like their RΙΙβlox/− littermates (Fig. 3 D–G), we suggest that the rescue of RΙΙβ expression in the hypothalamus by Rip2-Cre was required to reverse the lean phenotype.
The hypothalamus encompasses a diverse set of neurons in defined anatomical nuclei; many express leptin receptors and have been implicated in body weight regulation. To further define the hypothalamic cell types in which RΙΙβ deficiency influences adiposity, we tested a series of Cre transgenic mouse lines that activated RΙΙβ expression in subsets of hypothalamic neurons, including the paraventricular hypothalamic nucleus (PVH) [single-minded 1 (Sim1)-Cre], VMH [steroidogenic factor 1 (Sf1)-Cre], agouti-related peptide (AgRP) neurons (Agrp-Cre), and proopiomelanocortin (POMC) neurons (Pomc-Cre). All Cre lines gave efficient and region-specific activation of RΙΙβ expression. However, none of these lines reversed either the hyperactive or the lean phenotype of RΙΙβ KO mice (Fig. S4 A–C). These data suggested that RΙΙβ in hypothalamic cell types other than those defined by this subset of Cre recombinase transgenics might be playing a role, or perhaps that the deficiency of RΙΙβ is acting in multiple hypothalamic nuclei and the lean phenotype is not dependent on a single neuronal subtype.
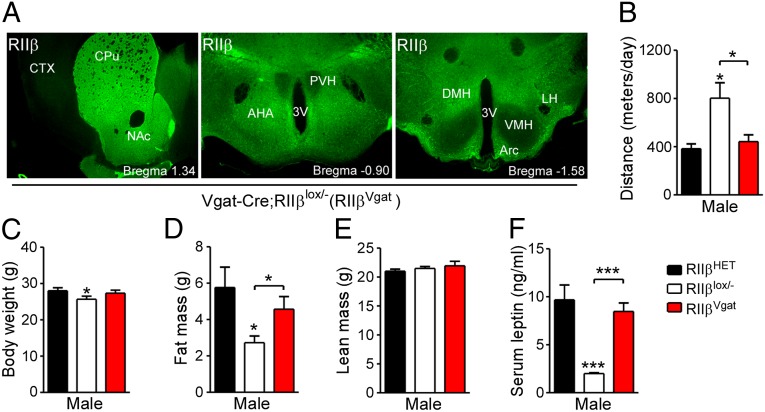
Leptin receptors (LepR) are expressed in both GABAergic and glutamatergic neurons of the hypothalamus as well as in other specific areas of the midbrain and brainstem. These leptin-responsive “first-order” neurons are thought to be key regulators of body weight, because a KO of the Lepr gene results in the rapid onset of hyperphagia, decreased energy expenditure, and subsequent obesity. The RIIβ reexpression studies described above implicate the hypothalamus as the site of the lean phenotype, but our efforts to pinpoint a specific cell type were unsuccessful. A recent study using a Vgat-ires-Cre to specifically eliminate the LepR demonstrated that LepRs in GABAergic inhibitory neurons are the key regulators of body weight (23). Using this Cre-expressing mouse line, we have reexpressed RIIβ only in GABAergic neurons to test whether this subset of neurons is responsible for the lean and hyperactive phenotypes of RIIβ KO mice. The Vgat-ires-Cre triggered reexpression of RIIβ in the DMH, ARC, and LH, but not the VMH and PVH regions of the hypothalamus (Fig. 6A), as expected, because these latter areas are primarily composed of glutamatergic neurons (23, 24). Reexpression of RIIβ is also strong in the striatum (Fig. 6A), as expected, because the MSNs are all GABAergic. Other regions of the brain, such as cortex, hippocampus, and thalamus, had much less RIIβ reexpression in the Vgat-ires-Cre/RΙΙβlox/− mice (Fig. S5A). RIIβ reexpression was not activated in BAT and WAT of the Vgat-ires-Cre/RΙΙβlox/− mice (Fig. S5B). As expected, due to expression in the striatum, the hyperactivity was completely reversed in the Vgat-ires-Cre/RΙΙβlox/− mice (Fig. 6B). The lean phenotype, as assessed by fat-pads weight (Fig. S5C), QMR scan (Fig. 6 C–E), and serum leptin levels (Fig. 6F), was rescued in the Vgat-ires-Cre/RΙΙβlox/− mice. We conclude from this result that the contribution of RIIβ-PKA to body weight regulation is dependent on GABAergic neurons in the hypothalamus that are distinct from the AgRP/neuropeptide Y (NPY) neurons that we can target with an Agrp-Cre transgenic driver.
Fig. 6.
Selective RIIβ reexpression in GABAergic neurons reverses the hyperactivity and leanness of RIIβ KO mice. (A) Immunohistochemistry for RIIβ expression in the striatum and hypothalamus of Vgat-ires-Cre/RIIβlox/− (RIIβVgat) mice. 3V, third ventricle; AHA, anterior hypothalamic area; CTX, cortex; CPu, caudate putamen; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; NAc, nucleus accumbens; PVH, paraventricular hypothalamus; VMH, ventromedial hypothalamus. (B) Locomotor activity, (C) body weight, (D) fat mass, and (E) lean mass as determined by QMR and (F) serum leptin levels of HET, RIIβlox/−, and RIIβVgat male mice at 16 wk of age (n = 6–12 for each genotype). Error bars represent SEM. **P < 0.01, ***P < 0.001, unpaired t test compared with HET controls or as indicated.
AAV1-Cre–Mediated RIIβ Reexpression in the Hypothalamus Reverses the Lean Phenotype.
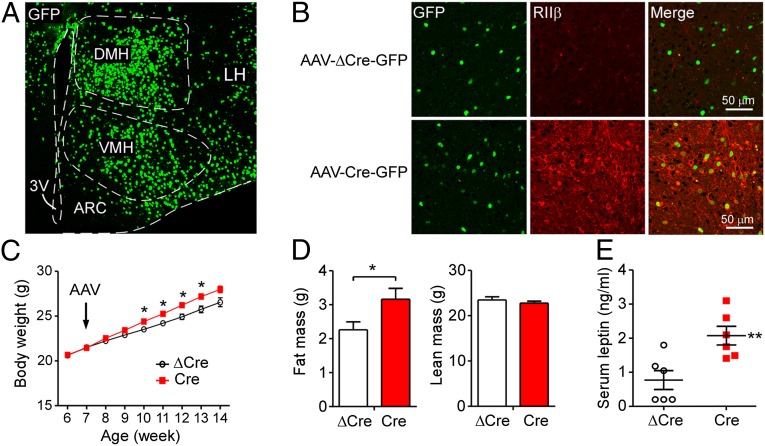
To activate RIIβ expression specifically in the hypothalamus, we injected a recombinant virus AAV1-Cre-GFP bilaterally into the hypothalamus of RIIβlox/lox mice. Fig. 7A shows that GFP-tagged Cre recombinase was expressed in multiple subregions of the hypothalamus, including the DMH, VMH, LH, and ARC. In these regions, RIIβ expression was efficiently activated by AAV-Cre infection but not by control AAV-ΔCre, which expressed an inactivated mutant Cre (Fig. 7B). The GFP-tagged Cre/ΔCre localized to the nucleus, whereas reexpressed RIIβ was localized in cytosol and neuronal projections (Fig. 7B). On regular chow diet, mice with hypothalamic AAV-Cre infection gained significantly more weight after surgery than control mice injected with the AAV-ΔCre (Fig. 7C). QMR analysis indicated that the greater weight gain was accompanied by more fat mass in AAV-Cre than AAV-ΔCre–injected RIIβlox/lox mice (Fig. 7D). Accordingly, mice with hypothalamic AAV-Cre infection had significantly higher levels of serum leptin than control mice (Fig. 7E). These results indicate that restoring RIIβ-PKA only to the hypothalamus rescues cAMP signaling and reverses the lean phenotype of RIIβ KO mice.
Fig. 7.
AAV1-Cre–mediated RIIβ reexpression in the hypothalamus increases adiposity. (A) A representative section of the hypothalamus with AAV1-Cre-GFP injection shows GFP expression in major subregions of the hypothalamus. 3V, third ventricle; ARC, arcuate nucleus; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; VMH, ventromedial hypothalamus. (B) Immunohistochemical staining shows RIIβ expression is activated in the hypothalamus of RIIβlox/lox mice by AAV1-Cre-GFP infection but not by the control virus, AAV1-ΔCre-GFP infection. (C) Body-weight changes of male RIIβlox/lox mice with AAV1-Cre-GFP or AAV1-ΔCre-GFP injection into the hypothalamus (n = 6 for each group). AAV1-Cre was injected at 7 wk of age, and the mice were killed at 17 wk. (D) Fat mass and LBM of mice in C as determined by QMR assays at 16 wk of age. (E) Serum leptin levels of mice in C at 17 wk of age.
Discussion
In this study, we demonstrate that the lean and hyperactive phenotypes of RIIβ KO mice can be rescued by reexpression of RIIβ in the brain, but are not affected by reexpression of RIIβ in white and brown adipose tissue. More detailed analysis reveals that the increased physical activity is dependent on loss of RIIβ in the D2R-expressing MSNs of the striatum, whereas the lean phenotype is dependent on disruption of RIIβ expression in the GABAergic neurons of the hypothalamus. Interestingly, the increased locomotor activity of RIIβ KO mice contributes little to their lean phenotype because the hyperlocomotor activity can be completely rescued by reexpression of RIIβ in the striatum, and the lean phenotype persists. Although we found this result surprising, it is consistent with previous observations that physical activity is not directly correlated to body fat content in mice (13), and that daily activity does not drive differences in EE between two groups of mice at room temperature (25).
A lean phenotype might develop in RIIβ mice if they were chronically hypophagic or displayed increased EE such that they were in negative energy balance, but this would be expected to lead to weight loss and failure to thrive. However, RIIβ KO mice actually appear to be slightly hyperphagic (4). We had previously reported that RIIβ KO mice have increased EE as determined indirectly by short-term measurements of oxygen consumption as a function of total body weight. In light of recent discussions on how best to normalize EE (26, 27), we have reevaluated EE by indirect calorimetry on RIIβ KO mice over a 2-d period. When EE is expressed either per mouse or per LBM, we find no significant difference between KO and WT males or females during the day (basal state). However, during the dark cycle when the mice are active, we found that males had a significant increase in EE and the females also trended toward higher EE. This finding suggests that RIIβ KO mice have increased EE during their period of hyperactivity, which is balanced by a slight increase in food intake.
The striatum regulates motor behaviors and is densely innervated by midbrain dopaminergic neurons. Dysfunction of either dopaminergic neurons or striatal MSNs are associated with the motor defects in Parkinson and Huntington disease (28). PKA is an important downstream effector of dopamine signaling. The classical model for the function of the G-protein coupled receptor expressing D1R and D2R MSNs suggests that activation of D1R MSNs (direct pathway) promotes locomotion, whereas activation of D2R MSNs (indirect pathway) inhibits locomotion. Dopamine positively modulates D1R MSNs but negatively modulates D2R MSNs, thus promoting locomotion by its action on both pathways. D1R is coupled to Gαs, leading to stimulation of the cAMP-PKA pathway; D2R is Gai-coupled, leading to a negative regulation of cAMP-PKA signaling (29). RIIβ-PKA is the major PKA subtype in the striatum (8). We have shown previously that RIIβ deficiency leads to a compensatory increase in type I R subunits but a dramatic decrease in total PKA activity in the striatum (7). This decrease in PKA activity is likely due to the release of free C subunit, which turns over rapidly when not complexed in a holoenzyme with R subunits (30). The RIIβ KO mice exhibit deficits in c-fos and dynorphin gene induction in D1R neurons and reduced induction of neurotensin and c-fos by D2R antagonists (7, 11), suggesting that both the direct and indirect pathways are affected by the RIIβ mutation. However, the rescue of the increased locomotor activity by selective RIIβ reexpression in D2R neurons but not in D1R neurons suggests that the indirect pathway plays a dominant role in the nocturnal hyperactivity phenotype.
Physical exercise and activity can be beneficial in preserving lean body mass and preventing obesity-related morbidities. However, research in humans and animal models indicate that physical activity is not necessarily related to obesity predisposition (12, 13). In contrast to the common belief that physical activity is a key factor in obesity development in humans, studies have shown that energy expenditure in activity may play less of a role in the development of obesity than anticipated (31). Inactivity may be a consequence rather than a cause of obesity in children (32). Over the long term, increased physical activity is often offset by increased calorie consumption to balance the energy equation (33). We found that the increased physical activity and reduced adiposity of RIIβ KO mice are independent phenotypes and that rescue of the increased locomotor activity by expression of RIIβ in D2R neurons did not rescue the lean phenotype.
We have previously shown that RIIβ deficiency significantly rescues the obesity of agouti yellow mice by decreasing their food intake and increasing their energy expenditure (4, 5), suggesting that RIIβ deficiency regulates body weight by modulating hypothalamic leptin and/or melanocortin pathways. We observed robust RIIβ expression in the hypothalamus and striatum with Rip2-Cre but little expression in other brain regions; reexpression of RIIβ by Rip2-Cre rescues both the lean and hyperactive phenotypes. In contrast, D32-Cre–induced RIIβ expression specifically in striatal MSNs completely rescues the hyperactivity without affecting the lean phenotype. These experiments implicate the hypothalamus as the anatomical site of RIIβ-PKA’s effect on adiposity, and this was confirmed directly by viral injection. Bilateral injection of AAV1-Cre into the hypothalamus induced RIIβ reexpression in RIIβlox/lox mice, causing an increase in body weight, adiposity, and serum leptin. In an attempt to further localize the site of RIIβ-PKA action, we selectively activated RIIβ expression using Cre-driver mice specific for the PVN (Sim1-Cre), VMH (Sf1-Cre), AgRP neurons (Agrp-Cre), or POMC neurons (Pomc-Cre), but found that none of these crosses were successful in rescuing the leanness of RIIβ KO mice. However, reexpression of RIIβ in GABAergic neurons with a recently developed Vgat-ires-Cre strain (23) completely rescued both the lean and hyperactive phenotypes.
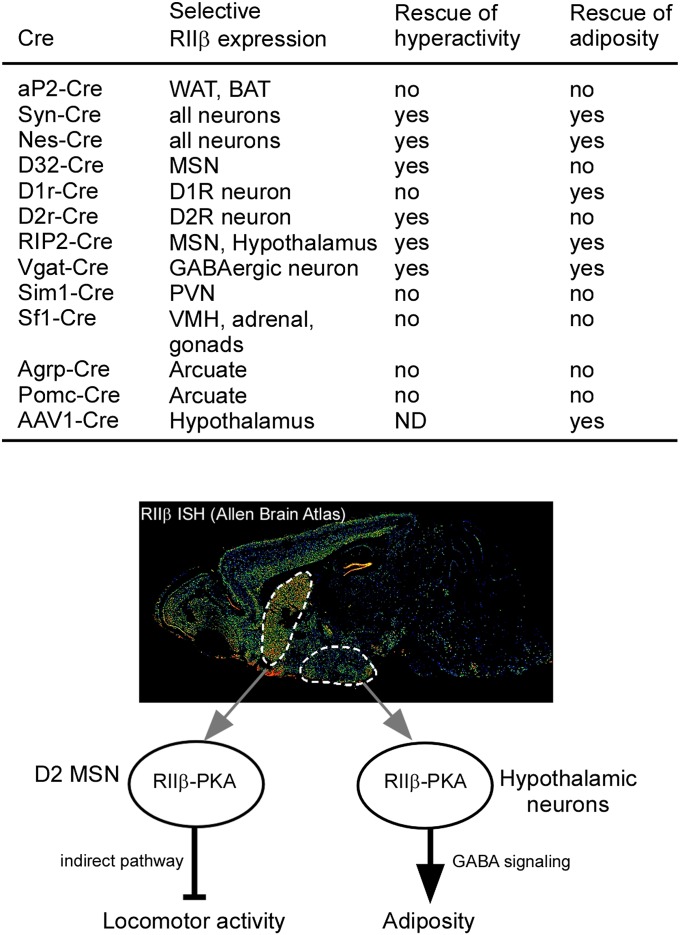
A summary of the Cre driver lines used in this study and their phenotype rescue is shown in Fig. 8. Vgat-ires-Cre activates RIIβ expression in multiple nuclei of the hypothalamus with the notable exception of the PVN and VMH, where glutamatergic neurons predominate (23, 24). In the arcuate nucleus, Vgat-ires-Cre was shown to be expressed in neurons, including AgRP neurons but not POMC neurons (23). These results demonstrate that RIIβ deficiency in glutamatergic neurons, including PVN, VMH, and POMC neurons, is not essential for the development of a lean phenotype. It is interesting to note that the vast majority of leptin’s antiobesity effects are also mediated by leptin receptors in GABAergic neurons rather than glutamatergic neurons (23). Recently, it was shown that GABA release from hypothalamic Rip2-Cre neurons regulates energy balance by stimulating thermogenesis and EE without affecting food intake (34). The overall phenotype of RIIβ KO mice suggests that they might have enhanced GABA release from Rip2-Cre neurons in response to leptin. We postulate that changes in PKA signaling in the hypothalamus of RIIβ KO mice has increased the strength or duration of the intracellular signals that emanate from LepR occupancy on GABAergic neurons; this has important therapeutic implications, because drugs targeting the cAMP system (phosphodiesterase inhibitors) are already used clinically, and protein kinases make excellent therapeutic targets (35).
Fig. 8.
Summary model. Summary of all Cre-induced RIIβ reexpression paradigms used in this study and their physiological effects. The diagram illustrates the differential regulation of locomotion and adiposity by RIIβ-PKA in striatal D2R neurons and hypothalamic GABAergic neurons. ND, not determined.
Materials and Methods
Animals.
Mice were housed at 22–24 °C with a 12-h light/dark cycle. Standard mouse chow (Picolab 20, 4.5% fat) and water were freely available except where otherwise indicated. All procedures were approved by the Institutional Care and Use Committee of the School of Medicine of the University of Washington. Mice carrying the conditional RIIβ allele were generated by inserting a loxP-flanked neomycin resistance gene (neo-STOP) into the RsrII site ∼50 bp upstream of the RIIβ ATG codon. Using standard ES cell procedures, germ-line–transmitting chimeric animals were obtained and then backcrossed with C57BL/6 for at least six generations. Offspring tail-genomic DNA was extracted and genotyped by PCR using three primers: 1, 5′AGGAGCTGGAGATGCTGCCAA3′; 2, 5′TCAGCACCTCCACCGTGAA3′; and 3, 5′GTGGTTTGTCCAAACTCATCAATGT3′. Primers 1 and 3 detect the LoxP-modified allele (as well as the knockout allele with non–loxP-modified neo-STOP cassette insertion at the same site), whereas primer 1 and 2 detect the WT RIIβ allele as well as the Cre-excised allele. During the breeding of Syn-Cre/RIIβlox/lox mice, we occasionally observed germ-line recombination, which could be detected by primer 1 and 2 (giving a PCR product 40-bp larger than that from WT alleles). These mice were eliminated from the studies. To minimize the occurrence of germ-line recombination, an alternative breeding strategy involving the RIIβ−/− mice was carried out for all of the other Cre transgenic lines (Fig. 1F). All Cre transgenic lines used in this study have been previously reported: aP2-Cre (16), Syn-Cre (17), Nes-Cre (19), D32-Cre (20), D1R-Cre (21), D2R-Cre (36), Rip2-Cre (37), Sim1Cre (38), Sf1Cre mice (39), Agrp-Cre (40), Pomc-Cre (41), and Vgat-ires-Cre (23).
Locomotor Activity and Indirect Calorimetry.
Locomotor activity of individual animals was measured at 16–20 wk of age in an activity chamber (47 × 25 × 21 cm) equipped with photobeams (San Diego Instruments). Ambulations are scored as interruption of consecutive beams and converted to meters based on the distance between the beams. EE and RER were measured using an open-circuit indirect calorimeter (Oxymax; Columbus Instruments). Eight mice were simultaneously measured at one time for a period of 48 h (two light and two dark cycles). Just before taking metabolic measurements for each mouse, lean tissue mass was assessed using QMR methods (EchoMRI-100; Echo Medical Systems) on the unsedated animal. VO2 and VCO2 for each mouse were standardized to its lean mass.
Body Weight and Adiposity.
Mice were killed with CO2, and the reproductive WAT pads were weighed and normalized to body weight. Body composition (fat and lean mass) was determined by QMR methods (EchoMRI-100; Echo Medical Systems).
Western Blot.
Intrascapular BAT, epididymal WAT, and brain samples were homogenized with a Polytron in lysis buffer (250 mM sucrose, 20 mM Tris⋅Cl (pH 7.5), 2 mM EDTA, 1% Triton X-100, 0.5% deoxycholic acid) supplemented with protease inhibitors (1 μg/mL leupeptin, 3 μg/mL aprotinin, 40 μg/mL soybean trypsin inhibitor), sonicated, and cleared by centrifugation (10,000 × g, 10 min). Protein concentration in the supernatant was determined by BCA assay (Pierce; 23227). Protein (10 µg) in 1× sample buffer [62.5 mM Tris·Cl (pH 6.8), 2% (wt/vol) SDS/5% glycerol/0.05% (wt/vol) bromophenol blue] was separated by 8% SDS/PAGE and transferred to nitrocellulose (Schleicher & Schuell) by electrophoresis. Blots were blocked [5% nonfat milk in Tris-buffered saline and Tween 20 (TBST)] for 2 h at room temperature and probed (2 h at room temperature or overnight at 4 °C) with mouse anti-RIIβ (BD Transduction Laboratories). Blots were washed in TBST. HRP-conjugated secondary antibodies (Jackson ImmunoResearch) were applied at 1:10,000 dilution in TBST plus 5% nonfat milk and incubated 1–2 h at room temperature. After washing, HRP was detected using an ECL assay kit (Amersham Biosciences).
Stereotaxic Viral Injection.
AAV1-Cre-GFP and AAV1-ΔCre-GFP (42) were bilaterally injected into the hypothalamus (2.2 × 1011 viral genome per microliter) of 7-wk-old male RIIβlox/lox mice. Two-site injections of 0.5 μL per site were performed for each side at the coordinates x = ±0.5, y = −1.4, and z = −5.6 and −5.0, which corresponds to ventral and dorsal hypothalamus, respectively. GFP fluorescence was used to identify the virally infected areas. RIIβ immunohistochemistry was used to show the activation of RIIβ expression. Body weight was monitored before and after virus injection for each mouse. Results from animals that received injections at the correct site as determined by GFP fluorescence were included in analyses.
Immunohistochemistry.
Adult mice were anesthetized with pentobarbital and perfused by cardiac puncture with PBS followed by ice-cold PBS-buffered 4% paraformaldehyde. Brains were removed and postfixed for 2 h followed by cryopreservation in 25% sucrose solution (wt/vol) overnight and subsequent freezing in OCT compound (Tissue-Tek). Cryosections (20 μm) were taken on a cryostat and allowed to air dry on slides, followed by processing or preservation at –80 °C. For immunohistochemistry, sections were washed for 10 min in PBS, followed by incubation in blocking solution (10% normal goat serum; 0.2% Triton X-100; 2% BSA; PBS) for at least 1 h at room temperature. Primary antibodies were applied in blocking solution and incubated overnight at 4 °C. Sections were washed at least three times with 5-min incubations in PBS plus 0.2% Triton X-100. Then a fluorescence-labeled secondary antibody was applied in blocking solution and incubated at room temperature for 2 h, followed by five washes with PBS plus 0.2% Triton X-100. Sections were mounted with VectaShield medium (Vector Laboratories) and analyzed on a confocal microscope (Keck Microscopy Facility, University of Washington).
Leptin Measurements.
Mice were killed by CO2 gas. Whole blood was collected by cardiac puncture. The serum was collected and assayed for leptin concentration by ELISA (Millipore; EZML-82K).
Statistical Analysis.
Statistical significance was determined using an unpaired two-tailed Student t test or ANOVA. Data are presented as the mean ± SEM.
Supplementary Material
Acknowledgments
The authors acknowledge the excellent technical assistance of Thomas Su, and thank Paul Amieux for helpful advice and editorial comments on the manuscript and James Allen and the Palmiter laboratory for providing the AAV1 virus. This research was supported by National Institutes of Health Grant GM032875 (to G.S.M.) and the Ellison Medical Foundation (W.C.L. and L.C.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219542110/-/DCSupplemental.
References
- 1.Cummings DE, et al. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382(6592):622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 2.Schreyer SA, Cummings DE, McKnight GS, LeBoeuf RC. Mutation of the RIIbeta subunit of protein kinase A prevents diet-induced insulin resistance and dyslipidemia in mice. Diabetes. 2001;50(11):2555–2562. doi: 10.2337/diabetes.50.11.2555. [DOI] [PubMed] [Google Scholar]
- 3.Enns LC, et al. Disruption of protein kinase A in mice enhances healthy aging. PLoS ONE. 2009;4(6):e5963. doi: 10.1371/journal.pone.0005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czyzyk TA, Sikorski MA, Yang L, McKnight GS. Disruption of the RIIbeta subunit of PKA reverses the obesity syndrome of Agouti lethal yellow mice. Proc Natl Acad Sci USA. 2008;105(1):276–281. doi: 10.1073/pnas.0710607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newhall KJ, Cummings DE, Nolan MA, McKnight GS. Deletion of the RIIbeta-subunit of protein kinase A decreases body weight and increases energy expenditure in the obese, leptin-deficient ob/ob mouse. Mol Endocrinol. 2005;19(4):982–991. doi: 10.1210/me.2004-0343. [DOI] [PubMed] [Google Scholar]
- 6.Nolan MA, Sikorski MA, McKnight GS. The role of uncoupling protein 1 in the metabolism and adiposity of RII beta-protein kinase A-deficient mice. Mol Endocrinol. 2004;18(9):2302–2311. doi: 10.1210/me.2004-0194. [DOI] [PubMed] [Google Scholar]
- 7.Brandon EP, et al. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998;18(10):3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 9.Planas JV, Cummings DE, Idzerda RL, McKnight GS. Mutation of the RIIbeta subunit of protein kinase A differentially affects lipolysis but not gene induction in white adipose tissue. J Biol Chem. 1999;274(51):36281–36287. doi: 10.1074/jbc.274.51.36281. [DOI] [PubMed] [Google Scholar]
- 10.Burton KA, et al. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94(20):11067–11072. doi: 10.1073/pnas.94.20.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams MR, et al. Loss of haloperidol induced gene expression and catalepsy in protein kinase A-deficient mice. Proc Natl Acad Sci USA. 1997;94(22):12157–12161. doi: 10.1073/pnas.94.22.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goris AH, Westerterp KR. Physical activity, fat intake and body fat. Physiol Behav. 2008;94(2):164–168. doi: 10.1016/j.physbeh.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996;60(1):37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 14.Keesey RE, Swiergiel AH, Corbett SW. Contribution of spontaneous activity to daily energy expenditure of adult obese and lean Zucker rats. Physiol Behav. 1990;48(2):327–331. doi: 10.1016/0031-9384(90)90322-u. [DOI] [PubMed] [Google Scholar]
- 15.Bjursell M, et al. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008;294(2):E251–E260. doi: 10.1152/ajpendo.00401.2007. [DOI] [PubMed] [Google Scholar]
- 16.He W, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rempe D, et al. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis. 2006;44(1):44–49. doi: 10.1002/gene.20183. [DOI] [PubMed] [Google Scholar]
- 19.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 20.Bogush AI, et al. DARPP-32 genomic fragments drive Cre expression in postnatal striatum. Genesis. 2005;42(1):37–46. doi: 10.1002/gene.20118. [DOI] [PubMed] [Google Scholar]
- 21.Heusner CL, Beutler LR, Houser CR, Palmiter RD. Deletion of GAD67 in dopamine receptor-1 expressing cells causes specific motor deficits. Genesis. 2008;46(7):357–367. doi: 10.1002/dvg.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarthy MV, et al. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest. 2007;117(9):2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448(3):217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- 25.Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab. 2012;16(5):665–671. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59(2):323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiyala KJ, et al. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59(7):1657–1666. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skaper SD, Giusti P. Transgenic mouse models of Parkinson’s disease and Huntington’s disease. CNS Neurol Disord Drug Targets. 2010;9(4):455–470. doi: 10.2174/187152710791556186. [DOI] [PubMed] [Google Scholar]
- 29.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 30.Amieux PS, et al. Compensatory regulation of RIalpha protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272(7):3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 31.Ebersole KE, et al. Energy expenditure and adiposity in Nigerian and African-American women. Obesity (Silver Spring) 2008;16(9):2148–2154. doi: 10.1038/oby.2008.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metcalf BS, et al. Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45) Arch Dis Child. 2011;96(10):942–947. doi: 10.1136/adc.2009.175927. [DOI] [PubMed] [Google Scholar]
- 33.Melzer K, Kayser B, Saris WH, Pichard C. Effects of physical activity on food intake. Clin Nutr. 2005;24(6):885–895. doi: 10.1016/j.clnu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Kong D, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151(3):645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen P. Protein kinases—the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1(4):309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 36.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27(37):9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 38.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3(12):e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Beutler LR, et al. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci USA. 2011;108(10):4206–4211. doi: 10.1073/pnas.1101424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.