Abstract
Despite the central importance of peripheral membrane proteins to cellular signaling and metabolic pathways, the structures of protein-membrane interfaces remain largely inaccessible to high-resolution structural methods. In recent years a number of laboratories have contributed to the development of an electron paramagnetic resonance (EPR) power saturation approach that utilizes site-directed spin labeling to determine the key geometric parameters of membrane-docked proteins, including their penetration depths and angular orientations relative to the membrane surface. Representative applications to Ca2+-activated, membrane-docking C2 domains are described.
Keywords: electron paramagnetic resonance, electron spin resonance, membrane proteins, site-directed spin labeling
PERSPECTIVES AND OVERVIEW
Membrane surfaces play a central regulatory role in cellular signaling pathways. The plasma membrane, for example, is one of the primary decision-making centers of the cell where a diverse array of receptors, channels, and signaling complexes make crucial decisions about which signaling pathways are switched on or off in response to extracellular and intracellular signals. Other critical decisions are made at the surfaces of intracellular reticular, Golgi, nuclear, and vacuolar membranes, where membrane protein complexes regulate vesicular and protein synthesis, assembly, trafficking, and degradation. Thus many, if not most, important cellular processes are regulated in part by signaling events occurring at membrane surfaces.
Many of the decisions made at membrane surfaces are initiated or modulated by a ubiquitous class of signaling proteins that transiently dock to the lipid bilayer during specific signaling events (17). These regulated peripheral proteins have two states: an aqueous state for which a high-resolution structure can often be obtained in a straightforward manner, and a lipid-bound state for which structural information is much more difficult to acquire. To date, no high-resolution structure of a peripheral protein bound to a lipid bilayer has yet been described. Yet a structural description of the membrane-bound state is essential for a mechanistic understanding of membrane docking and the subsequent regulation of signaling events. In addition, such structural information will likely facilitate the design of drugs engineered to block membrane docking and thereby inhibit certain signaling pathways.
Recent studies have used a combination of site-directed spin labeling and electron paramagnetic resonance (EPR) power saturation methods to define the membrane-docking depths and geometries of several functionally important peripheral signaling proteins (14, 15, 19, 23, 24). The method, termed the EPR membrane depth measurement, first couples an EPR spin label to selected positions on the protein of interest, and then measures the depth of the spin label in the membrane by monitoring its rate of collisions with extrinsic paramagnetic probes residing in the aqueous phase and in the lipid bilayer. The information provided by a sufficiently large library of spin label positions includes the identification of the protein residues and secondary structure elements that interact with the membrane, and their depth of penetration into the membrane. This information in turn enables the development of a model that describes the depth to which the full protein penetrates into the bilayer, as well as the angle of the docked protein relative to the membrane surface. The method can be carried out under physiological temperature and ionic conditions using small amounts of protein (1 nmol), together with synthetic membrane vesicles that recapitulate the standard bilayer structure and a lipid composition of a specific type of intracellular membrane. Another recently described method for analyzing peripheral protein-docking geometry in lipid bilayers uses solid-state nuclear magnetic resonance (NMR) chemical shifts to identify residues that detect local environmental changes upon membrane docking (39). This method has the advantage of not requiring the coupling of an extrinsic spin probe to the protein; however, it does require orders of magnitude more protein and lipids, and it does not directly measure membrane contact or depth of penetration into the membrane. Thus, the EPR membrane depth method provides the most detailed molecular pictures of membrane-docked proteins currently available.
The use of site-directed spin labeling and power saturation to study protein-membrane interactions was pioneered by studies of transmembrane proteins, membrane-binding peptides, and peripheral proteins in the Hubbell, Cafiso, and Robinson laboratories (2, 5, 6, 22, 33, 42). These studies have measured the accessibility of specific residues to the lipid and aqueous phases, which in turn identifies secondary structure elements on the basis of their characteristic patterns of solvent exposure and burial. Moreover, the measured accessibility parameters place limits on the depth and orientation of individual positions and secondary structure elements in the membrane, as well as identify where specific elements cross the membrane-water interface.
Three recent advances, together with the above pioneering power saturation studies, have led to the development of the current EPR membrane depth method for analyzing the docking of peripheral proteins of known structure to membranes. First, a new procedure has been developed to generate a standard curve relating the measured EPR power saturation parameter to known membrane depths, using spin labels coupled to specific positions on lipid acyl chains or on a transmembrane protein of known structure as calibration points (15, 24). This standard curve enables the membrane depth of an experimental spin label position to be interpolated from the basis of its measured EPR depth parameter. Second, crystallographic studies of spin labels coupled to lysozyme have revealed a common, stable geometry for the methanethiosulfonate spin label (MTSSL) side chain that aids in model building (21). Finally, the number of high-resolution NMR or crystal structures available for the soluble, membrane-free states of peripheral proteins is growing rapidly, making more proteins accessible to a detailed analysis of membrane docking by EPR. Together, these advances have made the refined EPR membrane depth method a powerful tool for generating detailed molecular pictures of a wide variety of peripheral proteins docked to the surface of lipid bilayers under physiological conditions.
The first three peripheral proteins analyzed by the refined EPR membrane depth method are each C2 domains isolated from essential signaling proteins: cytosolic phospholipase A2 (cPLA2), protein kinase Cα (PKCα), and the first C2 domain of synaptotagmin IA (SytIA). The C2 domain is a ubiquitous signaling motif (8, 16–18, 20, 35, 36, 38) typically activated by Ca2+ binding that drives docking to a specific cellular membrane (27, 29, 30). The resulting lipid association serves as a membrane anchor that localizes the other domains of the parent protein to the appropriate membrane surface for the completion of their essential signaling functions. C2 domains have been identified in more than 250 human proteins, making it one of the most common conserved signaling domains (Pfam database, http://www.sanger.ac.uk/Software/Pfam/). C2 domains control a broad array of essential intracellular processes including phosphorylation cascades, G protein circuits, vesicle targeting, synaptic vesicle fusion and neurotransmitter release, cell growth, cell cycle progression, cell migration, and inflammation. While some C2 domains lack Ca2+ regulation or dock to proteins rather than to membranes, most C2 domains characterized exhibit Ca2+-triggered membrane docking, as illustrated by the representative C2 domains considered herein. Analysis of these proteins by the EPR membrane depth method reveals different membrane-docking geometries and provides a physical explanation for the existence of two contrasting membrane-docking mechanisms employed by C2 domains to bind in a targeted fashion to the appropriate cellular membranes.
EXPERIMENTAL DESIGN OF THE EPR MEMBRANE DEPTH METHOD
Site-Directed Spin Labeling
EPR studies of a given peripheral membrane protein require the incorporation of spin labels into the protein at carefully selected locations. Previous studies of membrane proteins have successfully employed a form of site-directed cysteine chemistry and spectroscopy to introduce spin labels at specific sites (12). This approach, termed site-directed spin labeling, incorporates single cysteines on the protein surface via site-directed mutagenesis (1). The locations of cysteine substitutions may be scattered throughout the protein, or they may be focused in one region known to interact with the membrane. Sites on the protein surface are desirable for spin labeling because of their minimal effect on folding and stability, their greater efficiency of labeling, and their accessibility to collisions with probe molecules. Since each spin-labeled variant should possess only one labeled cysteine, native cysteines may interfere if present. Many proteins lack native cysteines, including the cPLA2, PKCα and SytIA C2 domains. When native cysteines are present, however, a cysteine-less version of the protein can be constructed by converting each cysteine to alanine or serine. Previous studies have indicated that such engineered cysteine-less proteins typically retain native structure and function (10, 25, 37, 40). Native cysteines that are protected from labeling by a highly buried location, by involvement in a disulfide bond, or by covalent modification such as myristoylation do not interfere with site-directed spin labeling and can be retained.
When a cysteine substitution is introduced into a protein, the cysteine can be labeled with a nitroxide probe such as MTSSL, which undergoes disulfide exchange with the cysteine to form a disulfide bond between the protein and the spin label as illustrated in Figure 1a. Several chemically different variants of MTSSL have been used in spin labeling studies (3), but the commercial version (Toronto Research Chemicals) is typically used because of its extensive characterization by many published studies and its ready availability. The disulfide exchange reaction can be performed in aqueous solution at physiological pH, so the protein can be labeled without an appreciable degradation or activity loss. Because the label is joined to the protein by a disulfide bond, the free cysteine variant of the protein can be easily regenerated by incubation of the spin-labeled protein with a reducing agent such as DTT.
Figure 1.
Use of site-directed spin labeling and power saturation to measure depth parameters (2, 13–15, 19, 23, 24). (a) Structure of the MTSSL nitroxide probe used for site-directed spin labeling, illustrating its coupling to a cysteine via a disulfide linkage. (b) The two gradients of paramagnetic probes used to define the depth parameter. Molecular oxygen is found at highest concentration in the bilayer hydrocarbon core because of its a polarity: Its concentration gradient points toward the membrane center. The zwitterionic nickel(II) ethylenediaminediacetic acid (NiEDDA) complex is found at highest concentration in the aqueous phase because of its high polarity: Its concentration gradient points away from the membrane. (c) Continuous-wave power saturation curves for a membrane-docked cysteine variant of cPLA2 C2 domain (Cys38-MTSSL) (24). The EPR signal amplitude is plotted as a function of the square root of microwave power. Saturation curves are shown for measurements of samples purged with nitrogen gas (closed circles), samples equilibrated with atmospheric oxygen (open circles), and samples containing 10 mM NiEDDA and purged with nitrogen gas (closed squares).
Once a library of spin-labeled cysteine mutants has been generated, it is important to test for perturbations caused by the modification (14, 15, 19, 24). Stability or activity assays can be used to resolve perturbed proteins from those that retain their native fold and function. The perturbed proteins are excluded from EPR analysis. Typically, incorporation of the spin label at a surface position yields little detectable effect, because the labeled side chain is similar in size to typical amino acids, is electrostatically neutral, and exhibits intermediate polarity and hydrophobicity. Even spin labels located at a membrane-docking surface are usually nonperturbing, because of the innocuous characteristics of the probe and the plasticity of the protein-membrane interface (14, 15, 19, 24).
EPR Analysis of Membrane Depth
Following the isolation and characterization of the library of spin-labeled proteins, EPR is used to investigate their interactions with membranes. Useful qualitative information about the environment and mobility of the spin label can be deduced by simulation of the EPR spectrum. However, for quantitating membrane depth the most instructive experiment is to measure the accessibility of spin labels to different paramagnetic probes and to use those accessibilities to determine the location of the spin label in the membrane interior (2). These accessibility studies utilize continuous-wave power saturation measurements, in which the signal amplitude of an EPR spectrum of the spin-labeled protein in its membrane-associated state is measured over a range of incident microwave powers. As illustrated in Figure 1c, the signal amplitude increases linearly with the square root of incident power, until it begins to saturate and decrease in intensity as the spin populations of the ground and excited states approach equality. The power at which the measured signal amplitude is half-saturated, P1/2, is proportional to the longitudinal relaxation rate of the spin label. Exposure to a paramagnetic probe increases the relaxation rate of the spin label and consequently increases the saturating power. Because this increase in relaxation is dominated by Heisenberg spin exchange, which occurs by collisions with the paramagnetic probe, the increase in saturating power is proportional to the rate of collisions with the probe. The relative accessibility of the spin label to the paramagnetic probe can be expressed by a parameter Π:
| 1. |
where P′1/2 (probe) and P′1/2(N2) are the corrected (2) half-saturating powers for samples containing probe or purged with dinitrogen to remove paramagnetic species (particularly dioxygen), respectively. The resulting Π parameter is directly proportional to the rate of collisions between the spin label and the paramagnetic probe. To measure membrane depth, it is useful to compare the collision rate of a probe in the membrane with that of an aqueous probe. Molecular oxygen, which selectively partitions into the hydrophobic core of the membrane because of its apolar nature, is typically used as the membrane probe, whereas a highly polar metal complex such as NiEDDA or Cr(C2O4)3− is typically used as the aqueous probe. The use of two paramagnetic probes, with concentration gradients in opposite directions as illustrated in Figure 1b, allows for the calculation of the depth parameter Φ:
| 2. |
which is positive for spin label locations deep in the membrane, where collisions with dioxygen dominate, and negative for spin label locations in the aqueous phase, where collisions with the metal complex dominate.
Use of the EPR Depth Parameter to Generate a Membrane-Docking Model
The development of a geometric model for the membrane-docked protein requires the EPR depth parameters to be converted to physical distances relative to the membrane surface. To relate the depth parameter to a distance, it is necessary to measure the depth parameters for spin labels with membrane depths that have been determined by independent methods. Studies of peripheral membrane proteins have utilized spin labels coupled to either phospholipid acyl chains at different carbons or lipid-exposed positions on bacteriorhodopsin (14, 15, 19, 24). The depth parameters for the protein of interest can then be compared with the depth parameters of the standard spin labels through the construction of a calibration curve. However, the construction of a membrane depth calibration curve for peripheral proteins is sometimes complicated by two factors. First, the control spin labels for which membrane depths are known from independent measurements are typically located in the membrane interior, whereas a spin label coupled to a peripheral membrane protein is sometimes located outside this range. In such a case, the membrane depth of the protein-bound spin label must be extrapolated from the standard points, rather than interpolated. Second, the behavior of the depth parameter with respect to membrane depth varies in different regions of the membrane. In the membrane interior, the depth parameter (Equation 2) has been shown to decrease as a simple linear function of the distance from the center of the membrane for bacteriorhodopsin and other membrane proteins (2, 13, 23, 24). Moving through the membrane-water interface into aqueous solution, however, the depth parameter begins to asymptotically approach the constant value it achieves in the aqueous phase far from the membrane surface. A hyperbolic tangent function has provided a reasonable first approximation of this distance-dependence (15):
| 3. |
This equation plots the depth parameter Φ as a function of x, the distance from the plane of the membrane phosphates. The constants A and D set the bulk values of Φ in the water and hydrocarbon, while B and C define the slope and intercept of the linear region of the function, respectively. Like the depth parameter, this function is linear in certain regions of the membrane interior and exhibits an asymptotic approach to the aqueous value of the depth parameter observed beyond the membrane-water interface. However, unlike experimentally measured depth parameters, the function asymptotically approaches its maximum at the center of the membrane, and use of the function requires knowledge of this maximum value. This value may be estimated, but it is difficult to verify experimentally.
Careful measurements of the depth parameter for membrane-exposed sites on the transmembrane proteins bacteriorhodopsin and BtuB indicate that the depth parameter closely approximates a purely linear, rather than asymptotic, approach to a maximum at the membrane center (2, 13). In contrast to the hyperbolic tangent function, a simple hyperbolic function faithfully describes both the linear behavior of the depth parameter in the interior of the membrane and the asymptotic approach of the depth parameter to a constant value at sites distant from the membrane (23; N.J. Malmberg, J.A. Corbin & J.J. Falke, manuscript in preparation):
| 4. |
In this equation, which also plots the depth parameter Φ as a function of the distance of the spin label from the membrane phosphates x, the constants A and B represent the slope and intercept of the linear region of the curve, C represents the value of the depth parameter at sites distant from the membrane, and D reflects the curvature of the junction between the two regions of the curve. Within the current margins of experimental error, this hyperbolic function correctly describes the empirically observed behavior of the depth parameter throughout its full membrane and aqueous ranges (2, 13). The linear behavior of the depth parameter in the membrane interior indicates that the ratio of membrane to aqueous paramagnetic probe concentrations (typically dioxygen and NiEDDA, respectively) decays exponentially from a peak value at the membrane center. In this region, the hyperbolic function fits the depth parameters of the standard spin labels used for calibration with greater accuracy than does the hyperbolic tangent curve. Figure 2 illustrates the shape of the hyperbolic function and its ability to fit experimental membrane depths obtained for the cPLA2 C2 domain and a set of spin-labeled lipids. The hyperbolic function provides a better fit than does the hyperbolic tangent function (23, 24). Similarly, a hyperbolic function provides a better fit than does a hyperbolic tangent function for a recently measured, extensive set of depth parameters obtained for the PKCα C2 domain (23; N.J. Malmberg, J.A. Corbin & J.J. Falke, manuscript in preparation). Overall, it now appears that the hyperbolic function is the best choice for fitting the distance dependence of experimental depth parameters.
Figure 2.
Plot of measured depth parameters versus modeled membrane depth for a membrane-docking C2 domain (24). The depth parameters of spin labels incorporated into the cPLA2 C2 domain are plotted as a function of the modeled distance from the headgroup phosphate plane. Filled circles indicate spin label positions in the membrane interior where the depth parameter varies linearly: These depth parameters were used in the linear fitting procedure that generated the membrane-docking model. Open circles indicate spin label positions outside the linear region that were excluded from the fitting procedure. The side chain conformations of six spin labels were adjusted to optimize the linear fit. Open squares indicate the measured depth parameters for phosphatidylcholine lipids possessing spin labels at different carbons in the A2 fatty acid, plotted as a function of the known distance of the fatty acid carbon from the plane of membrane phosphates (squares). The solid curve represents the best-fit hyperbolic function (Equation 4), illustrating the ability of this function to accurately reproduce the distance-dependence of the depth parameter.
Four recent studies of membrane-docked C2 domains have successfully employed a self-consistent modeling approach to overcome the difficulties presented by the complex dependence of the depth parameter on distance. The highest resolution studies of membrane-docking C2 domains have measured depth parameters for up to 24 site-directed spin label positions on the region of protein surface that lies within or near the membrane in the docked state, as well as depth parameters determined for calibration spin labels on standard lipids or on a standard transmembrane protein (14, 15, 19, 24). The measurement of as many depth parameters as possible serves to optimize the subsequent best-fit analysis that determines (a) the unknown parameters of the modeled function and (b) the geometric parameters of the protein relative to the membrane surface while satisfying (c) the constraints of the calibration points. Because all these features are optimized simultaneously, the approach yields a self-consistent model for membrane insertion. Such an algorithm has been successfully employed with hyperbolic tangent or simple hyperbolic functions to generate self-consistent membrane insertion models for the C2 domains of cPLA2 and SytIA (14, 15) and most recently for the C2 domain of PKCα (23; N.J. Malmberg, J.A. Corbin&J.J. Falke, manuscript in preparation). Alternatively, when a sufficient number of depth parameters can be determined for positions in the membrane interior at which the depth parameter function exhibits linear behavior, a simpler approach can be employed. This approach is illustrated by a study of the cPLA2 C2 domain that measured depth parameters for 13 spin label locations in the membrane interior, 9 on the C2 domain and 4 on the standard lipids (24). In the latter study, a simple linear function yielded a self-consistent membrane insertion model for the membrane-docked C2 domain.
Each of these studies employed the following detailed procedures to dock the structure of the spin-labeled C2 domain to the membrane using the constraints provided by EPR membrane depth measurements (14, 15, 23, 24). The modeling begins with the experimentally determined protein structure, which is modified to incorporate models of spin labels at each of the individual labeling sites. The three-dimensional coordinates of the spin labels are tabulated and serve as a basis for subsequent determinations of the model of membrane association. The labeled protein coordinates are then rotated and translated to optimize the correlation between membrane depth and the selected hyperbolic, hyperbolic tangent, or linear function. The orientation and depth of the protein relative to the membrane surface are defined relative to an arbitrarily chosen plane in coordinate space. As translations parallel to the membrane surface or rotations about the membrane normal result in equivalent positions and orientations of the protein, respectively, these two characteristics can be defined minimally by rotations about the two orthogonal axes parallel to the membrane surface, followed by a translation along the axis perpendicular to the membrane. In the determination of the orientation and position of the membrane protein, the positions of the membrane protein spin labels must be treated differently from the depths of the spin-labeled standards so that the depths of the standard spin labels are not altered by rotation or translation of the membrane protein. This is accomplished by treating the standard spin labels in a fundamentally different way, either by incorporating the standard depth parameter-distance pairs into the calculation of the best-fit calibration curve after the protein model has been rotated and moved, or by utilizing separate rotation and translation variables for the standard and unknown spin labels, holding constant the variables for the calibration spin labels. Overall, the depth parameters of the spin-labeled lipids strongly constrain the penetration depth of the protein in the membrane, and the depth parameters of the protein spin labels provide the primary constraints on the rotations of the domain and the unknown parameters of the best-fit function. The errors of the penetration depth and angular orientation are determined by generating controlled displacements from the optimized model and testing their effects on goodness of fit.
It is important to address spin label conformations in a systematicway during the modeling procedure (14, 15, 23, 24). Typically the initial model uses the same spin label conformation for all positions, in order to begin with a starting structure that is as unbiased as possible. In this case the dihedral angles of the first two bonds of each spin label with respect to the protein backbone are set to a gauche (+) conformation to mimic the most common spin label conformation seen in crystal structures of spin-labeled T4 lysozyme (21). The spin label position in space is then determined by the location of the nitroxide nitrogen, which carries much of the density of the unpaired electron and is magnetically coupled to the electron. Once an initial model for the membrane-associated protein geometry has been produced, it is instructive to readjust the conformations of spin labels that deviate substantially from the resulting calibration curve and to recalculate the geometry model. Rotations about the first two bonds of the spin label with respect to the protein backbone have the largest effect on the position of the modeled spin label. The optimized model will not usually change greatly, but the geometric parameters may be more precisely defined. This optimization helps ascertain whether the deviation of a particular spin label from the calibration curve could be the result of the uncertainty in its side chain conformation. The final, optimized spin label conformations generated for the self-consistent docking model of the cPLA2 C2 domain are illustrated in Figure 3 (see color insert), which also summarizes the depth parameters measured for all 24 spin label positions with a color scale.
Figure 3.
Orientation and depth of the C2 domain of cPLA2 with respect to a membrane surface (24). The crystal structure of the C2 domain of cPLA2 (31) is represented as the cyan ribbon, with two Ca2+ ions shown as yellow spheres. The horizontal lines represent the planar boundaries of the headgroup and hydrocarbon regions of the bilayer. Protein spin labels oriented in their final optimized conformations are colored according to their measured depth parameters, with positive depth parameters indicated by increasing red, and negative depth parameters indicated by increasing blue. Figure generated by Insight (Accelrys).
Limitations of the Approach
The resolution of the EPR membrane depth approach is limited by several factors. One limitation is provided by the conformation and motion of the spin label, which is best circumvented by the use of a large number of labeling positions so that the results do not depend strongly on any one spin label site. Another limitation arises from the assumption that the high-resolution structure of the protein, determined in a solution or crystal environment in the absence of membrane, does not change greatly upon membrane association. This assumption has been shown to be valid for the cPLA2 C2 domain, which undergoes only minimal structural changes upon Ca2+ binding and membrane docking (24). Finally, the method depends on concentration gradients of the paramagnetic probes that vary in a continuous, differentiable way and that vary only along the membrane normal. The presence of protein bound to the membrane may change the local concentrations of both molecular oxygen and metal complex, particularly for proteins that insert deeply into the membrane. However, this perturbation is minimized by the rapid diffusion coefficients of the small-molecule paramagnetic probes utilized by the method.
REPRESENTATIVE APPLICATIONS OF THE EPR MEMBRANE DEPTH METHOD
Early Studies of Membrane-Associated Proteins and Peptides
The use of EPR power saturation to probe membrane depth and accessibility began with a study of bacteriorhodopsin (2). This pioneering study first proposed the exponential nature of chemical gradients in the membrane, as well as the current operational definition of the depth parameter (Equation 2), to linearize the measured collision rates. Distances from the headgroup phosphate plane to the amino acids located at the different spin label sites were estimated from the bacteriorhodopsin crystal structure, enabling the development of a calibration curve that plots the measured depth parameter as a function of distance. The results confirmed that the depth parameter varies linearly with depth inside the membrane.
One of the first studies utilizing the depth parameter to determine membrane geometry was carried out for MARCKS, a membrane-associated signaling peptide (33). Although limited by the small number of available labeling sites, the experiments revealed that the N terminus of the peptide is located in the aqueous headgroup region of the membrane and that the C terminus is embedded more deeply in the membrane. Additional EPR experiments have studied the interactions of MARCKS with its target lipid PI(4,5)P2 (34).
The first studies to use power saturation to define the membrane-docking geometry of a peripheral membrane protein focused on bee venom phospholipase A2 and the C2 domain of cPLA2 bound to lipid vesicles (5, 22). The vesicles were composed of pure, anionic phosphatidylmethanol to generate an electrostatic gradient of paramagnetic Cr(C2O4)3− ions that extends into the aqueous phase well beyond the membrane surface. The resulting models compared favorably with independent studies comparing the depths of penetration of the two domains. However, the angular orientation of the membrane-docked cPLA2 C2 domain differs from that deduced by subsequent studies, most likely in part because the first study utilized pure phosphatidylmethanol, a nonphysiological lipid that generates a membrane with a significantly higher negative charge density than physiological membranes. In addition, these early studies utilized only one concentration gradient for positioning, namely that of the aqueous paramagnetic probe Cr(C2O4)3, rather than two opposing concentration gradients in the aqueous and membrane phases.
Recent Applications to C2 Domains
The EPR membrane depth method has recently been applied to three C2 domains of known structure, illustrating the newest refinements of the approach. Each of these studies has employed the two opposing concentration gradients of the paramagnetic probes O2 and NiEDDA to define the depth parameter as indicated in Figure 1b and Equation 2. The refinements include the use of either a hyperbolic, hyperbolic tangent, or linear function to describe the dependence of the measured depth parameters on the distance of membrane penetration. In addition, the modeling strategy simultaneously varies both the depth and orientation of the protein, as well as the parameters of the selected function, to generate a self-consistent, best-fit model of the membrane-docked protein.
Two of these studies focused on the C2 domain of cPLA2. The initial work utilized 13 spin-labeled cysteine variants of the isolated C2 domain, wherein the labeling sites were scattered throughout the protein surface (15). This study employed the hyperbolic tangent function to develop a self-consistent model of the membrane-docking geometry. The results indicated that the two Ca2+ ions in the domain, which are responsible for high-affinity membrane binding, are located in the headgroup region of the membrane, but the angular orientation of the domain was not well determined, since only four positions yielded positive depth parameters associated with membrane insertion. A second, higher resolution study utilized 24 spin labels, all located in the membrane-interacting loops of the domain, to measure the highest density of depth parameters possible within the membrane interior (24). This study generated a self-consistent docking model using a simple linear function to describe the dependence of the depth parameter on distance within the membrane interior. Since the modeling focused on the linear range of the depth parameter in the membrane interior, only the nine protein spin label positions yielding positive depth parameters indicative of membrane insertion, together with the depth parameters for four spin-labeled lipids, were employed in the analysis. The advantage of this approach is that the depth parameter is most sensitive to distance in the linear region, thus the positive depth parameters selected for the modeling are the best-defined data points, and the analysis avoids all uncertainties regarding the shape of the nonlinear region. The results of this study provide evidence that the conformations of the membrane-docking loops remain essentially unchanged when the Ca2+-occupied protein docks to the membrane, likely due to the extensive crosslinks between loops maintained by the coordination of the two Ca2+ ions. The refined docking model, illustrated in Figure 3, places the two Ca2+ ions within 0 ± 1 Å of the headgroup phosphate plane, and places the body of the C2 domain at an angle roughly 52 ± 5° from the membrane normal, or significantly closer to an orientation perpendicular to the membrane than does the initial model.
A recent study of the first C2 domain of synaptotagmin I (SytIA) utilized spin labels incorporated at 19 positions throughout the membrane-docking loops (14). Because this domain does not insert into the hydrophobic interior of the domain, but instead lies near the membrane-water interface, where the dependence of depth parameter on distance is nonlinear, a linear function would be inappropriate and the hyperbolic tangent function was used to describe the distance-dependence of the depth parameter in modeling studies. All 19 positions were used in the determination of the self-consistent docking model, although five sites exhibited depth parameters that were too close to the asymptotic value of the depth parameter in the bulk aqueous phase to be useful in defining distance. The resulting model indicates that the Ca2+ ions, like those of the C2 domain of cPLA2, reside within −1 ± 3 Å of the membrane phosphates and that the β-strands adopt a tilt of 71 ± 5° from the membrane normal. Thus, the orientation of the domain is 19° more parallel to the membrane surface than observed for the cPLA2 C2 domain.
The C2 domain of protein kinase Cα (PKCα) also has been investigated in two recent studies. The initial study utilized 18 spin labels scattered throughout the protein surface (19). The resulting docking model placed the Ca2+ ions in the headgroup region of the membrane, similar to the other C2 domains. However, the β-strands lie nearly parallel to the membrane surface, based on fluorescence and EPR data for labels on one of the β-sheets. This model was limited by the small number of spin label positions located in the membrane (only three), such that the best-fit analysis was dominated by data points in the aqueous region, where the distance-dependence of the depth parameter is relatively weak. Thus, a second study was undertaken that utilized 24 spin labels incorporated throughout the membrane-docking loops and in the β-sheet facing the membrane surface (23; N.J. Malmberg, J.A. Corbin & J.J. Falke, manuscript in preparation). Using a simple hyperbolic function, this study developed a self-consistent docking model that places the two Ca2+ ions at a distance of 2 ± 2 Å from the headgroup phosphate plane and that places the β-strands at 68 ± 7° from the membrane normal.
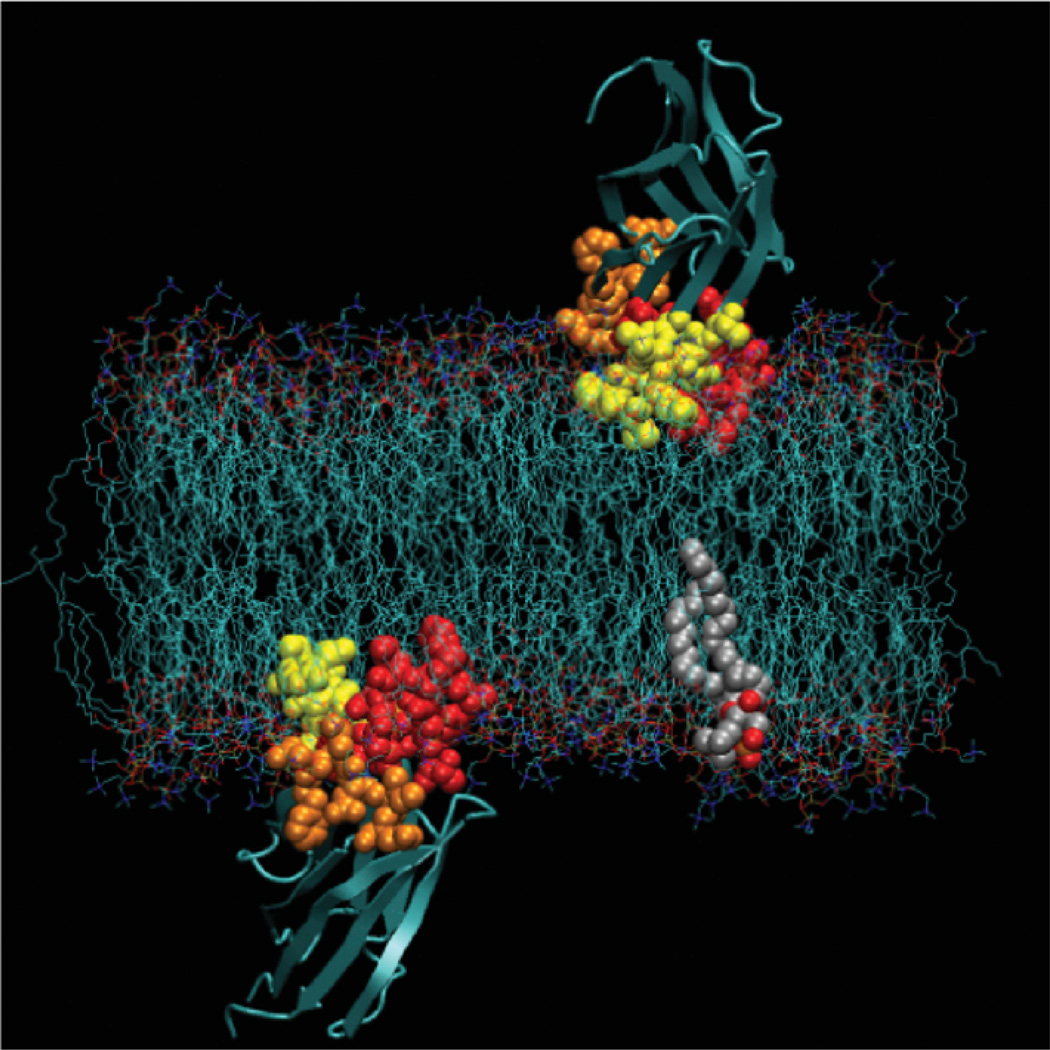
The observed docking models for the three C2 domains (summarized in Table 1 and Figure 4, see color insert) correlate nicely with independent observations of the functional similarities and differences between the domains. That all the models place the Ca2+ ions near the membrane phosphates is consistent with the proposal that Ca2+-dependent membrane docking is mediated at least in part by an electrostatic switch, such that binding of the Ca2+ ions to the membrane-docking loops triggers an electrostatic attraction to the negative charge of the headgroup phosphates (26). The different depths of side chain penetration for the three domains (cPLA2 C2 inserts most deeply, followed by PKCα C2 and SytIA C2) explain the strikingly different membrane-docking mechanisms previously observed for the three domains: cPLA2 C2 exhibits a hydrophobic mechanism that involves deep side chain insertion into the bilayer hydrocarbon core, whereas PKCα and SytIA exhibit an electrostatic mechanism that involves only shallow interactions with the lipid headgroups (30). Interestingly, the trend exhibited by the relative membrane dissociation rates (cPLA2 < PKCα < SytIA) (30) is the inverse of that exhibited by the penetration depths (14, 15, 19, 23, 24), presumably because more time is required to dissociate a deeply penetrating domain than a domain bound to the surface of the headgroup layer. Finally, the greater tilt of the PKCα and SytIA C2 domains toward the membrane surface, relative to the cPLA2 C2 domain, which is oriented closer to the membrane normal, could play an important role in regulating these domains (14, 15, 19, 23, 24). The PKCα C2 domain has been proposed to bind phosphatidylinositol lipids such as PI(4,5)P2 with a lysine-rich cluster of residues on one face of the domain (9). These lysine residues are important for docking to phospholipid membranes and may be responsible for mediating regulation of the C2 domain by interactions with signaling lipids.
TABLE 1.
Comparison of the docking orientations and depths of Ca2+ ions for three membrane-docking C2 domainsa
| C2 domain | β-strand tilt (°) | Average Ca2+ depth (Å) | Deepest label (Å) |
|---|---|---|---|
| cPLA2 | 52 ± 5 | 0 ± 1 | 12 |
| SytIA | 71 ± 5 | −1 ± 3 | 6 |
| PKCα | 68 ± 7 | 2 ± 2 | 9 |
A comparison of the tilt angles and depths of insertion for three different C2 domains (cPLA2, SytIA, and PKCα) studied by continuous-wave power saturation EPR experiments (14, 15, 19, 23, 24). The tilt angle of the β-strands is defined by the angle between a vector defined by two α-carbons in the longest β-strand of the domain and the membrane normal. The Ca2+ depth represents the average distance of the Ca2+ ions from the membrane phosphates in the model (two for cPLA2 and PKCα, three for SytIA). The deepest label parameter indicates the distance from the plane of phosphates for the most deeply buried spin label in the model.
Figure 4.
Comparison of the membrane-docking geometries of the cPLA2, PKCα, and SytIA C2 domains (14, 15, 19, 23, 24). The cPLA2 C2 domain penetrates significantly farther into the hydrocarbon core, explaining its hydrophobic docking mechanism, and the PKCα and SytIA C2 domains interact primarily with the charged headgroup region, explaining their electrostatic docking mechanism. The cPLA2 C2 domain is oriented with its β-strands closer to the membrane normal than the other two domains. For all three domains the Ca2+ ions lie near the headgroup phosphate plane. Figure was generated by MacPymol (DeLano Scientific).
FUTURE APPLICATIONS
Modeling the Effects of Protein Docking on Lipids
Although the EPR membrane depth method provides an accurate picture of the membrane-docking depth and geometry of a given peripheral protein, it does not specify the positions and conformations of the surrounding lipid molecules. Insertion of a protein into the headgroup region, or especially into the hydrocarbon core, must significantly alter the local structure of the lipids and bilayer, but unfortunately such perturbations are inaccessible to current structural methods. However, the docking geometry provided by the EPR approach can be used as a starting point for molecular dynamics simulations of the protein-membrane complex, designed to generate a qualitative structural picture of the inserted protein and the surrounding lipid molecules.
Such an effort has begun for the cPLA2 C2 domain (Steven White, Douglas Tobias, and Simon Jaud at the University of California, Irvine; Joseph Falke at the University of Colorado, Boulder). The approach starts with a pre-equilibrated and solvated 288-lipid 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) bilayer. From each monolayer, 11 lipids are removed and the crystallographic coordinates (31) of two cPLA2 C2 domains are inserted into these cavities using the EPR distance constraints (24). After hydrating and relaxing the system, a molecular dynamics run is carried out until the system reaches equilibrium. Subsequently, a 10-ns dynamic simulation reveals that the unconstrained system is stable and that the membrane-docking loops penetrate nearly to the center of the bilayer and have occasional contacts with lipids from the opposite leaflet that extend transiently into the proximal leaflet. Figure 5 (see color insert) illustrates the resulting atomic model for the protein-membrane interface. The membrane-docking loops exhibit extensive hydrophobic contacts with lipid acyl chains, which accounts for the previously observed hydrophobic docking mechanism of the domain (7, 11, 26, 28, 31, 32). The two Ca2+ ions lie at the depth of the headgroup phosphate plane, but several lipid headgroups are pushed underneath the Ca2+-binding loops such that the phosphate of one lipid provides outer sphere coordination to the Ca2+ ions. Despite the considerable fluctuations observed in the protein backbone during the simulation, the bulky Ca2+-binding loops block access of the headgroup phosphates to the Ca2+ ions, preventing direct inner sphere coordination (Figure 5). This finding was unexpected, since crystallographic studies of thePKCα C2 domain in complex with soluble headgroup analogues reveal direct Ca2+ coordination by lipid phosphates (41). However, in hindsight it makes sense because the PKC loops lack the α-helix present in cPLA2 loop 1 and are thus considerably more open in conformation.
Figure 5.
Molecular model of cPLA2 C2 domain docked to a lipid bilayer showing two views of the domain in the preferred docking geometry. The three Ca2+-binding loops are shown in CPK format with loop 1 in red, loop 2 in orange, and loop 3 in yellow. Also highlighted in CPK is a representative phospholipid (S. Jaud, S. White, J. Falke & D. Tobias, unpublished data). The model was generated by equilibrating the C2 domain in the membrane at the depth and angular orientation specified by the EPR membrane depth analysis (24), followed by unconstrained molecular dynamics that revealed that the system was stable for the full length of the molecular dynamics simulation (10 ns; see text).
Overall, the EPR membrane depth measurement provides the essential geometric information needed to place a peripheral protein at the correct depth and orientation in the membrane prior to molecular dynamics (MD) simulations. The subsequent MD simulations determine whether the EPR model is energetically stable and provide a qualitative picture of the effects of protein docking on the local structure and dynamics of lipids. Together, the EPR approach and careful MD simulations provide the most detailed molecular structural and dynamical picture currently available for membrane-docked peripheral proteins. Future applications of these methods to a broad spectrum of membrane-docked proteins will ultimately provide a wealth of comparative information that will greatly enhance the molecular understanding of membrane-binding mechanisms for diverse peripheral proteins. Of course, to avoid overinterpretation, it is important to be aware of the significant approximations employed by each of the methods.
Analyzing Changes in Docking Geometry Triggered by Regulation
Another important future application of EPR membrane depth measurements will be to analyze functionally critical changes in the docking geometry of membrane-associated peripheral proteins that are triggered by regulatory events. For example, current models propose that the PKCα and SytIB C2 domains, in their Ca2+-activated state, can dock to membranes containing anionic lipids such as PS via their three Ca2+-binding loops. However, both C2 domains are proposed to also possess a PI(4,5)P2 (or PIP2)-binding site on the β3–4 hairpin, which lies on the side of the domain (4, 9). In the case of the PKCα C2 domain, it is proposed that the Ca2+-activated C2 domain first binds to PS in the membrane and then undergoes a two-dimensional search of the membrane surface to locate its comparatively rare target lipid (PIP2). This PIP2 recruitment targets the C2 domain to lipid rafts where other important signaling proteins are located (E. Evans & J.J. Falke, manuscript in preparation). The reverse order of binding is proposed for the SytIB C2 domain, which is predicted to dock first to its membrane-bound target lipid PIP2 via the β3–4 hairpin, and then to the membrane during a Ca2+ signal via the occupied Ca2+-binding loops (4). The second Ca2+-triggered step is proposed to initiate synaptic vesicle fusion with the plasma membrane and neurotransmitter release. For both C2 domains, the putative direct interaction with membrane-bound target lipid PIP2 is likely to significantly alter the depth or orientation of the C2 domain in the membrane. To directly test such regulatory models, and to define the nature of the signal-induced reorientation at the membrane surface, future studies will use the EPR membrane depth measurement to analyze the docking geometry of each domain in the presence and absence of PIP2. Such studies will provide views of membrane-docked peripheral proteins in different signaling states, thereby revealing structural information central to a molecular understanding of their regulatory mechanisms.
Applications to Other Classes of Membrane-Docking Proteins
While the initial applications of the EPR membrane depth approach have provided useful structural information about the geometries of membrane-docked C2 domains, there are many other classes of peripheral proteins for which no such information is yet available. Other important signaling domains that dock to membranes in a regulated fashion include the PH, FYVE, PX, C1, and FERM domains and the EF-hand motif (17). For example, the PH domain is the most common type of regulated membrane-docking motif in the human genome, in which it is found in more than 320 proteins (Pfam database, http://www.sanger.ac.uk/Software/Pfam/). A number of high-resolution structures have been determined for complexes between PH domains and soluble target lipid headgroups (16), but no structural information yet exists for a PH domain bound to its target lipid on a membrane surface. PH domains could, in principle, have strong interactions with the background lipids, and such interactions could play an important role in defining the mechanism of membrane association, ranging from a hydrophobic mechanism involving insertion into the bilayer hydrocarbon core to an electrostatic mechanism for interactions with charges and polar groups in the headgroup region. Notably, interactions with background lipids play a central role in a two-dimensional search mechanism that a representative PH domain uses to more rapidly find its rare target lipid PIP3 on a membrane surface (9a), but the molecular nature of these functionally important interactions remains largely unknown. Such interactions with background lipids would likely modulate the affinity and bound state lifetime of the PH domain-membrane complex and thereby play an important role in tuning the activation parameters as appropriate for cellular signaling events. In short, the EPR membrane depth method will continue to provide unique information regarding the membrane-docking, activation, and regulatory mechanisms of a wide array of peripheral membrane proteins.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Drs. David Cafiso and Wayne Hubbell for helpful conversations, and NIH grant GM063235 for funding.
Contributor Information
Nathan J. Malmberg, Email: nathan.malmberg@colorado.edu.
Joseph J. Falke, Email: falke@colorado.edu.
LITERATURE CITED
- 1.Altenbach C, Flitsch SL, Khorana HG, Hubbell WL. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989;28:7806–7812. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- 2.Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA. 1994;91:1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altenbach C, Oh KJ, Trabanino RJ, Hideg K, Hubbell WL. Estimation of interresidue distances in spin labeled proteins at physiological temperatures: experimental strategies and practical limitations. Biochemistry. 2001;40:15471–15482. doi: 10.1021/bi011544w. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 5.Ball A, Nielsen R, GelbMH,Robinson BH. Interfacial membrane docking of cytosolic phospholipase A2 C2 domain using electrostatic potential-modulated spin relaxation magnetic resonance. Proc. Natl. Acad. Sci. USA. 1999;96:6637–6642. doi: 10.1073/pnas.96.12.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barranger-Mathys M, Cafiso DS. Membrane structure of voltage-gated channel forming peptides by site-directed spin-labeling. Biochemistry. 1996;35:498–505. doi: 10.1021/bi951985d. [DOI] [PubMed] [Google Scholar]
- 7.Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2. Identification of essential calcium ligands and hydrophobic membrane binding residues. J. Biol. Chem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- 8.Cho W. Membrane targeting by C1 and C2 domains. J. Biol. Chem. 2001;276:32407–32410. doi: 10.1074/jbc.R100007200. [DOI] [PubMed] [Google Scholar]
- 9.Corbalan-Garcia S, Garcia-Garcia J, Rodriguez-Alfaro JA, Gomez-Fernandez JC. A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Calpha. J. Biol. Chem. 2003;278:4972–4980. doi: 10.1074/jbc.M209385200. [DOI] [PubMed] [Google Scholar]
- 9a.Corbin JA, Dirks RA, Falke JJ. GRP1 pleckstrin homology domain: activation parameters and novel search mechanism for rare target lipid. Biochemistry. 2004;43:16161–16173. doi: 10.1021/bi049017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culham DE, Hillar A, Henderson J, Ly A, Vernikovska YI, et al. Creation of a fully functional cysteine-less variant of osmosensor and proton-osmoprotectant symporter ProP from Escherichia coli and its application to assess the transporter’s membrane membrane orientation. Biochemistry. 2003;42:11815–11823. doi: 10.1021/bi034939j. [DOI] [PubMed] [Google Scholar]
- 11.Davletov B, Perisic O, Williams RL. Calcium-dependent membrane penetration is a hallmark of the C2 domain of cytosolic phospholipase A2 whereas the C2A domain of synaptotagmin binds membranes electrostatically. J. Biol. Chem. 1998;273:19093–19096. doi: 10.1074/jbc.273.30.19093. [DOI] [PubMed] [Google Scholar]
- 12.Falke JJ, Sternberg DE, Koshland DE. Site-directed sulfhydryl chemistry and spectroscopy: applications in the aspartate receptor system. Biophys. J. 1986;49:20a. [Google Scholar]
- 13.Fanucci GE, Cadieux N, Piedmont CA, Kadner RJ, Cafiso DS. Structure and dynamics of the beta-barrel of the membrane transporter BtuB by site-directed spin labeling. Biochemistry. 2002;41:11543–11551. doi: 10.1021/bi0259397. [DOI] [PubMed] [Google Scholar]
- 14.Frazier AA, Roller CR, Havelka JJ, Hinderliter A, Cafiso DS. Membrane-bound orientation and position of the synaptotagmin I C2A domain by site-directed spin labeling. Biochemistry. 2003;42:96–105. doi: 10.1021/bi0268145. [DOI] [PubMed] [Google Scholar]
- 15.Frazier AA, Wisner MA, Malmberg NJ, Victor KG, Fanucci GE, et al. Membrane orientation and position of the C2 domain from cPLA2 by site-directed spin labeling. Biochemistry. 2002;41:6282–6292. doi: 10.1021/bi0160821. [DOI] [PubMed] [Google Scholar]
- 16.Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Curr. Opin. Cell. Biol. 2001;13:146–152. doi: 10.1016/s0955-0674(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 17.Hurley JH, Misra S. Signaling and subcellular targeting by membrane-binding domains. Annu. Rev. Biophys. Biomol. Struct. 2000;29:49–79. doi: 10.1146/annurev.biophys.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley JH, Tsujishita Y, Pearson MA. Floundering about at cell membranes: a structural view of phospholipid signaling. Curr. Opin. Struct. Biol. 2000;10:737–743. doi: 10.1016/s0959-440x(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 19.Kohout SC, Corbalan-Garcia S, Gomez-Fernandez JC, Falke JJ. C2 domain of protein kinaseCalpha: elucidation of the membrane docking surface by site-directed fluorescence and spin labeling. Biochemistry. 2003;42:1254–1265. doi: 10.1021/bi026596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41:11411–11424. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langen R, Oh KJ, Cascio D, Hubbell WL. Crystal structures of spin labeled T4 lysozyme mutants: implications for the interpretation of EPR spectra in terms of structure. Biochemistry. 2000;39:8396–8405. doi: 10.1021/bi000604f. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Nielsen R, Murray D, Hubbell WL, Mailer C, et al. Docking phospholipase A2 on membranes using electrostatic potential-modulated spin relaxation magnetic resonance. Science. 1998;279:1925–1929. doi: 10.1126/science.279.5358.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmberg NJ. PhD thesis. Boulder: Univ. Colo.; 2004. Spectroscopic characterization of membrane docking C2 domains; p. 167. [Google Scholar]
- 24.Malmberg NJ, Van Buskirk DR, Falke JJ. Membrane-docking loops of the cPLA2 C2 domain: detailed structural analysis of the protein-membrane interface via site-directed spin-labeling. Biochemistry. 2003;42:13227–13240. doi: 10.1021/bi035119+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansoor SE, McHaourab HS, Farrens DL. Determination of protein secondary structure and solvent accessibility using site-directed fluorescence labeling. Studies of T4 lysozyme using the fluorescent probe monobromobimane. Biochemistry. 1999;38:16383–16393. doi: 10.1021/bi991331v. [DOI] [PubMed] [Google Scholar]
- 26.Murray D, Honig B. Electrostatic control of the membrane targeting of C2 domains. Mol. Cell. 2002;9:145–154. doi: 10.1016/s1097-2765(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 27.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalefski EA, Falke JJ. Location of the membrane-docking face on the Ca2+-activated C2 domain of cytosolic phospholipase A2. Biochemistry. 1998;37:17642–17650. doi: 10.1021/bi982372e. [DOI] [PubMed] [Google Scholar]
- 29.Nalefski EA, Slazas MM, Falke JJ. Ca2+-signaling cycle of a membrane-docking C2 domain. Biochemistry. 1997;36:12011–12018. doi: 10.1021/bi9717340. [DOI] [PubMed] [Google Scholar]
- 30.Nalefski EA, Wisner MA, Chen JZ, Sprang SR, Fukuda M, et al. C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity. Biochemistry. 2001;40:3089–3100. doi: 10.1021/bi001968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J. Biol. Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 32.Perisic O, Paterson HF, Mosedale G, Lara-Gonzalez S, Williams RL. Mapping the phospholipid-binding surface and translocation determinants of the C2 domain from cytosolic phospholipase A2. J. Biol. Chem. 1999;274:14979–14987. doi: 10.1074/jbc.274.21.14979. [DOI] [PubMed] [Google Scholar]
- 33.Qin Z, Cafiso DS. Membrane structure of protein kinase C and calmodulin binding domain of myristoylated alanine rich C kinase substrate determined by site-directed spin labeling. Biochemistry. 1996;35:2917–2925. doi: 10.1021/bi9521452. [DOI] [PubMed] [Google Scholar]
- 34.Rauch ME, Ferguson CG, Prestwich GD, Cafiso DS. Myristoylated alanine-rich C kinase substrate (MARCKS) sequesters spin-labeled phosphatidylinositol 4,5-bisphosphate in lipid bilayers. J. Biol. Chem. 2002;277:14068–14076. doi: 10.1074/jbc.M109572200. [DOI] [PubMed] [Google Scholar]
- 35.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 36.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 37.Sahin-Toth M, Frillingos S, Lawrence MC, Kaback HR. The sucrose permease of Escherichia coli: functional significance of cysteine residues and properties of a cysteine-less transporter. Biochemistry. 2000;39:6164–6169. doi: 10.1021/bi000124o. [DOI] [PubMed] [Google Scholar]
- 38.Tomsig JL, Creutz CE. Copines: a ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell. Mol. Life Sci. 2002;59:1467–1477. doi: 10.1007/s00018-002-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuzi S, Uekama N, Okada M, Yamaguchi S, Saito H, Yagisawa H. Structure and dynamics of the phospholipase C-delta1 pleckstrin homology domain located at the lipid bilayer surface. J. Biol. Chem. 2003;278:28019–28025. doi: 10.1074/jbc.M300101200. [DOI] [PubMed] [Google Scholar]
- 40.van Iwaarden PR, Pastore JC, Konings WN, Kaback HR. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991;30:9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]
- 41.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wertz SL, Savino Y, Cafiso DS. Solution and membrane bound structure of a peptide derived from the protein kinase C substrate domain of neuromodulin. Biochemistry. 1996;35:11104–11112. doi: 10.1021/bi961248x. [DOI] [PubMed] [Google Scholar]