Abstract
Objective
Apoptosis signal-regulating kinase 1 (ASK1)–interacting protein-1 (AIP1) is a signaling adaptor molecule implicated in stress and apoptotic signaling induced by proinflammatory mediators. However, its function in atherosclerosis has not been established. In the present study, we use AIP1-null (AIP1−/−) mice to examine its effect on atherosclerotic lesions in an ApoE-null (ApoE−/−) mouse model of atherosclerosis.
Approach/Results
ApoE−/− control mice developed atherosclerosis in the aortic roots and descending aortas upon Western-type diet for 10 weeks, while the atherosclerotic lesions are significantly augmented in ApoE−/−AIP1−/− double knockout (DKO) mice. DKO mice show increases in plasma inflammatory cytokines with no significant alterations in body weight, total cholesterol levels or lipoprotein profiles. Aortas in DKO mice show increased inflammation and endothelial cell (EC) dysfunction with NF-κB activity, correlating with increased accumulation of macrophages in the lesion area. Importantly, macrophages from DKO donors are not sufficient to augment inflammatory responses and atherogenesis when transferred to ApoE-KO recipients. Mechanistic studies suggest that AIP1 is highly expressed in aortic EC but not in macrophages, and AIP1 deletion in EC significantly enhance oxidized LDL-induced NF-κB signaling, gene expression of inflammatory molecules and monocyte adhesion, suggesting that vascular EC are responsible for the increased inflammatory responses observed in DKO mice.
Conclusions
Our data demonstrate that loss of AIP1 in aortic EC primarily contributes to the exacerbated lesion expansion in the ApoE−/−AIP1−/− mice, revealing an important role of AIP1 in limiting inflammation, EC dysfunction and atherosclerosis.
Keywords: Atherosclerosis, inflammation, endothelial cell, lipids and lipoproteins, macrophage, AIP1
INTRODUCTION
Myocardial infarction caused by atherosclerosis of coronary arteries remains the leading cause of death in the United States. Atherosclerosis involves plaque formation in the arterial wall and is characterized by inflammation, lipid accumulation, smooth muscle cell proliferation/neointima expansion, cell death and fibrosis 1, 2. Subendothelial retention and accumulation of lipoproteins could be converted to atherogenic remnant lipoproteins and low-density lipoprotein (LDL) 3, 4. A key early inflammatory response to retained lipoproteins is activation of vascular endothelial cells (EC), which may be enhanced by oxidative modifications of these lipoproteins. Activated EC increase expressions of adhesion molecules and chemokines which in turn promote infiltration of immune cells, including macrophages, neutrophils, master cells, dendritic cells and T cells 2, 5. Macrophages take up native and oxidized LDL to become lipid-laden foam cells and together with other cells release atherogenic cytokines and other activators such as reactive oxygen species (ROS) that exacerbate EC dysfunction (characterized by a reduction in amount of bioavailable nitric oxide) and EC apoptosis, and promote atherosclerotic plaque development 6. Therefore, the phenotypic changes in EC may be an early event during initiation and progression of atherosclerosis 2, 7, 8. Defining genes and pathways critical in EC function may provide therapeutic targets for treatment of atherosclerosis.
We have identified AIP1, a protein highly expressed in vascular endothelium, as a novel signaling scaffolding protein that mediates EC changes induced by inflammatory stimuli 9–14. AIP1 was initially identified as an ASK1-interacting protein as a positive regulator in TNF-induced JNK MAPK signaling 9. It contains multiple functional domains including PH, C2, Ras-GAP (therefore is a new member of the Ras-GAP family) at the N-terminal half while a period-like domain, a proline-rich region, a coiled-coil and leucine-zipper (CC/LZ) as well as a phospho-serine for 14-3-3 and Akt binding at its C-terminal half 15. Thereafter we have shown that AIP1 can act an inhibitor of TNF induced NF-κB activation by inhibiting RIP1 and IKK kinase activity 10, 11. It has been proposed that AIP1 differentially regulates TNFR1 signaling complexes. Specifically, AIP1 inhibits membrane-bound and IKK-activating TNFR1 complex I while promoting the JNK-activating complex (we named the AIP1 complex) 10. Interestingly, we have shown that AIP1 suppresses TLR4-mediated activation of both the JNK and NF-κB signaling pathways in EC 13. We further show that AIP1 functions as a novel Arf6-GAP to negatively regulate phosphatidylinositol 4, 5-bisphosphate (PIP2)-dependent assembly of the TLR4-TIRAP-MyD88 signaling complex. Recently, we have shown that AIP1 in vascular smooth muscle cells inhibits IFN-γ-induced JAK2 signaling pathways 14. Therefore, AIP1 appears to function as a general suppressor of inflammatory signaling.
In supporting our in vitro studies, analyses from AIP1-null mice (AIP1−/−) demonstrate AIP1 functions as an inflammatory suppressor. AIP1−/− mice are viable without obvious defects in vascular development. However, AIP1−/− mice show dramatically enhanced inflammatory responses in ischemic hindlimb, inflammatory sponge, and graft arteriosclerosis models 12, 14. Interestingly, a recent human genome-wide association study (GWAS) has identified a sequence variant within intron 1 of the AIP1 (DAB2IP) gene (rs7025486) conferring susceptibility to coronary artery disease (CAD), including abdominal aortic aneurysm, peripheral vascular disease, early onset of myocardial infarction and pulmonary embolism 16. How AIP1 is involved in CAD such as in atherosclerosis is still unknown. In the present study, we determine the effects of AIP1 global deletion on atherosclerotic progression in an ApoE-null mouse model. The ApoE-null or the LDL receptor-null mice which has impaired LDL clearance, are the most commonly used mouse models of atherosclerosis. Moreover, hyperlipidemia exacerbates inflammation and EC dysfunction in the ApoE-null mouse which are important early events for the development of atherosclerosis 17–19. Our current data suggest that AIP1 suppresses atherosclerosis progression by limiting hyperlipidemia-induced inflammation and vascular endothelial dysfunction.
RESULTS
A global deletion of AIP1 facilitates hyperlipidemia-induced atherosclerosis in ApoE−/− mouse model
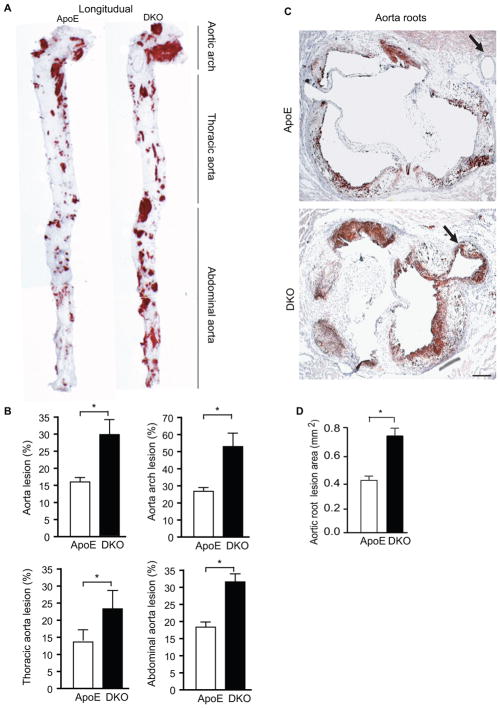
To study the functional role of AIP1 in atherogenesis, we subjected the ApoE−/−AIP1−/− double knockout (DKO) and corresponding ApoE−/− mice to a Western-type diet containing 1.25% cholesterol for 10 weeks. Lipid-rich and atherosclerotic plaques were visualized by opening the aortas longitudinally followed by oil red O staining. AIP1 deletion significantly increased the number and size of aortic plaques at 10-week in the ApoE−/− mice (Fig. 1A). Lesion areas were greater throughout the aortic arch, thoracic aorta and abdominal region (Fig. 1A–B). We also quantified lesion areas in cross-sections of the aortic roots. AIP1 deletion increased the lesion area in the aortic roots compared to that observed in ApoE−/− mice (Fig. 1C with quantification in 1D). Moreover, severe atherosclerosis was detected in the coronary artery of DKO (indicated by arrows in Fig. 1C). This phenotype is atypical for the ApoE−/− mice, but has been reported in the ApoE−/− mice after feeding with Western-type diet for 10 months 20, 21, and in ApoE−/− mice that lack additional gene such as low-density lipoprotein (LDL) receptor 22, scavenger receptor B1 (SR-BI) 23 or Akt1 gene 24, or ApoE−/− bred to mice expressing urokinase in the macrophages 25.
Figure 1. AIP1 deletion facilitates early atherosclerosis progression in ApoE−/− mice.
ApoE−/− and DKO adult mice were fed with Western-type diet for 10 weeks. A–B. Whole aortas were harvested, opened longitudinally followed by en face staining with oil red O. Representative photomicrographs are shown in A with the aortic arch, thoracic aorta and abdominal aorta indicated. Quantifications of atheroma (%) in total and each aortic segment are shown in B. Data are mean ± SEM from 10 mice per group. *, p<0.05, comparing ApoE−/− and DKO groups. C–D. Cross-sections from the aortic roots stained with Oil red-O. Representative photomicrographs are shown in C. Scale bar: 100 μm. Quantification of atheroma area is shown in D. Six sections (150 μm apart for each section) per mouse from 10 mice in each group. Data are presented are mean±SEM, *, p<0.05.
AIP1 deletion increases lesion expansion and macrophage infiltration in the aorta and innominate artery in hyperlipidemic ApoE−/− mice
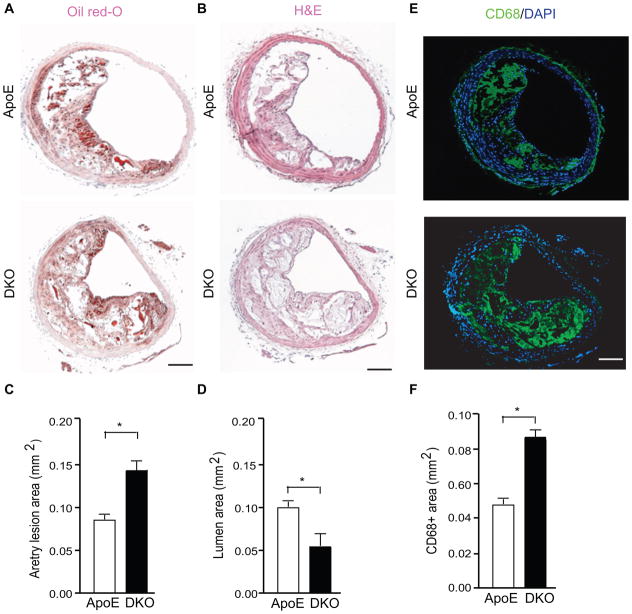
In addition to aortas and coronary lesions, we also compared the lesions in the innominate (branchiocephalic) arteries. We found that AIP1 deletion significantly increased atheroma areas with reduced lumens at 10 weeks (Fig. 2A–B for H&E and oil-red staining with quantifications in 2C–D). The increased plaque areas correlated with increased macrophage (CD68+) infiltration in branchiocephalic arteries (Fig. 2E with quantification in 2F) and aortic roots (Supplemental Fig. IA). Smooth muscle cell numbers were not significantly altered by AIP1 deletion (Supplemental Fig. IB). Thus, the genetic loss of AIP1 increases aortic and coronary atherogenesis and macrophage infiltrations, and does not involve the smooth muscle cells. We also measured the circulating monocytes (which infiltrate into tissue and become macrophages) in the blood of ApoE and DKO mice by complete blood count. No differences were detected between the ApoE−/− and DKO mice in circulating monocytes (Supplemental Fig. IC), suggesting that a higher number of macrophages in the DKO plaque was likely due to enhanced monocyte infiltration into the vascular wall of DKO mice.
Figure 2. AIP1 deletion increases lesion expansion and macrophage infiltration in the innominate artery in the ApoE−/− mice.
A–C. Representative histological analysis of cross-sections from the brachiocephalic arteries stained with hematoxylin and eosin (H&E), oil red-O, CD68 (a macrophage marker). Scale bar: 50 μm. Quantifications of atheroma area, lumen area and CD68+ area are shown in D–F. Six sections (150 μm apart for each section) per mouse from 5 mice in each group. Data are presented are mean±SEM, *, p<0.05.
AIP1 deletion has no effects on body weight, serum cholesterol and triglyceride levels, and lipoprotein profiles in hyperlipidemic ApoE−/− mice
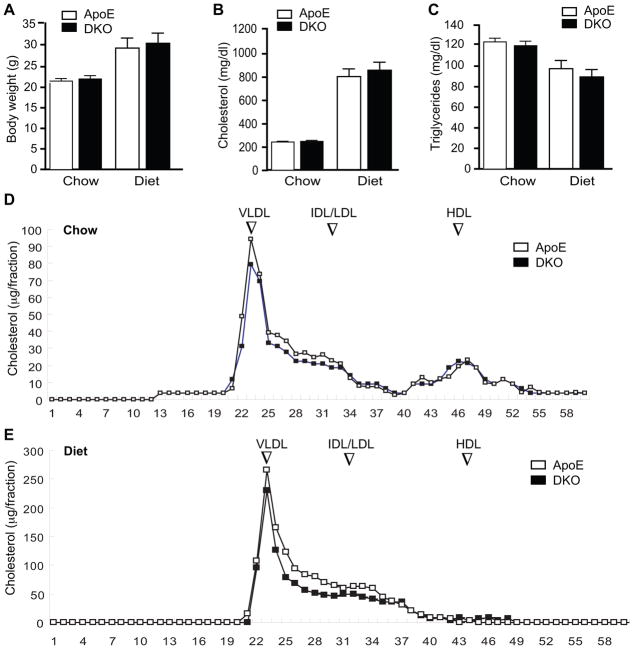
Since lipid metabolism contributes significantly to atherosclerosis, we next examined the effects of AIP1 on multiple metabolic parameters in mice fed with chow or Western-type diet for 10 weeks. We did not detect obvious differences between the ApoE−/− and DKO in body weight, serum cholesterol and triglyceride levels (Fig. 3A–C). Lipoprotein profiles of VLDL, IDL/LDL and HDL were also similar between the two groups (Fig. 3D–E). These data suggest that augmented atherosclerotic lesion formation by AIP1 deletion occurred independent of the plasma lipid profiles in the DKO mice.
Figure 3. No effects of AIP1 deletion on body weight, serum cholesterol and triglyceride levels, and lipoprotein profiles in ApoE−/− mice.
ApoE−/− and DKO adult mice were fed with chow or Western-type diet for 10 weeks. A–C. Body weight, total plasma cholesterol and triglyceride levels after fasting were measured. Data are presented are mean±SEM, n=8. D–E. Lipoprotein profiles. The lipid distribution in plasma lipoprotein fractions from apoE−/− and DKO mice under chow (D) or diet (E) were assessed by fast-performance liquid chromatography (FPLC) gel filtration with 2 Superose 6 HR 10/30 columns (Pharmacia). Peaks for VLDL, IDL/LDL and HDL are indicated. Note: HDL was reduced upon Western-type diet in both ApoE−/− and DKO mice.
AIP1 deletion augments hyperlipidemia-induced inflammatory cytokines with enhanced NF-κB signaling in aortas of the ApoE−/− mice
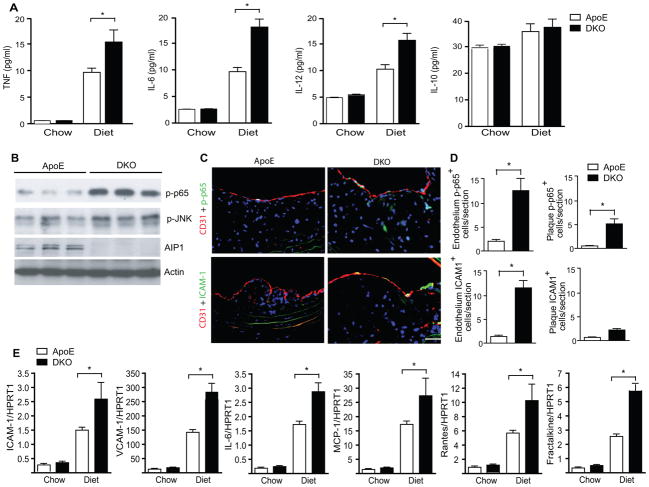
Inflammation is a key component during the initiation and progression of atherosclerosis 2. We measured atherogenic cytokines such as TNF, IL-6 and IL-12 as well as the atheroprotective cytokine IL-10 1, 26. TNF, IL-6 and IL-12, but not IL-10, were drastically increased in the ApoE−/− mouse plasma after 10 weeks on a Western-type diet; this increase was further augmented in the DKO mice (Fig. 4A). The augmented expression of proinflammatory cytokines could be due to increased number of macrophages in the atheroma plaque observed in the DKO aorta, or enhanced activation of transcriptional factor NF-κB and JNK-dependent c-Jun/ATF2 that drive gene expression of TNF, IL-6 and IL-12 27–30. Therefore, we measured activities of NF-κB and JNK in the aortic lysates with phospho-p65- and p-JNK-specific antibodies. Phopho-p65 and phospho-JNK were undetectable in chow-fed aortas (not shown). However, activation of NF-κB (phopho-p65) and JNK were strongly induced in aortas from ApoE−/− mice on a Western-type diet, and were further augmented in the DKO aortas (Fig. 4B). We further determined the cell types expressing active NF-κB in atheroma plaques by immunofluoresence staining with phopho-p65. The number of phospho-p65 positive cells was increased in DKO aortas where the majority was detected in vascular endothelium compared to the macrophage-rich atheroma plaques (Fig. 4C with quantifications in 4D). Consistently, NF-κB-dependent EC adhesion molecule ICAM-1 was also augmented by AIP1 deletion in the DKO compared to the ApoE−/− mice (Fig. 4C with quantifications in 4D). We also examined the NF-κB-dependent gene expression of macrophage recruitment chemokines MCP-1, Rantes and fractalkine (CX3CL1) by qRT-PCR from ApoE and DKO aortas. Diet-induced gene expression of MCP-1, Rantes and fractalkine was also significantly augmented by AIP1 deletion (Fig. 4E). These data suggest that augmented atherosclerotic lesion formation by AIP1 deletion was likely attributed to enhanced EC inflammatory responses in the DKO mice.
Figure 4. AIP1 deletion increases plaque and systemic inflammatory responses.
ApoE−/− and DKO adult mice were fed with chow or Western-type diet for 10 weeks. A. Effects of AIP1 deletion on proinflammatory cytokine production. TNF, IL-6, IL-12 and IL-10 in plasma were measured by ELISA. Data are presented are mean±SEM, n=8 mice in each group. B. Inflammatory responses in the plaques. NF-κB and JNK signaling in atherosclerotic plaques were measured by immunoblotting with phospho-p65- and p-JNK-specific antibodies. Total AIP1 and β-actin proteins were also determined. N=3 mice per group C. Enhanced NF-κB signaling and ICAM-1 expression in DKO vascular endothelium. NF-κB activity and ICAM-1 expression in brachiocephalic arteries lesions from ApoE−/− and DKO mice were stained with anti-p-P65 and anti-ICAM-1. CD31 was used for EC staining. Representative images are shown in panel C. Scale bar: 50 μm. Macrophages within the endothelium layer and the plaque were quantified in D. Data are mean±SEM from 6 mice per group. *, p<0.05. E. Transcripts for macrophage recruitment chemokines MCP-1, Rantes and CX3CL1 were quantified by qRT-PCR and normalized to hypoxanthine guanine phosphoribosyltransferase (HPRT) from ApoE and DKO aortas. Data are mean±SEM from 5 mice per group. *, p<0.05.
Bone marrow cells from ApoE−/−AIP1−/− donors are not sufficient to enhance inflammatory responses and atherogenesis when transferred to ApoE-KO recipients
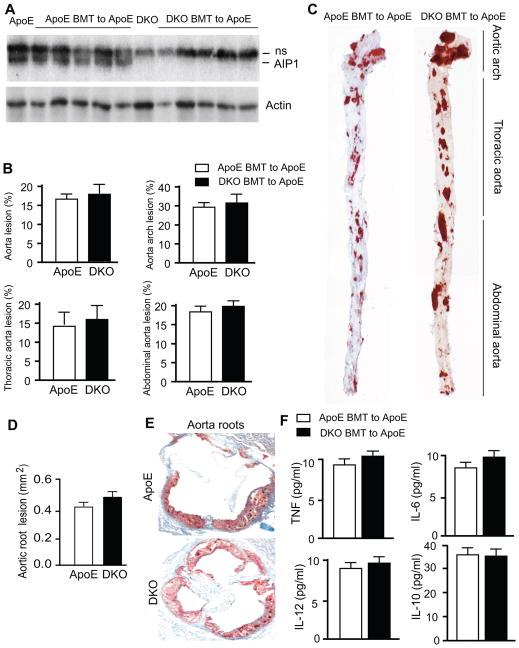
To confirm whether an AIP1-dependent mechanism in macrophages accounts for the increase of atherosclerosis in the DKO mice, we performed bone marrow transplantation (BMT) experiments. To verify our BMT procedure, we transplant bone marrow cells from WT C57BL/6 or AIP1-KO mice (CD45.2+) into irradiated Pep3B recipients (CD45.1+) as we performed previously 31. The engraftment ratio in BM of recipient mice was analyzed by flow cytometry analysis using CD45.2 and CD45.1 surface marker after 6 weeks of BMT. Engraftments reached >95% (percentage of CD45.2+ cells in the recipients. No difference was detected between WT and AIP1-KO bone marrow cells in the reconstitution of recipient BM (n=4). Bone marrow cells from ApoE−/− or DKO mice were transplanted into 8-week-old, lethally irradiated ApoE−/− mice. 6 weeks post-BMT, we confirmed the complete bone marrow reconstitution by genotyping of peripheral blood cells using the AIP1 WT or KO allele-specific primers. Results show that the KO, but not WT allele-specific PCR band, was detected in DKO BMT to ApoE−/− recipients. The complete bone marrow reconstitution was confirmed by Western blot with an AIP1-specific antibody (Fig. 5A). We also performed complete blood tests using the peripheral blood from basal ApoE−/, DKO and BMT ApoE mice. Results indicated that bone marrows in BMT mice were completely reconstituted as measured for hemoglobin, hematocrit, numbers of red blood cell, platelet, total white blood cell as well as individual cell types such as neutrophil, lymphocyte and monocyte. More importantly, no significant increases in peripheral monocytes and lymphocytes were observed (compare Basal vs BMT in Supplemental Table I). We did not observe significant increases in tissue macrophage infiltration 32, suggesting that BMT procedure did not promoted inflammation in the recipients. The BMT mice were then fed with Western-type diet containing 1.25% cholesterol for 10 weeks. Lipid-rich and atherosclerotic plaques were visualized by opening the aortas longitudinally followed by oil red O staining as described. Lesions throughout the aortic arch, thoracic aorta and abdominal region as well as the cross-sections of the aortic roots were similar between the ApoE−/− mice with transplantation of DKO bone marrow cells (BMT DKO to ApoE) and the ApoE−/− mice with transplantation of ApoE−/− bone marrow cells (BMT ApoE to ApoE) (Fig. 5B–C for aortas; Fig. 5D–E for aorta roots). Serum cytokines between the two groups were also similar (Fig. 5F). These data demonstrate that the loss of AIP1 in bone marrow-derived cells (macrophages) does not account for the enhanced aortic atherosclerosis in DKO mice.
Figure 5. AIP1-KO macrophages do not accelerate atherogenesis and alter inflammatory cytokines in ApoE-KO recipients.
A. Characterization of bone marrow transplantation (BMT). Bone marrow cells from ApoE−/− or DKO mice were transplanted into ApoE−/− recipient mice. Successful BMT was verified in six weeks post-BMT by genotyping the peripheral blood cells for the AIP1 gene (not shown). Peripheral blood cell lysates were also determined for AIP1 protein expression by Western blot with anti-AIP1. Peripheral blood cells from ApoE and DKO mice without BMT were used as controls. AIP1 band is indicated. ns: non-specific bands. B–C. The BMT mice were fed with Western-type diet for 10 weeks, whole aortas were harvested, opened longitudinally followed by en face staining with oil red O. Quantifications of atheroma (%) in total and each aortic segment are shown in B, and representative photomicrographs are shown in C with the aortic arch, thoracic aorta and abdominal aorta indicated. Data are mean±SEM from 8 mice per group. D–E. Cross-sections from the aortic roots stained with Oil red-O. Quantification of atheroma area is shown in D, and representative photomicrographs are shown in E. Six sections (150 μm apart for each section) per mouse from 8 mice in each group. Data are presented are mean±SEM. F. Concentration of cytokines: TNF, IL-6, IL-10, IL-12 in plasma of BMT mice with 10 weeks Western-type diet. n = 8 mice in each group. No significant differences between ApoE and DKO BMT groups.
AIP1 deletion augments hyperlipidemia-induced EC dysfunction at early phases of atherosclerosis
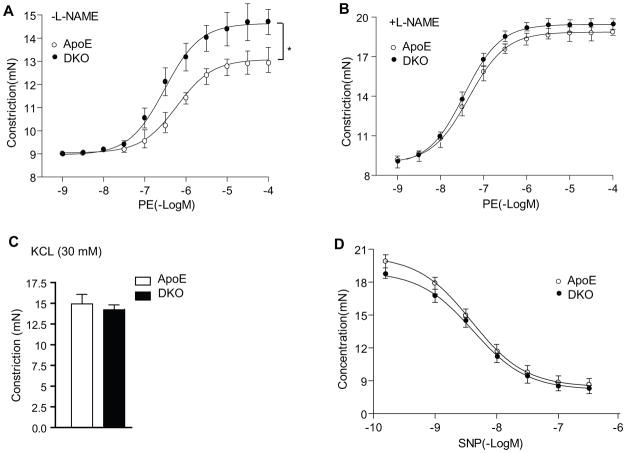
Vascular EC is the primary cell type limits inflammation, and EC dysfunction has been implicated as an early step for atherosclerosis development. To determine if augmented EC dysfunction is a potential mechanism of enhanced atherosclerosis in the DKO mice, aortas from the ApoE−/− and DKO adult mice were harvested for vessel function assays after only 2 weeks on the Western-type diet when few atherosclerotic lesions could be detected. Vascular reactivity of isolated aortic rings was determined by examining the responses to the vasoconstrictor phenylepherine (PE) and the NOS inhibitor L-NAME 33. Aortas from DKO mice were more responsive to PE compared to the ApoE−/− mice (Fig. 6A). Aortas from DKO mice were more responsive to PE, as a 3-fold lower concentration of PE was required to elicit constriction of the isolated aortic rings (EC50=4×10−7 in DKO vs 1.2×10−6 in ApoE−/−). To determine if this difference was due to the basal release of eNOS-derived NO in DKO versus ApoE−/− vessels, aortic rings were preconstricted with submaximal dose of PE, and the NOS inhibitor L-NAME (100 μM) was added at the peak of the constriction to remove endogenous NO tone. Upon removal of the basal NO by the presence of L-NAME, both vessels exhibited similar constriction response to PE (EC50=7.0×10−8 for DKO vs EC50=7.5×10−8 for ApoE−/−)(Fig. 6B). These effects on vasomotion occurred selectively in the endothelium because the vasoconstrictive responses to KCl and relaxation in response to the NO donor drug SNP were similar between the two groups (Fig. 6C–D). We did not observe obvious differences in chow-fed ApoE−/− and DKO mice (Supplemental Fig. II). These data suggest that Western-type diet induces EC dysfunction (reduction in basal NO activity) in the ApoE−/− mice, and this diet-induced EC dysfunction can be further augmented by AIP1 deletion at an early phase of atherosclerosis.
Figure 6. AIP1 deletion augments EC dysfunction at early phases of atherosclerosis.
ApoE−/− and DKO adult mice were fed with Western-type diet for 2 weeks, and aortas were harvested for vessel function assays. A. Aortic rings were contracted with PE at a full range of doses (10−9-10−4 M). Constriction force (mN) is shown. B. Aortic rings were incubated with a NOS inhibitor L-NAME (100 μM) to remove basal NO synthesis and then contracted with PE as in A. C. AIP1 deletion has no effects on vessel constriction in response to KCl. Aortic rings were contracted with 50 mM of KCl. D. AIP1 deletion has no effects on vessel relaxation to the NO donor drug SNP. Aortic rings were incubated with a NOS inhibitor L-NAME to remove basal NO synthesis followed by a precontraction with PE as in A, and were then relaxed with SNP at a full range of doses (10−9-10−6 M). Data in A–D are presented are mean±SEM, with n=5 animals and eight aortic rings per animal, *, p<0.05 indicate that statistically significant by comparing DKO versus ApoE−/−.
AIP1 deletion strongly augments oxLDL-induced inflammatory responses in vascular EC
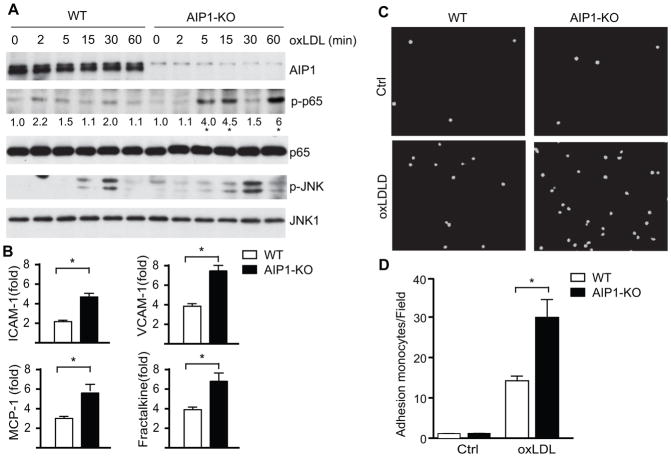
We have previously shown that AIP1 regulates cellular signaling in EC induced by proinflammatory mediators TNF, IL-1β and TLR ligands. Specifically, TLR ligands (LPS for TLR4 or Pam3CSK4 for TLR2)-induced activations of the MAPK and NF-κB signaling pathways were augmented in AIP1-KO EC 13. We determined if AIP1 regulates signaling by oxLDL, a major proinflammatory mediator derived from LDL, using aortic EC isolated from both WT (C57BL/6) and AIP1-KO mice. oxLDL induced activation of NF-κB and JNK in aortic EC as measured by phopho-p65 and phopho-JNK, respectively. Moreover, AIP1 deletion strongly augmented oxLDL-induced NF-κB signaling with a weaker effect on oxLDL-induced JNK activation (Fig. 7A), consistent with the observations in the atherosclerotic aortas (see Fig. 4). In contrast, we observed only small effects of AIP1 deletion on oxLDL-induced NF-κB and JNK signaling in peritoneal macrophages (Supplemental Fig. IIIA). Of note, AIP1 expression in macrophages, similar to peripheral blood cells (see Fig. 5A), was very low compared to aortic EC. This was further confirmed by qRT-PCR (Supplemental Fig. IIIB). We then examined the oxLDL-induced gene expression of macrophage recruiting adhesion molecules (ICAM-1 and VCAM-1) and chemokines (MCP-1 and fractalkine) in ApoE−/− and DKO EC. oxLDL-induced gene expressions of adhesion molecules and chemokines were significantly augmented by AIP1 deletion (Fig. 7B).
Figure 7. AIP1 deletion in EC significantly enhances oxLDL-induced NF-κB signaling in EC, but not in macrophages.
1×106 of WT (C57BL/6) and AIP1-KO mouse aortic EC were treated with 100 μg/ml oxLDL for the indicated times. Phospho- and total p65 and p-JNK1/2 were determined by immunoblotting with the respective antibodies. Total JNK1, AIP1 and β-actin were also determined. Representative blots from three independent experiments are shown. The quantification of the ratios of p-p65/p65 are presented, with untreated WT as 1.0. *, p<0.05 comparing WT vs AIP1-KO. B. WT and AIP1-KO mouse aortic EC were treated with 100 μg/ml oxLDL for 12 h. Transcripts for EC adhesion molecules (ICAM-1 and VCAM-1) and macrophage recruitment chemokines (MCP-1 and fractalkine) were quantified by qRT-PCR and normalized to hypoxanthine guanine phosphoribosyltransferase (HPRT). oxLDL-induced gene expression (fold of increase) are presented. Data are mean±SEM from three independent experiments. *, p<0.05. C–D. AIP1 deletion enhances leukocyte-endothelial adhesion. Isolated WT and AIP1-KO mouse aortic EC were seeded in 6 well plate (5×106 cells/well) as confluent monolayer for 24 h, followed by treatment with 100 μg/ml oxLDL for the other 24 h. Afterwards, RAW 264.7 cells, a mouse leukaemic monocyte macrophage cell line, were pre-stained with live cell tracker CMFDA and loaded onto mouse aortic EC layer (1×106 cells/well) for 1 h. Non-adherent cells were then removed by washing with PBS and adherent cells underwent immunofluorescence microscopy, with quantification of fluorescein isothiocyanate (FITC) units. Representative images are shown in panel C, with quantifications in panel D. Data are mean±SEM from three independent experiments. *, p<0.05.
We finally performed in vitro assays to determine the effects of AIP1 deletion on EC-leukocyte adhesion, a critical early step in inflammatory cell recruitment 2, 34. Fluorescently pre-labeled mouse monocytes were seeded onto confluent primary cultured mouse aortic EC that were pre-treated with oxLDL. AIP1 deletion in mouse EC significantly augmented oxLDL-induced monocyte attachment (Fig. 7C with quantifications in 7D). Taken together, these data suggest that AIP1 deletion in EC primarily attributed to enhanced EC activation and macrophage infiltration, leading to accelerated atherosclerosis progression in DKO mice.
DISCUSSION
The major finding of this study is that a global deletion of AIP1 in vivo enhances hyperlipidemia-induced atherosclerotic progression in an ApoE-null mouse model of atherosclerosis. AIP1 deletion promotes a proatherogenic phenotype without significantly altering metabolic parameters, including body weight, total cholesterol levels or lipoprotein profiles. Mechanistic studies suggest that AIP1 deletion significantly augments LDL-induced NF-κB/JNK proinflammatory signaling in vascular EC but not in macrophages. This augmented proinflammatory signaling contributes to enhanced EC activation/dysfunction, increased macrophage accumulation in the lesion area and exacerbated atheroma growth. In supporting of this conclusion, macrophages from AIP1-deficient donors do not augment inflammatory responses and atherogenesis in ApoE-KO recipients. Therefore, our current study uncovers a critical role of AIP1 in vascular EC in limiting inflammation, EC dysfunction and atherosclerosis.
Our current study further supports our general theme that AIP1 functions as a critical cellular inhibitor of inflammation in the vasculature. We have previously examined the role of AIP1 in inflammatory responses in several mouse models, including a sponge granuloma model 12 and transplant graft arteriosclerosis models 14. In the sponge granuloma model, poly(vinyl alcohol) (PVA) sponges are implanted into mice subcutaneously, and the invasion of the sponge by host cells and the formation of new tissue permit the quantification of inflammatory and angiogenic responses. AIP1 global deletion increases invasion of macrophages, foreign body responses as well as neovascularization 12. However, these previous studies focused on how AIP1 directly regulates angiogenic receptor VEGFR2, while the mechanism for increased microphage infiltration observed in AIP1-KO mice had not been further investigated. Graft arteriosclerosis (also called allograft vasculopathy), the major cause of late cardiac allograft failure, is characterized by arterial intimal hyperplasia due to recruitment and proliferation of smooth muscle cells within the intima, resulting in luminal obstruction and allograft ischemia. Evidences from human studies and animal models suggest that IFN-γ, a proinflammatory cytokine produced by effector T cells, is a critical mediator for smooth muscle cell proliferation. We have shown that AIP1 deletion enhances intimal formation and graft arteriosclerosis by downregulating IFN-γ-JAK2-STAT1/3–dependent migratory and proliferative signaling in smooth muscle cells. Interestingly, AIP1-mediated inhibition of IFN-γ signaling seems to be smooth muscle cell-specific as we do not observe any effects of AIP1 deletion on IFN-γ signaling in EC 14. Therefore, our current study demonstrates a specific function of AIP1 in the vascular EC, which is primarily responsible for limiting hypoerlipidemina-induced inflammatory responses and atherosclerotic lesion progression in atherosclerosis model. This conclusion is supported by the following evidences: 1) Although the ApoE−/−AIP1−/− DKO mice exhibit increased atherosclerotic lesion with increased macrophage infiltration compared to ApoE−/− mice, smooth muscle cell contents are not significantly altered by AIP1 deletion; 2) AIP1 deletion has no effects on body weight, serum cholesterol and triglyceride levels, and lipoprotein profiles in hyperlipidemic ApoE−/− mice; 3) AIP1 deletion augments hyperlipidemia-induced EC dysfunction at early phases of atherosclerosis; 4) finally, AIP1 deletion augments both hyperlipidemia-induced inflammatory cytokines and macrophage-recruiting chemokines with enhanced NF-κB signaling in ApoE−/− mice aortas, and oxLDL-induced NF-κB signaling in vascular EC but not in macrophages. This vascular EC-specific effect is likely due to the fact that AIP1 is abundant in vasculature but is very low in macrophages. We have recently generated EC-specific AIP1 knockout mice that will be useful in defining the function of EC-expressed AIP1 in limiting atherogenesis.
ApoE-null mouse is a widely used model for atherosclerosis. Aortas in these mice show increased oxidative stress and inflammation, and decreased endothelial function 17–19, 33. Atherogenic stimuli such as oxLDL induce NF-κB signaling in EC, which drives expression of adhesion molecules on EC, mediating interactions of monocytes with EC during the initiation of atherosclerosis. Indeed, we observe that AIP1 deletion in vascular EC augments oxLDL-induced NF-κB (and JNK) signaling, gene expression of adhesion molecules, chemokines and monocyte adhesion. Of note, AIP1 deletion has similar effects on oxLDL and TLR2/4 signaling in EC suggest a shared function of AIP1 in these pathways. One possible explanation is that oxLDL utilizes TLR2/4 to activate NF-κB and JNK pathways in vascular EC 35. OxLDL also utilizes unique receptor(s) to induce intracellular signaling in EC. It has been shown that lectin-like oxLDL receptor-1 (LOX-1), a membrane protein structurally distinct from scavenger receptors on endothelial surfaces, is involved in oxLDL-induced NF-κB activation, endothelial dysfunction and atherogenesis 36–38. It will be interesting to investigate if and how AIP1 plays a role in LOX-1-mediated signaling. In addition to NF-κB and JNK signaling, atherogenic stimuli such as oxLDL also induce intracellular production of ROS in EC, which in turn may cause EC dysfunction 39–41. We have previously shown that the ApoE−/− mice exhibit increased oxidative stress, reduced NO bioavailability and EC dysfunction, and EC specific expression of anti-oxidant protein mitochondrial thioredoxin-2 (Trx2) reduces ROS, preserves EC function and reduces atherosclerosis progression in the ApoE−/− model 33. Conversely, the current study indicates that AIP1 deletion augments hyperlipidemia-induced EC dysfunction (reduction of basal NO activity) at an early phase of atherosclerosis, and AIP1 could limit atherogenic stimuli-induced oxidative stress in vascular endothelium. The mechanism by which AIP1 regulates intracellular ROS requires further study.
Taken together, our current study in mouse model of atherosclerosis provides evidence to support the human genome-wide association study (GWAS) that AIP1 is identified as a susceptibility gene for coronary artery diseases16. A future analysis of the regulation of AIP1 expression during atherosclerosis should help to determine if AIP1 is a potential therapeutic target for the prevention of atherosclerosis and other vascular diseases.
Supplementary Material
SIGNIFICANCE.
Using AIP1 knockout mice, we show that AIP1, a signaling adaptor molecule implicated in stress and apoptotic signaling induced by proinflammatory mediators, prevents atherosclerotic lesions in an ApoE-null mouse models of atherosclerosis.
The augmented atherosclerosis is due to AIP1 function in vascular EC, but not macrophages.
AIP1 is highly expressed in aortic EC but not in macrophages; AIP1 in EC inhibits NF-κB signaling to limit inflammation.
Our study in mouse model of atherosclerosis provides evidence to support the human genome-wide association study (GWAS) that AIP1 is identified as a susceptibility gene for coronary artery diseases.
Acknowledgments
We thank Drs. Jordan S. Pober and Renjing Liu for discussion and editing.
Source of funding: This work was supported by grants from NIH grants R01HL065978, R01 HL085789 and R01HL109420.
Footnotes
Disclosure: None.
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50 (Suppl):S388–393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 8.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. Aip1 mediates tnf-alpha-induced ask1 activation by facilitating dissociation of ask1 from its inhibitor 14-3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Zhang R, Luo Y, D’Alessio A, Pober JS, Min W. Aip1/dab2ip, a novel member of the ras-gap family, transduces traf2-induced ask1-jnk activation. J Biol Chem. 2004;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W. Rip1-mediated aip1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ask1-jnk/p38 activation. J Biol Chem. 2007;282:14788–14796. doi: 10.1074/jbc.M701148200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, Sessa WC, Min W. Aip1 functions as an endogenous inhibitor of vegfr2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan T, Liu T, Zhang H, Tang S, Min W. Aip1 functions as arf6-gap to negatively regulate tlr4 signaling. J Biol Chem. 2010;285:3750–3757. doi: 10.1074/jbc.M109.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Qin L, Zhang H, He Y, Chen H, Pober J, Tellides G, Min W. Aip1 prevents graft arteriosclerosis by inhibiting ifn-γ-dependent smooth muscle cell proliferation and intimal expansion. Cir Res. 2011;109:418–427. doi: 10.1161/CIRCRESAHA.111.248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie D, Gore C, Liu J, Pong RC, Mason R, Hao G, Long M, Kabbani W, Yu L, Zhang H, Chen H, Sun X, Boothman DA, Min W, Hsieh JT. Role of dab2ip in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2010;107:2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gretarsdottir S, Baas AF, Thorleifsson G, et al. Genome-wide association study identifies a sequence variant within the dab2ip gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 18.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. Apoe-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 21.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo e. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 22.Caligiuri G, Levy B, Pernow J, Thoren P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 1999;96:6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of sr-bi expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein e-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozen AE, Moriwaki H, Kremen M, DeYoung MB, Dichek HL, Slezicki KI, Young SG, Veniant M, Dichek DA. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, Libby P, Hansson GK, Shortman K, Dong C, Gabrilovich D, Gabrysova L, Howes A, O’Garra A. Highlights of 10 years of immunology in nature reviews immunology. Nat Rev Immunol. 2011;11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De LL, Johnson DR, Whitley MZ, Collins T, Pober JS. Camp and tumor necrosis factor competitively regulate transcriptional activation through and nuclear factor binding to the camp-responsive element/activating transcription factor element of the endothelial leukocyte adhesion molecule-1 (e-selectin) promoter. J Biol Chem. 1994;269:19193–19196. [PubMed] [Google Scholar]
- 28.Min W, Pober JS. Tnf initiates e-selectin transcription in human endothelial cells through parallel traf-nf-kappa b and traf-rac/cdc42-jnk-c-jun/atf2 pathways. J Immunol. 1997;159:3508–3518. [PubMed] [Google Scholar]
- 29.Collins T, Cybulsky MI. Nf-kappab: Pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker RG, Hayden MS, Ghosh S. Nf-kappab, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Ji W, Zhang H, Renda MJ, He Y, Lin S, Cheng EC, Chen H, Krause DS, Min W. Senp1-mediated gata1 desumoylation is critical for definitive erythropoiesis. J Exp Med. 2010;207:1183–1195. doi: 10.1084/jem.20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, Zhang R, Luo D, Li X, Chi H, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of bmx/etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–2355. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 36.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 37.Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Pastorino AM, Gaviraghi G, Lo Cascio V. Oxidized low-density lipoprotein increases the production of intracellular reactive oxygen species in endothelial cells: Inhibitory effect of lacidipine. J Hypertens. 1998;16:1913–1919. doi: 10.1097/00004872-199816121-00010. [DOI] [PubMed] [Google Scholar]
- 38.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-ldl) binding to ox-ldl receptor-1 in endothelial cells induces the activation of nf-kappab through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 39.Greig FH, Kennedy S, Spickett CM. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic Biol Med. 2012;52:266–280. doi: 10.1016/j.freeradbiomed.2011.10.481. [DOI] [PubMed] [Google Scholar]
- 40.Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: A pro-inflammatory factor in vascular disease. Biochem J. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- 41.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.