Abstract
The α2 adrenergic receptor antagonist yohimbine (YO) increases transmitter release from noradrenergic (NA) terminals in cortical and subcortical brain regions, including the bed nucleus of the stria terminalis (BST). YO activates the HPA stress axis and is potently anxiogenic in rats and humans. We previously reported that hindbrain NA neurons within the caudal nucleus of the solitary tract (NST-A2/C2) and ventrolateral medulla (VLM-A1/C1) that innervate the anterior ventrolateral (vl)BST contribute to the ability of YO to activate the HPA stress axis in rats. To determine whether the same NA pathway also contributes to YO-induced anxiogenesis in the elevated plus maze (EPMZ), a selective saporin ribotoxin conjugate (DSAP) was microinjected bilaterally into the anterior vlBST to destroy its NA inputs. Sham-lesioned controls were microinjected with vehicle. Two experiments were conducted to determine DSAP lesion effects on EPMZ behavior. DSAP lesions did not alter maze behavior in rats after i.p. saline, and did not alter the significant effect of prior maze experience to reduce exploratory and open arm maze activities. However, in maze-naïve rats, DSAP lesions abolished YO anxiogenesis in the EPMZ. Postmortem immunocytochemical analyses confirmed that DSAP consistently ablated caudal NST-A2/C2 and VLM-A1/C1 neurons that innervate the anterior vlBST. DSAP lesions did not destroy non-NA inputs to the anterior vlBST, and produced inconsistent cell loss within the pontine locus coeruleus (A6 cell group) that was unrelated to YO anxiogenesis. Thus, the ability of YO to increase anxiety-like behavior in the EPMZ depends on hindbrain NA neurons that target the anterior vlBST.
Keywords: norepinephrine, anxiety, rat, nucleus of the solitary tract, ventrolateral medulla, locus coeruleus
Introduction
Noradrenergic (NA) signaling within the hypothalamus, cortex, and subcortical limbic forebrain is necessary for the full expression of physiological and behavioral responses to emotional and stressful events (Onaka & Yagi, 1998; Palkovits et al., 1999; Delfs et al., 2000; Cecchi et al., 2002a; Cecchi et al., 2002b; Morilak et al., 2003; Watanabe et al., 2003; Fendt et al., 2005; Morilak et al., 2005; Banihashemi & Rinaman, 2006). Dysregulated NA signaling is implicated in a variety of stress-related disorders, including general anxiety disorder, panic disorder, and post-traumatic stress disorder (Southwick et al., 1999; Sullivan et al., 1999b). Clinical and pre-clinical studies have focused on a potential role for altered NA signaling from the pontine locus coeruleus (LC-A6 cell group) to the medial prefrontal cortex (mPFC) and basolateral amygdala (BLA) in generating symptoms of anxiety in humans and experimental animals (Charney et al., 1983; Bremner et al., 1996a; b; Sullivan et al., 1999a; Charney et al., 2000; Barlow, 2002; Crespi, 2009). However, while the mPFC and BLA clearly contribute to processes underlying attention, arousal, and emotional regulation, the anterior ventrolateral bed nucleus of the stria terminalis (vlBST) appears to be more directly involved in the ability of increased NA signaling to trigger anxiety-like behavior. NA inputs from the hindbrain nucleus of the solitary tract (NST-A2/C2 cell groups) and ventrolateral medulla (VLM-A1/C1 cell groups) to the anterior vlBST are recruited by stressful and emotional stimuli (Aston-Jones et al., 1999; Dayas et al., 2001; Myers & Rinaman, 2002; Zhu & Onaka, 2002; Bailey et al., 2003; Myers & Rinaman, 2005; Bienkowski & Rinaman, 2008; Rinaman, 2011), and NA acts within the vlBST to promote avoidance and anxiety-like behavior (Cecchi et al., 2002a; Cecchi et al., 2007). The anterior vlBST also receives direct input from other brainstem and telencephalic regions that process emotionally-relevant information (Shin et al., 2008; Bienkowski & Rinaman, 2012), and projects in turn to hypothalamic, amygdalar, and brainstem targets that mediate physiological and behavioral responses to threatening stimuli (Walker et al., 2003; Walker et al., 2009; Bienkowski & Rinaman, 2012).
Drugs such as yohimbine (YO) that increase peripheral and central NA transmission are potently anxiogenic, and YO is used clinically as a probe to identify abnormal physiological and affective responses to increased NA signaling (Charney et al., 1983; Gurguis et al., 1997; Sullivan et al., 1999a). Systemic YO also robustly increases anxiety-like behavior in rats (Pellow et al., 1985; Pellow et al., 1987; Baldwin et al., 1989; Braun et al., 2011) while activating cFos expression in NST-A2/C2 and VLM-A1/C1 neurons that provide NA input to the anterior vlBST (Myers et al., 2005). Indeed, YO-induced cFos expression within the anterior vlBST depends on NA inputs from these hindbrain neurons (Banihashemi & Rinaman, 2006). A previous report indicates that pharmacological blockade of adrenergic receptors within the anterior vlBST in rats reverses the anxiogenic effects of immobilization stress (Cecchi et al., 2002a), which increases NA signaling in the BST and other brain regions (Pacák et al., 1995; Cecchi et al., 2002a; Morilak et al., 2003). However, Cecchi and colleagues also reported that adrenergic receptor blockade did not alter baseline anxiety-like behavior (Cecchi et al., 2002a). These findings suggest that the ability of stress to increase anxiety-like behavior depends on increased NA signaling within the anterior vlBST. However, no studies have examined whether selective depletion of NA inputs to an otherwise intact anterior vlBST is sufficient to alter anxiety-like behavior in rats under baseline conditions or in response to treatments that increase NA signaling.
Based on available evidence, we hypothesize that vlBST-projecting NA neurons are necessary for the ability of YO to increase anxiety-like behavior in rats (i.e., are necessary for the ability of YO to increase “state” anxiety), but do not participate in establishing baseline “trait” anxiety-like behavior. To challenge this hypothesis, behavior was assessed in the elevated plus maze (EPMZ) in sham-lesioned rats and in rats with selective bilateral loss of vlBST-projecting NA neurons, with EPMZ tests conducted under baseline conditions or after systemic YO treatment. The EPMZ is a well validated and widely-used instrument to evaluate emotionality in rodents, including baseline trait anxiety and treatment-induced state anxiety (Pellow et al., 1985; Hogg, 1996; Wall & Messier, 2001; Carobrez & Bertoglio, 2005; Walf & Frye, 2007; Goes et al., 2009; Braun et al., 2011). The first experiment compared the effects of systemic YO and vehicle treatment on EPMZ behavior in sham control and lesioned rats. In a second experiment, we examined whether loss of vlBST-projecting NA neurons alters the pronounced effect of prior maze experience to alter maze behavior during a subsequent test, a phenomenon observed previously in non-lesioned rats (File et al., 1990; File & Jr., 1993; Treit et al., 1993; Bertoglio & Carobrez, 2000; Bertoglio & Carobrez, 2004; Albrechet-Souza & Brandao, 2010). It has been suggested that the reduced exploratory behavior and open arm activities displayed by rats during maze re-exposure is indicative of learned avoidance, and can be used to assess memory acquisition and retention (Bertoglio & Carobrez, 2000; Bertoglio & Carobrez, 2004; Albrechet-Souza & Brandao, 2010; Galvis-Alonsoa et al., 2010). Therefore, we sought to determine whether vlBST-projecting NA neurons participate in this process of emotional learning and memory.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN; 290–300g) were singly housed in hanging wire-bottom stainless steel cages in an AAALAC-accredited facility (20–22°C, 12 h light/dark, lights on at 7:00 a.m.), with ad libitum access to pelleted chow (Purina #5001) and tap water. Rats were acclimated to this environment for at least one week before stereotaxic surgery, described below (BST lesions). Experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and are consistent with the U.S. Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Data from 50 rats are included in this report.
BST lesions
As in our previous study (Banihashemi & Rinaman, 2006), we used a NA-specific ribotoxin to selectively lesion NA (and adrenergic) neurons projecting to the anterior ventrolateral (vl)BST. The toxin comprises an antibody raised against dopamine beta hydroxylase (DbH) conjugated to saporin toxin (DSAP, Advanced Targeting Systems, San Diego, CA). After binding to vesicular DbH exposed at sites of NA release within the DSAP injection site, saporin is internalized and transported retrogradely to inactivate ribosomes and selectively destroy NA neurons within 10–14 days (Ippoliti et al., 1992; Madden et al., 1999; Fraley & Ritter, 2003; Rinaman, 2003; Ritter et al., 2003; Banihashemi & Rinaman, 2006; Madden et al., 2006; Bienkowski & Rinaman, 2008). Rats were anesthetized by inhalation of isoflurane (Halocarbon Laboratories, River Edge, NJ; 1–3% in oxygen) and placed into a stereotaxic frame in the flat-skull position. DSAP (21.4 ng/100nl) or 0.15M NaCl vehicle alone (100nl; sham controls) was delivered bilaterally into the anterior vlBST by pressure (20.0 nl/min for 5 min) using a 1.0 μl Hamilton syringe driven by a motorized nano-injector (Stoelting Co., Wood Dale, Illinois). The syringe was left in place for 10 min after each injection to minimize back-diffusion of injectate up the needle track. The vlBST microinjection coordinate target sites were 0.3 mm posterior, 1.8 mm lateral and 7.4 mm ventral to bregma, with the injector advanced at a 10-degree angle from vertical to avoid passing the needle through the anterior dorsolateral BST. These coordinates were based on the rat brain atlas of Paxinos and Watson (Paxinos & Watson, 1997). As in our previous study (Banihashemi & Rinaman, 2006), injections were designed to deliver DSAP into the anterolateral and fusiform subnuclei of the anterior vlBST, where NA inputs are most dense (see Fig. 4).
Figure 4.
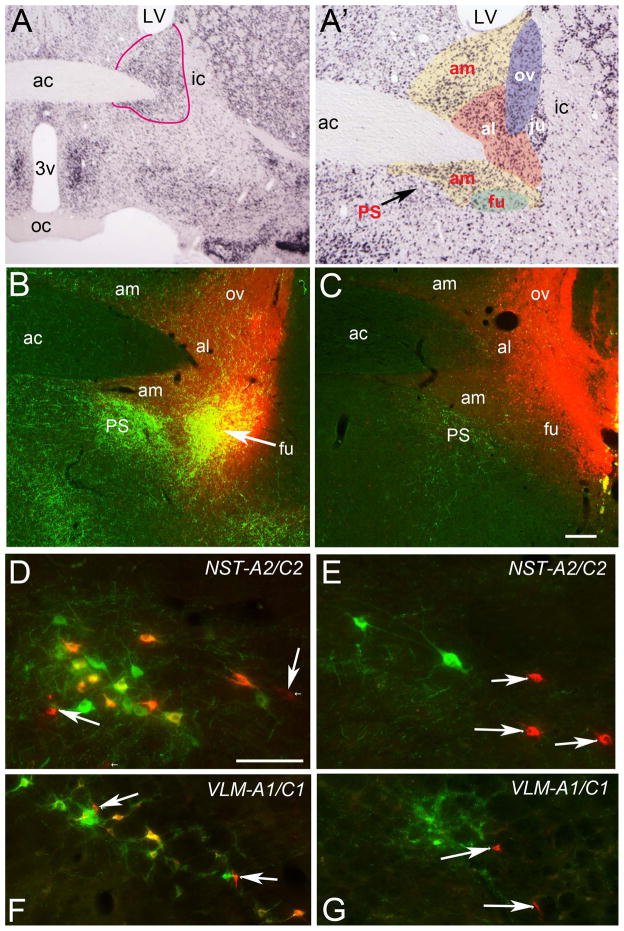
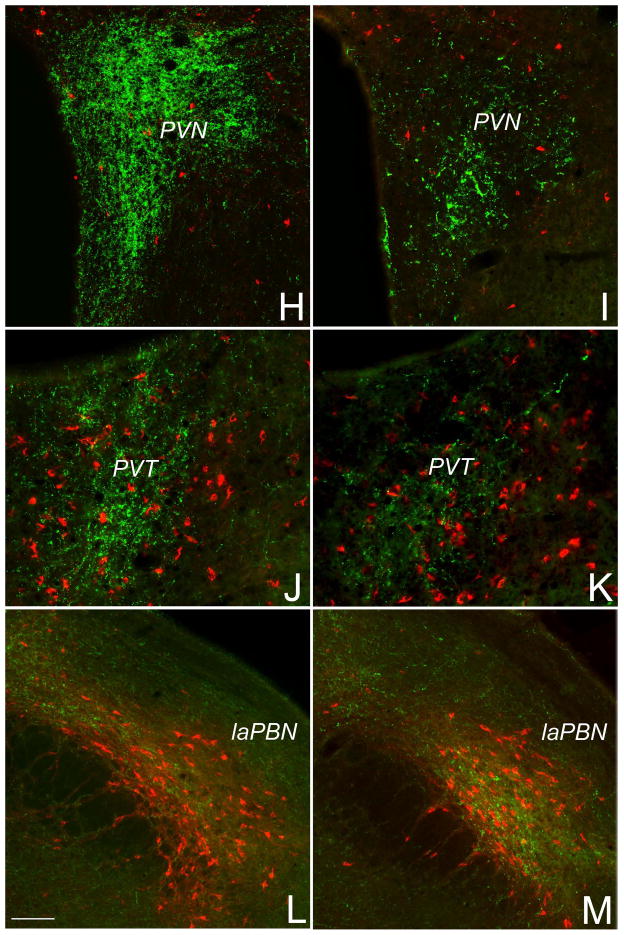
Anatomical analysis of DSAP lesion effects. A, NeuN-immunostained section through the anterior BST from a non-injected control rat. The anterior BST (circled in red) is shown at a higher magnification in A′, where individual BST subnuclei are differentially colored and labeled. The anterior vlBST comprises the ventral am, ventral al, and fu subnuclei. B, photomicrograph of the anterior vlBST in a sham-lesioned rat microinjected with a cocktail of saline+CTB (red). Dense green DbH-positive terminals are evident within the vlBST injection site. C, photomicrograph of the same region in a rat injected with a cocktail of DSAP+CTB. DbH immunolabeling (green) is markedly reduced within the anterior vlBST and adjacent PS subnucleus of the preoptic area. In the sham-lesioned rat, while many NST-A2/C2 (D) and VLM-A1/C1 neurons (F) are double-labeled (yellow-to-orange) for both DbH and CTB, several purely red non-NA neurons also are CTB-labeled (arrows). In DSAP rats, double-labeled NA neurons are absent within the NST (E) and VLM (G), although purely red (non-NA) CTB-labeled neurons remain (arrows). Non-NA neurons also were retrogradely labeled by CTB (red) within the PVN (H, I), PVT (J, K), and laPBN (L, M), with no apparent difference between with sham-lesioned (H, J, L) or DSAP-lesioned rats (I, K, M). Compared to green DbH immunolabeling in sham-lesioned rats, DbH immunolabeling was reduced within the PVN and PVT in DSAP rats. DbH immunolabeling was similar within the laPBN in sham and DSAP rats, suggesting that NA inputs to the laPBN arise from neurons that do not also innervate the anterior vlBST. Scale bar in C = 100 microns, applies also to B. Scale bar in D = 100 microns, applies also to E-K. Scale bar in L = 100 microns, applies also to M. Abbreviations not defined in text: ac, anterior commissure; al, anterolateral; am, anteromedial; fu, fusiform; ju, juxtacapsular; LV, lateral ventricle; ov, oval; PS, parastrial; 3v, third ventricle.
To enhance visualization of vlBST injection sites and to reveal the central distribution of neurons projecting to those sites, a subset of rats used in Experiment 1 (described below) received vlBST microinjections of DSAP (n=2) or vehicle (n=2) mixed in a cocktail with a retrograde neural tracer, cholera toxin βsubunit (CTB, 0.15%, List Biological Laboratories, Inc. Campbell, California). CTB in the injectate did not interfere with DSAP lesion efficacy (see Fig. 4) and had no independent effect on EPMZ behavior.
Behavioral testing on the EPMZ
Behavioral tests were conducted during the light phase of the photoperiod, between 9:00 am–12:00 pm. Rats were weighed, gently handled, and adapted to daily intraperitoneal (i.p.) saline injections for one week before behavioral testing.
The EPMZ comprised two open arms (45 × 10 cm) with transparent 1 cm-high edging, and two closed arms (45 × 10 × 48 cm) extending from a common central platform (10 × 10 cm) elevated 90 cm above the floor. Two 25 W cool white fluorescent bulbs were affixed to the wall 150 cm above the maze at a 45° angle from its center. On test days, rats were injected with sterile saline (3.0 ml/kg BW, i.p.) or YO (1.0 mg/3.0ml/kg BW, Sigma, St. Louis, MO) in random order, and immediately replaced in their home cage. Thirty minutes later, rats were individually transported in a plastic tub into the adjacent testing room and placed onto the center of the EPMZ with their heads facing an open arm. The experimenter then left the room, and the rat’s behavior was video recorded for 5 min. Rats were then returned to their home cage. The EPMZ was cleaned with a mild odor-neutralizing cleanser and allowed to dry between rats.
Video records were scored off-line by a trained experimenter who was blind to surgery and treatment conditions. Maze behaviors were analyzed according to previously described procedures (Walf & Frye, 2007). Quantified variables included [1] time spent in the open arms, closed arms, or center platform; [2] number of entries made into open and closed arms; and [3] time engaged in head-dipping (HD) behavior within an open arm. Ratios for open arm time:total time and open arm entries:total entries also were calculated for each 5 min test. A rat was considered to have entered an open or closed maze arm when all four paws initially occupied it. HD was defined as the rat extending its head outward and downward along the side of an open arm. By convention, a significant decrease in open arm time and/or open arm entries is considered to reflect increased anxiety-like behavior, whereas HD is an open arm exploratory behavior that is viewed as being inversely proportional to anxiety (Fernandes & File, 1996; Walf & Frye, 2007; Braun et al., 2011).
In Experiment 1, maze-naïve rats was tested only once in the EPMZ, two weeks after surgery (n=22 DSAP, n=15 sham), to assess maze behavior after either i.p. saline or YO. Two rats within each surgical lesion group were those that received co-microinjection of CTB together with DSAP or saline vehicle into the vlBST, as described above (BST lesions). Experiment 2 used a within-subjects paradigm in which each rat was tested twice on the EPMZ, with behavior compared between the first and second test. For this purpose, a new group of rats was initially tested two weeks after surgery (n=8 DSAP, n=5 sham) for baseline EPMZ behavior after i.p. saline, and one week later the same rats were re-tested after i.p. saline. This experiment sought to confirm the lack of a DSAP lesion group effect on baseline EPMZ behavior in maze-naïve rats after i.p. saline (as documented in Experiment 1; see Results), and further sought to determine whether DSAP lesions impacted the ability of prior maze experience to alter subsequent maze behavior.
EPMZ data analysis
Behavioral data from Experiment 1 were analyzed by 2-way multivariate ANOVA, with lesion group (sham vs. DSAP) and i.p. injection (saline vs. YO) as the independent variables. Behavioral data from Experiment 2 were analyzed using repeated measures multivariate ANOVA, with EPMZ exposure [maze test 1 vs. test 2] as the within-subjects variable, and lesion group as the between-subjects variable. When F values indicated significant interactions between lesion group and i.p. injection and/or main effects of either independent variable, the ANOVA was followed by post hoc t-comparisons between groups of interest. Effects and differences were considered statistically significant when P < 0.05. Data points were flagged as outliers if they exceeded two standard deviations from the group mean. Data from two rats in Experiment 1 and one rat in Experiment 2 were excluded as outliers, and are not included in the reported group sample sizes or statistics. Data in figures and tables are displayed as group mean ± standard error.
Perfusion and immunohistochemistry
To evaluate the placement and extent of vlBST-targeted NA lesions, DSAP and sham control rats were anesthetized with sodium pentobarbital (Nembutal; 50 mg/kg, i.p.) and transcardially perfused with physiological saline followed by a fixative solution containing 4% paraformaldehyde, 1.4% lysine, and 0.3% sodium metaperiodate in 0.1 M sodium phosphate buffer (McLean & Nakane, 1974). Fixed brains were removed, postfixed overnight in the same fixative, and cryoprotected in 20% sucrose solution for 24–48 hours. Using a freezing stage sliding microtome (Leica), brains were cut into six adjacent series of 35 μm-thick coronal sections from the upper cervical spinal cord through the forebrain rostral to the corpus callosum. Free-floating sections were either collected into buffer (0.1 M sodium phosphate, pH 7.4) and processed immediately for immunohistochemistry, or were collected into cryopreservant solution (Watson et al., 1986) and stored at −20°C for later processing.
Sections were rinsed for 1 h in several changes of buffer before initiating immunocytochemical procedures. Antisera were diluted in buffer containing 0.3% Triton X-100 and 1% normal donkey serum. To assess lesion extent, one set of tissue sections from each rat was processed for single immunoperoxidase localization of DbH using a mouse monoclonal antibody (1:50K; Millipore, Temecula, CA), biotinylated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch), Vectastain Elite ABC immunoperoxidase reagents (Vector Laboratories), and diaminobenzidine (DAB, Sigma Aldrich).
A second set of tissue sections from rats that received vlBST microinjections of CTB+DSAP or CTB+vehicle was processed for dual immunofluorescence to localize CTB (using goat anti-CTB, 1:5000, List Biological Laboratories) and mouse anti-DbH (1:5000), in order to simultaneously visualize vlBST injection sites, non-lesioned retrogradely labeled neurons projecting to those sites, and DbH-positive neurons and fibers. CTB immunofluorescence was visualized using CY3-conjugated AffiniPure donkey anti-goat IgG (1:300, Jackson ImmunoResearch), and DbH was visualized using DyLight 488-conjugated AffiniPure donkey anti-mouse IgG (1:300, Jackson ImmunoResearch). After rinsing, tissue sections were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Houston, TX), allowed to dry overnight, dehydrated and defatted in graded ethanols and xylene, and coverslipped using Cytoseal 60 (VWR, West Chester, PA).
We also assessed whether potential loss of NA neurons within the pontine LC (A6 cell group) was related to the inability of YO treatment to increase anxiety-like EPMZ behavior in DSAP rats. For this purpose, an additional set of tissue sections from selected rats (n=7 sham, n=12 DSAP) in which EPMZ behavior was examined after YO treatment was processed for single immunofluorescent localization of DbH as described above, but using CY3-conjugated donkey anti-mouse IgG (1:300, Jackson ImmunoResearch).
Quantification of DSAP lesion extent
DSAP lesion placement and extent was assessed qualitatively in each rat by inspecting DbH immunolabeling within the BST, PVN, NST, VLM, and LC. In a subset of rats from Experiment 1 (n=18 DSAP, n=7 sham), DbH-positive neurons within the caudal visceral NST and caudal VLM were counted using a light microscope (Nikon, ECLIPSE E40) with a 20X objective. For this purpose, DbH-positive neurons were counted in ten sections (spaced by 210 μm) spanning the A2 and A1 NA cell groups, from the upper cervical spinal cord through the level at which the NST pulls away from the medial wall of the fourth ventricle (i.e., bregma levels −13.0 to −14.9 mm). The number of DbH-positive NST (A2/C2) and VLM (A1/C1) neurons at each rostrocaudal level was determined bilaterally in each case. Counts from individual rats were combined and averaged within each lesion group to obtain mean NST and VLM DbH counts at each rostrocaudal level. The number of DbH-positive neurons remaining within the NST and VLM was analyzed separately using repeated measures ANOVA, with lesion group (DSAP vs. sham) as the between-subjects factor, and rostrocaudal level as the within-subjects factor. When F values indicated a significant interaction and/or main effect of lesion group and rostrocaudal level on DbH counts, each ANOVA was followed by post hoc t-comparisons. Effects and differences were considered statistically significant when P < 0.05. Extremely dense DbH immunolabeling within the pontine LC precluded accurately assessing the number of resident NA neurons in sham or DSAP rats. However, photomicrographs of DbH immunolabeling within the LC at a specific, matched rostrocaudal level (i.e., bregma level −10.0 mm) obtained from sham (n=16) and DSAP rats (n=12) were used to determine qualitatively whether individual DSAP rats displayed a reduced area of DbH labeling within the LC compared to sham rats.
Results
Experiment 1: DSAP lesion effects on EPMZ behavior in maze-naïve rats after i.p. YO vs. saline
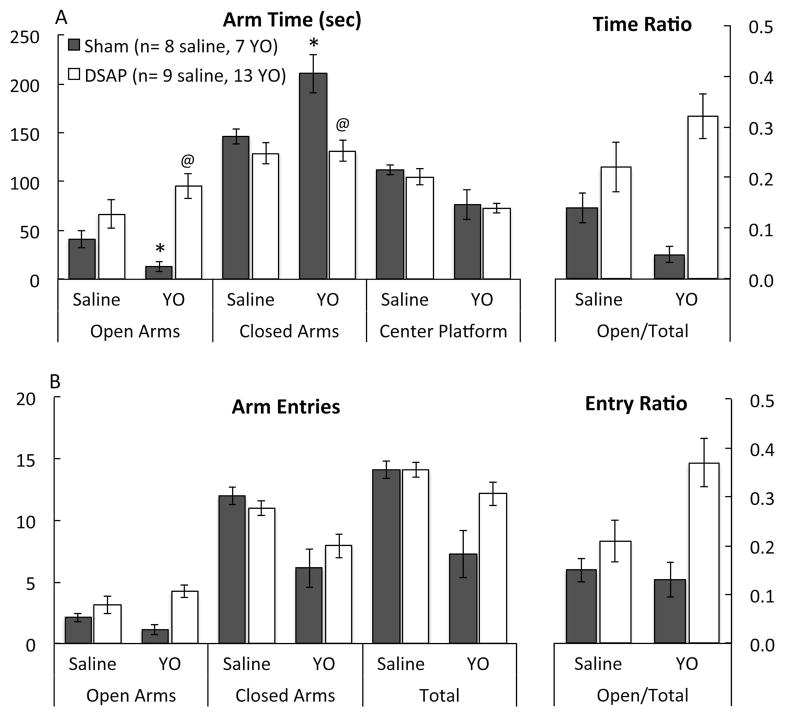
Two weeks after microinjecting DSAP or vehicle into the vlBST, maze-naïve rats from each lesion group were tested in the EPMZ, 30 min after i.p. injection of either saline or YO. As reported in Table 1, two-way multivariate ANOVA revealed a significant interaction between lesion group and i.p. injection on open arm time, closed arm time, and open arm:total time ratio. Surgical lesion group had a significant main effect on 5-min cumulative open arm time, closed arm time, open arm:total time ratio, open arm entries, open arm:total entries ratio, and HD behavior (Table 1). I.p. treatment had a significant main effect on cumulative closed arm time, center time, closed arm entries, total entries, and HD behavior (Table 1).
Table 1.
Two-way multivariate ANOVA (Experiment 1) and two-way repeated measures ANOVA (Experiment 2) main effects, interactions, and P values. See Figures 1–3 for graphical display of data and post-hoc comparisons. Statistically significant effects are highlighted in bold font.
| Measures | Experiment 1 (n=37) | Experiment 2 (n=13) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| See Figures 1 & 2 for post hoc comparisons | See Figures 1 & 3 | for post hoc comparisons | ||||
| Source | F (3, 33) | P | Source | F (1, 11) | P | |
| Open Time | Lesion | 17.24 | 0.00 | Lesion | 1.8 | 0.21 |
| ip treatment | 0.01 | 0.93 | Test | 23.9 | 0.00 | |
| Lesion * ip treatment | 4.81 | 0.04 | Lesion * Test | 2.2 | 0.17 | |
|
| ||||||
| Closed Time | Lesion | 12.11 | 0.00 | Lesion | 0.2 | 0.66 |
| ip treatment | 5.26 | 0.03 | Test | 33.1 | 0.00 | |
| Lesion * ip treatment | 4.43 | 0.04 | Lesion * Test | 1.0 | 0.35 | |
|
| ||||||
| Center Time | Lesion | 1.39 | 0.25 | Lesion | 0.4 | 0.53 |
| ip treatment | 13.52 | 0.00 | Test | 0.6 | 0.47 | |
| Lesion * ip treatment | 0.05 | 0.83 | Lesion * Test | 0.2 | 0.69 | |
|
| ||||||
| Time Ratio (Open/Total) | Lesion | 17.24 | 0.00 | Lesion | 1.8 | 0.21 |
| ip treatment | 0.01 | 0.93 | Test | 23.9 | 0.00 | |
| Lesion * ip treatment | 4.81 | 0.04 | Lesion * Test | 2.2 | 0.17 | |
|
| ||||||
| Open Entries | Lesion | 12.50 | 0.00 | Lesion | 1.0 | 0.34 |
| ip treatment | 0.06 | 0.80 | Test | 26.0 | 0.00 | |
| Lesion * ip treatment | 3.10 | 0.09 | Lesion * Test | 2.1 | 0.17 | |
|
| ||||||
| Closed Entries | Lesion | 0.00 | 0.98 | Lesion | 0.0 | 0.95 |
| ip treatment | 15.99 | 0.00 | Test | 1.7 | 0.23 | |
| Lesion * ip treatment | 1.01 | 0.32 | Lesion * Test | 0.5 | 0.50 | |
|
| ||||||
| Total Entries | Lesion | 3.50 | 0.07 | Lesion | 0.0 | 0.98 |
| ip treatment | 13.81 | 0.00 | Test | 2.5 | 0.14 | |
| Lesion * ip treatment | 3.55 | 0.07 | Lesion * Test | 1.0 | 0.34 | |
|
| ||||||
| Entry Ratio (Open/Total) | Lesion | 10.92 | 0.00 | Lesion | 0.4 | 0.52 |
| ip treatment | 2.04 | 0.16 | Test | 29.5 | 0.00 | |
| Lesion * ip treatment | 3.78 | 0.06 | Lesion * Test | 2.4 | 0.15 | |
|
| ||||||
| HD | Lesion | 17.25 | 0.00 | Lesion | 4.2 | 0.06 |
| ip treatment | 7.73 | 0.01 | Test | 30.2 | 0.00 | |
| Lesion * ip treatment | 0.03 | 0.87 | Lesion * Test | 5.6 | 0.04 | |
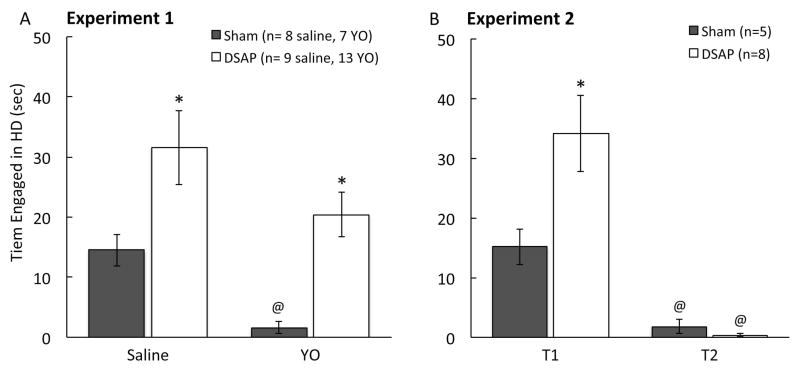
Post hoc comparisons confirmed that the only significant difference between sham and DSAP rats after i.p. saline was in HD behavior, which was expressed more frequently by DSAP rats than by sham rats (Fig. 1A). All other EPMZ measures were similar between sham and DSAP rats after i.p. saline (Fig. 2). Conversely, after YO treatment, open arm time was significantly lower, closed arm time was significantly higher, and the open arm:total time ratio was significantly lower in sham vs. DSAP rats (Fig. 2). HD behavior was virtually abolished in sham rats after YO treatment (Fig. 1A). YO-treated DSAP rats displayed more HD behavior than YO-treated sham rats, but less than saline-treated DSAP rats (Fig. 1A).
Figure 1.
Open-arm head dipping (HD) behavior expressed by rats in Experiments 1 and 2 (see Table 1 for multivariate ANOVA results). A, DSAP lesioned rats displayed more HD behavior than sham control rats regardless of i.p. treatment (*P < 0.05, DSAP vs. sham). YO significantly reduced HD behavior in sham rats (@P < 0.05, YO vs. saline), but not in DSAP rats. B, DSAP-lesioned maze-naïve rats displayed more HD behavior than maze-naïve sham control rats during maze test 1 (T1; *P < 0.05, DSAP vs. sham). HD behavior was significantly reduced in both lesion groups during maze test 2 (T2; (@P < 0.05, T2 vs. T1).
Figure 2.
Experiment 1: DSAP lesions attenuate the anxiogenic effects of YO (1.0 mg/kg body weight) in maze-naïve rats (see Table 1 for multivariate ANOVA results). A, DSAP lesion abolished the YO-induced suppression of EPMZ open arm time and the increase in closed arm time observed in YO-treated sham rats. Post-hoc comparisons: *P < 0.05 for i.p. YO vs. i.p. saline in sham-lesioned rats; @P < 0.05 for DSAP vs. sham rats after i.p. YO. B, Arm entry data are displayed. None of the interaction terms (i.e., lesion x i.p. treatment) reached significance for arm entry data (see Table 1). However, ANOVA indicated a significant main effect of lesion group on open arm entries and the open:total entry ratio, and a significant main effect of i.p. treatment on closed arm and total entries (see Table 1). Group sizes are indicated in panel A.
Experiment 2: DSAP lesion effects on EPMZ behavior in maze-naïve and maze-experienced rats after i.p. saline
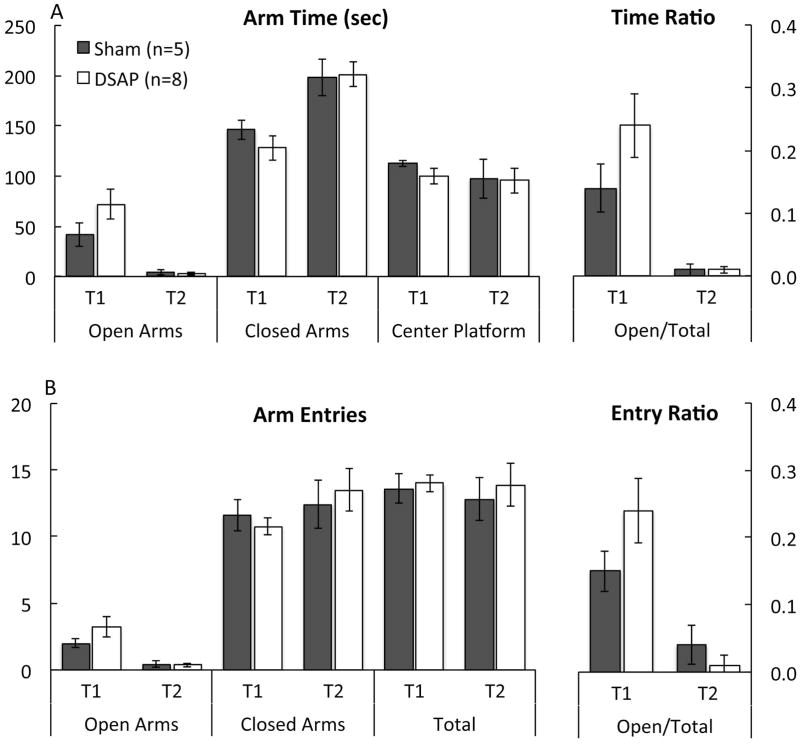
In a new group of DSAP and sham-lesioned rats, post-surgical maze behavior after i.p. saline was evaluated in a test and retest paradigm, with the two post-surgical EPMZ tests (T1 vs. T2) separated by one week. As reported in Table, repeated measures multivariate ANOVA revealed no significant main effect of lesion group on any assessed parameter. HD behavior was the only measure for which there was a significant interaction between lesion group and maze experience (Table 1). There was a significant main effect of prior maze experience to reduce open arm time, to increase closed arm time, and to increase the open arm:total time ratio (Fig. 3A). Prior maze experience also reduced open arm entries and the open arm:total entries ratio (Fig. 3B), and reduced HD behavior (Fig. 1B). Prior maze experience did not affect time spent in the center platform (Fig. 3A), or the number of closed and total arm entries (Fig. 3B). These results confirm the lack of DSAP lesion effect on baseline EPMZ behavior, consistent with results from Experiment 1 using different rats. Experiment 2 results also demonstrate that under baseline conditions (i.e., after i.p. saline), prior exposure to the EPMZ significantly reduced open arm activity during a subsequent test, regardless of DSAP lesion group. Post hoc comparisons confirmed that maze-naïve DSAP rats spent significantly more time engaged in HD behavior compared to maze-naïve sham rats during initial maze exposure (T1; Fig. 1B), similar to results obtained in Experiment 1 (Fig. 1A), whereas no lesion group difference remained for HD behavior during T2 (Fig. 1B).
Figure 3.
Experiment 2: DSAP lesions do not affect baseline (i.e., drug-free) EPMZ behavior in maze-naïve or maze-experienced rats (see Table 1 for a summary of repeated measures multivariate ANOVA main effects, interactions, and P values). There was no significant interaction between lesion group and test on arm time or entry data (see Table 1), and so post-hoc tests were not performed. However, there was a significant main effect of test (T1 vs. T2) on open arm time, closed arm time, and open:total time ratio, and also on open entries and open:total entry ratio (see Table 1). During first-time (T1) maze exposure, sham-lesioned and DSAP rats displayed similar open arm, closed arm, and center platform times (A), as well as similar arm entries (B). Compared to T1 results, T2 results were characterized by significantly reduced open arm time, increased closed arm time, and a reduced open:total time ratio (A), and by reduced open arm entries and a reduced open:total entry ratio (B). These experience-induced effects on maze behavior were similar in sham and DSAP rats. Group sizes are indicated in panel A.
DSAP lesion effects on DbH immunolabeling and CTB retrograde tracing
Microinjections of DSAP or vehicle were designed to target DbH-rich terminal fields within the anterior vlBST (Fig. 4A, B). In two sham-lesioned control rats that were microinjected with CTB+vehicle, a dense plexus of DbH-positive fibers and terminals was evident within the vlBST microinjection site and adjacent parastrial nucleus of the preoptic area (Fig. 4B), and also within the paraventricular nucleus of the hypothalamus (PVN; Fig. 4H), the paraventricular thalamus (PVT; Fig. 4J), and the lateral parabrachial nucleus (laPBN; Fig. 4L). Many tracer-labeled neurons within the NST and VLM were DbH-positive in sham rats (Fig. 4D, F), whereas tracer-labeled neurons within the PVT and PBN were not (Fig. 4J, L). A small number of tracer-labeled neurons were observed within the pontine LC, including a subset (i.e., 5 neurons total across the rostrocaudal extent of the LC) that were DbH-positive in sham rats. In two rats that received CTB+DSAP microinjections into the vlBST, DbH immunolabeling was sparse or absent within the injection site (Fig. 4C), coupled with a notable loss of DbH terminal immunolabeling within the adjacent parastrial nucleus (Fig. 4C), and loss of retrogradely-labeled, DbH-positive neurons within the NST (Fig. 4E) and VLM (Fig. 4G). Reduced DbH terminal labeling also was noted within the PVN (Fig. 4I) and PVT (Fig. 4K), consistent with earlier reports of collateralized axonal projections from NST and VLM neurons that target the diencephalon and limbic forebrain (Banihashemi & Rinaman, 2006; Bienkowski & Rinaman, 2008; 2012). As in sham rats, retrogradely-labeled, DbH-negative neurons in DSAP rats were present in many brain regions known to target the vlBST (Shin et al., 2008; Bienkowski & Rinaman, 2012), including the NST (Fig. 4E), VLM (Fig. 4G), PVT (Fig. 4J), and laPBN (Fig. 4M). There were no obvious differences between lesion groups in the number of DbH-negative retrogradely labeled neurons in these regions, although cell counting was not performed.
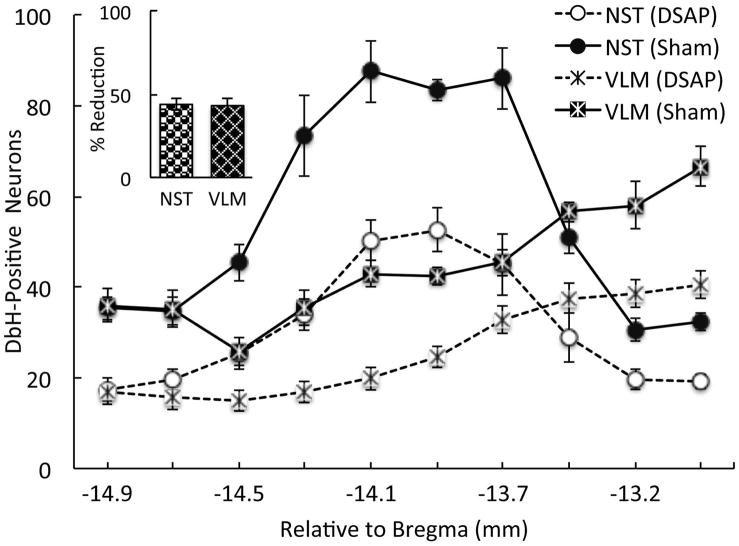
Tissue sections from a subset of rats were used to count NA (i.e., DbH-positive) neurons remaining within the NST and VLM in rats after DSAP or sham lesion surgery (Fig. 5). Repeated-measures ANOVA revealed a significant interaction between lesion group and rostrocaudal level for the NST (F 9, 207 = 4.6; P < 0.01), but no significant interaction for the VLM (F 9, 207 = 1.7; P = 0.09). Thus, the magnitude of DSAP-induced NA neuronal loss within the NST varied depending on NST rostrocaudal level, whereas DSAP-induced loss of NA neurons within the VLM did not vary across levels. There was a significant within-subjects effect of rostrocaudal level on NA cell counts within the NST (F 9, 207 = 49.2, P < 0.01) and the VLM (F 9, 207 = 44.7, P < 0.01), and a significant between-subjects effect of DSAP lesion on the number of NA neurons within the NST (F 1, 23 = 53.8; P < 0.01) and VLM (F 1, 23 = 42.0; P < 0.01). As expected, DSAP lesions produced a significant loss of NA neurons in both medullary regions (Fig. 5). Post hoc comparisons confirmed that DSAP-induced loss of DbH-positive NA neurons was significant at each rostrocaudal level examined (P < 0.05 at each level for both NST and VLM; Fig. 5). Across all rostrocaudal levels, DSAP lesions produced an average 43.9% loss of DbH-positive NST neurons, and an average 43.1% loss of DbH-positive VLM neurons (Fig. 5, inset) compared to counts in sham controls.
Figure 5.
Anterior vlBST-targeted DSAP lesions reduced the number of DbH-positive NA neurons remaining within the NST-A2/C2 and VLM-A1/C1 cell groups compared to counts in sham-lesioned controls. Inset: Average percent reduction of DbH-positive neurons in DSAP rats (n=18) across ten rostrocaudal levels, relative to counts in sham controls (n=7). The percent reduction of NA neurons in each DSAP rat was calculated using the average counts in sham rats as 100%. Line graph symbols represent group means. Post hoc analyses confirmed that NA cell count differences between DSAP and sham rats were significant at every rostrocaudal level of the NST and VLM (P < 0.05 at each level).
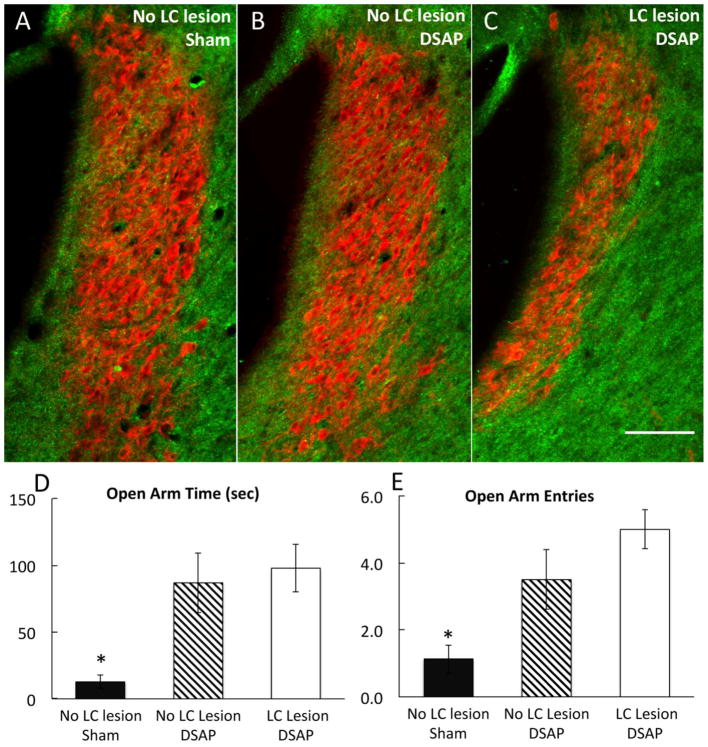
As reported near the beginning of this section, very few tracer-labeled neurons were observed within the pontine LC in rats that received CTb injections into the anterior vlBST. However, the LC area occupied by DbH immunolabeling was noticeably reduced in some (but not all) DSAP rats compared to LC DbH labeling in sham-lesioned controls. Loss of DbH immunolabeling within the LC varied among DSAP rats, presumably due to variability in the extent to which DSAP microinjections targeting the anterior vlBST extended into overlying or adjacent brain regions that receive NA input from the LC, and/or the extent to which DSAP diffused into the lateral ventricle [which can kill LC neurons (Wrenn et al., 1996)]. To determine whether loss of LC neurons contributed to the loss of YO anxiogenesis in DSAP rats, EPMZ data from a subgroup of YO-treated DSAP rats that displayed visibly reduced LC DbH immunolabeling (n=6; see Fig. 6B) were compared with EPMZ data from YO-treated sham-lesioned rats (n=7; Fig. 6A), and with EPMZ data from another subgroup of YO-treated DSAP rats (n=6) with no apparent loss of LC DbH immunolabeling (Fig. 6C). Compared to results in YO-treated sham rats, open arm time and open arm entries were significantly higher in both subgroups of DSAP rats after YO treatment, evidence for less anxiety-like behavior in DSAP rats [Fig. 6D, E; main group effect on open arm time, F 2,16 = 8.5, P < 0.01; main group effect on open arm entries, F 2,16 = 8.7, P < 0.01]. Importantly, time spent in the open arms of the maze (Fig. 6D) and the number of entries into the open arms (Fig. 6E) did not differ between the two subgroups of DSAP rats after YO treatment (post-hoc t-tests, P > 0.05 for each variable), regardless of the presence or absence of visible LC neuronal loss. The total number of DbH-positive neurons remaining within the NST and VLM also did not differ between the two DSAP subgroups (NST: 320±33 vs. 330±27.4 neurons, P = 0.97; VLM: 246±18.3 vs. 260±44 neurons, P = 0.94), whereas in both DSAP subgroups the loss of NST and VLM NA neurons was significant compared to total counts of DbH-positive neurons in sham-lesioned rats (NST: 598±18.7 neurons; VLM: 475±7.6 neurons; for both regions, P < 0.00 for counts in sham rats vs. counts in each DSAP subgroup).
Figure 6.
Immunofluorescent localization of DbH (red) within the pontine LC (A6 NA cell group; bregma level −10.0 mm) in a sham-lesioned rat (A; no LC lesion), a DSAP rat from the subgroup displaying no apparent loss of DbH-positive LC neurons (B; no LC lesion), and a DSAP rat from the subgroup in which a loss of DbH-positive LC neurons is evident (C; LC lesion). Scale bar in C = 100 microns, applies also to A and B. Bar graphs illustrate time spent in the open arms of the EPMZ (D) and the number of open arm entries (E) by rats in each group after YO treatment (data represent group means ± SE). After YO treatment, sham-lesioned rats (n=16) spend significantly less time in the EPMZ open arms and make fewer open arm entries compared to both subgroups of YO-treated DSAP rats (n=6 per subgroup; *P < 0.01, sham vs. DSAP). Differences between DSAP subgroups were not significant.
Discussion
Results from previous studies have implicated NA inputs to the BST in mediating the physiological and behavioral effects of anxiogenic, stressful, and aversive stimuli (Aston-Jones et al., 1999; Delfs et al., 2000; Cecchi et al., 2002a; Cecchi et al., 2002b; Morilak et al., 2003; Fendt et al., 2005; Schweimer et al., 2005; Banihashemi & Rinaman, 2006). The present study is the first to demonstrate that selective depletion of NA inputs to an otherwise intact anterior vlBST is sufficient to block the anxiogenic effects of YO. We further localize the anatomical source of this selective and behaviorally effective depletion to hindbrain NA neurons, rather than to the pontine LC. Strikingly, the loss of YO anxiogenesis after DSAP lesions occurs without any change in baseline EPMZ behavior, and without altering the ability of prior maze experience to alter subsequent maze behavior. We recently reported that the anxiogenic effect of YO also requires direct connections between the alBST and the central nucleus of the amygdala (CeA) in rats, although bilateral BST-CeA disconnection does not alter EPMZ behavior under baseline conditions (Cai et al., 2012). Collectively, our findings support the view that the ability of YO to promote anxiety-like behavior in the EPMZ requires NA signaling within the anterior vlBST, and also requires direct BST-CeA connections (Cai et al., 2012). Conversely, neither NA inputs nor direct BST-CeA interactions appear necessary for shaping EPMZ behavior under baseline (i.e., no drug/no additional stress) conditions.
Postmortem immunocytochemical analysis confirmed that DSAP lesions destroyed DbH-positive neurons within the caudal NST and VLM that provide NA input to the anterior vlBST, without destroying non-NA inputs to the same region. The present report is the first to combine DSAP lesions with retrograde labeling in order to support the neurochemical specificity of the lesions. Quantitative analysis revealed that approximately 43% of DbH-positive NA neurons within the NST-A2/C2 and VLM-A1/C1 regions were lost after bilateral DSAP lesions. While significant, this extent of NA cell loss is somewhat less than that obtained in a prior study in which 59–69% of NST and VLM NA neurons were destroyed after bilateral DSAP injections into the vlBST (Banihashemi & Rinaman, 2006). The apparently reduced DSAP lesioning efficacy observed in the present study may be due to differences in the lots/batches of ribotoxin used in each study. Nevertheless, DSAP lesions in the present study were effective and neurochemically specific, and robustly disrupted the anxiogenic effects of YO. Interestingly, the more complete vlBST DSAP lesions produced in our earlier study markedly reduced HPA stress axis responses to YO, but did not reduce the ability of YO to inhibit food intake or to support the formation and expression of conditioned flavor avoidance (Banihashemi & Rinaman, 2006). Although some DSAP rats in the present study also displayed a visible loss of DbH-positive NA neurons within the pontine LC (A6 cell group), the presence or absence of an LC lesion was unrelated to the blockade of YO anxiogenesis displayed by all DSAP rats. Considered together, our findings support the view that hindbrain NA neurons that innervate the anterior vlBNST play a special role in mediating the anxiogenic and HPA axis-activating effects of YO, whereas the same NA neurons are not involved in mediating YO-induced hypophagia or conditioned avoidance. Our results also challenge the proposed role of pontine LC neurons in mediating YO anxiogenesis (e.g., (Crespi, 2009), insofar as LC neuronal loss was unrelated to the ability of YO to induce anxiety-like behavior in DSAP-lesioned rats.
Anatomical analysis of DbH immunolabeling patterns in the present study confirmed previous reports (Woulfe et al., 1988; Banihashemi & Rinaman, 2006; Schiltz & Sawchenko, 2007; Bienkowski & Rinaman, 2008; 2012) that at least a subset of NA neurons projecting to the vlBST have axons that collateralize to provide NA input to other forebrain regions, including the thalamus, hypothalamus, and amygdala. Accordingly, loss of vlBST-projecting hindbrain NA neurons in DSAP rats also resulted in loss of some NA axonal inputs to other brain regions, which could contribute to the behavioral and physiological effects of the lesion (Banihashemi & Rinaman, 2006). Nevertheless, as reviewed below, the available evidence supports the view that NA signaling within the vlBST is most critical for the anxiogenic effects of YO. The observed loss of DbH-positive fibers and terminals in forebrain regions other than the vlBST (e.g., PVN, PVT) was always partial rather than complete, suggesting that the more complete loss of NA inputs to the anterior vlBST had the greatest impact on experimental outcomes. Additional experiments in which DSAP injections are targeted to other forebrain regions that receive collateralized axonal input from vlBST-projecting hindbrain NA neurons will be necessary to determine whether NA signaling to those regions contributes importantly to YO anxiogenesis, although results from such studies would be impacted similarly by an expected (but less complete) loss of NA inputs to the anterior vlBST. DSAP lesions in this study are best described as destroying NA neurons that target the vlBST. The behavioral effects of these lesions should not be strictly attributed to loss of NA inputs to the vlBST, but rather to the loss of hindbrain NA neurons that provide these inputs.
Experiment 1 examined the effect of DSAP lesion on baseline EPMZ behavior after i.p. saline, and on behavioral responses to YO. DSAP lesions did not alter baseline EPMZ behavior, except for an interesting effect to increase the incidence of open-arm HD behavior (discussed further, below). However, DSAP lesions abolished the ability of YO to reduce open arm time and to increase closed arm time. Experiment 2 used a new group of rats to confirm the absence of lesion effect on baseline EPMZ behavior after i.p. saline, and further demonstrated that DSAP lesions did not alter the known effect of prior EPMZ experience to alter EPMZ behavior during a subsequent test, a phenomenon observed previously in non-lesioned rats (File et al., 1990; File & Jr., 1993; Treit et al., 1993; Bertoglio & Carobrez, 2000; Bertoglio & Carobrez, 2004; Albrechet-Souza & Brandao, 2010). Indeed, test/retest EPMZ data in the present study are very similar to data reported by Albrechet-Souza and Brandao (see their Figure 5B) (Albrechet-Souza & Brandao, 2010). Those authors also reported distinct behaviors displayed by rats during initial and subsequent EPMZ exposure that are associated with distinct patterns of brain activity. For example, maze-experienced rats display significantly more neuronal cFos activation within the ventral medial prefrontal cortex, BLA, and central amygdala after EPMZ re-testing compared to cFos activation displayed by maze-naïve rats after initial testing (Albrechet-Souza et al., 2008; Albrechet-Souza & Brandao, 2010). It has been suggested that the reduced exploratory behavior and open arm activities displayed by rats during maze re-exposure is indicative of learned avoidance, and can be used to assess memory acquisition and retention (Bertoglio & Carobrez, 2000; Bertoglio & Carobrez, 2004; Albrechet-Souza & Brandao, 2010; Galvis-Alonsoa et al., 2010). In the present study, DSAP lesions had no significant impact on the development of avoidance learning that presumably underlies the qualitative shift in emotional state suggested by behavioral changes between Test 1 and Test 2. These novel results discount any significant contribution by hindbrain NA neurons that target the anterior vlBNST to this process of learned avoidance.
Interestingly, open-arm HD behavior was significantly more frequent in maze-naïve DSAP rats vs. sham controls after either saline or YO treatment, providing some evidence for increased “exploration” and reduced trait anxiety under baseline conditions in DSAP rats. The frequency of HD in the EPMZ has been reported to be inversely proportional to anxiety (Fernandes & File, 1996; Walf & Frye, 2007; Braun et al., 2011). However, HD was the only behavioral measure that differed between surgical groups after i.p. saline; thus, the weight of evidence supports the view that loss of NA input to the anterior vlBST has relatively little impact on baseline maze behavior. This conclusion is consistent with a previous report that pharmacological blockade of adrenergic receptors within the anterior BST does not alter baseline EPMZ behavior, although it does reverse the anxiogenic effects of prior immobilization stress (Cecchi et al., 2002a), which increases NA signaling within the BST and other brain regions (Pacák et al., 1995; Morilak et al., 2003).
DSAP lesions presumably did not reduce the peripheral sympathomimetic effects of YO, and combined results from our earlier cFos studies indicate that similar DSAP lesions targeting the anterior vlBST do not suppress the ability of YO to activate pontine LC-A6 neurons or to activate the cortical or subcortical targets of these neurons (Myers et al., 2005; Banihashemi & Rinaman, 2006). Our findings do not challenge the well-known contribution of LC-A6 neural signaling pathways (i.e., the “LC-NE system”) in regulating arousal and emotionality (Chrousos & Gold, 1992; Charney & Deutch, 1996; Sullivan et al., 1999a; Charney et al., 2000), but they provide an impetus for increased research focus on how NA signaling pathways from the hindbrain participate in generating stress-related anxiety. This expanded view should ultimately contribute to more cogent hypotheses regarding the origin and neuropathophysiology of anxiety disorders.
Acknowledgments
The authors thank Li Cai, Emily Maier, and Victoria Maldovan for expert technical assistance. Research supported by the National Institutes of Health #MH59911.
Footnotes
The authors declare no conflicts of interest.
References
- Albrechet-Souza L, Borelli KG, Brandao ML. Activity of the medial prefrontal cortex and amygdala underlies one-trial tolerance of rats in the elevated plus maze. Journal of Neuroscience Methods. 2008;169:109–118. doi: 10.1016/j.jneumeth.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L, Brandao ML. Hormonal and cognitive factors associated with the exploratory behavior of rats submitted to repeated sessions of the elevated plus-maze. Psychology & Neuroscience. 2010;3:43–52. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? Journal of Psychopharmacology. 2003;17:252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Johnston AL, File SE. Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. European Journal of Pharmacology. 1989;159:211–215. doi: 10.1016/0014-2999(89)90709-7. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. The Guilford Press; New York: 2002. [Google Scholar]
- Bertoglio LJ, Carobrez AP. Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behavioural Brain Research. 2000;108:197–203. doi: 10.1016/s0166-4328(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Scopolamine given pre-Trial 1 prevents the one-trial tolerance phenomenon in the elevated plus-maze Trial 2. Behav Pharmacol. 2004;15:45–54. doi: 10.1097/00008877-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and the anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Structure & Function. 2012 doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague–Dawley rats: Effects of anxiolytic and anxiogenic agents. Pharmacology Biochemistry and Behavior. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical Studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical Studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Cai L, Bakalli H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience. 2012;223:200–208. doi: 10.1016/j.neuroscience.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and Biobehavioral Reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. b1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002a;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002b;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Charney DS, Bremner JDDE, Redmond J. Noradrenergic neural substrates for anxiety and fear. Clinical associations based on preclinical research 2000 [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Critical Reviews in Neurobiology. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DEJ. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Science. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concept of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Crespi F. Anxiolytics antagonize yohimbine-induced central noradrenergic activity: A concomitant in vivo voltammetry–electrophysiology model of anxiety. Journal of Neuroscience Methods. 2009;180:97–105. doi: 10.1016/j.jneumeth.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. European Journal of Neuroscience. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. The Journal of Neuroscience. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacology Biochemistry and Behavior. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- File SEHZ., Jr Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology. 1993;111:491–494. doi: 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- File SE, Mabbutt PS, Hitchcott PK. Characterisation of the phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology (Berl) 1990;102:98–101. doi: 10.1007/BF02245751. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. 2003;144:75–83. doi: 10.1210/en.2002-220659. [DOI] [PubMed] [Google Scholar]
- Galvis-Alonsoa OY, Garciac AMB, Orejarenaa MJ, Lampreae MR, Botelhof S, Conded CA, Moratoc S, Garcia-Cairascoa N. A combined study of behavior and Fos expression in limbic structures after re-testing Wistar rats in the elevated plus-maze. Brain Research Bulletin. 2010;81:595–599. doi: 10.1016/j.brainresbull.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Goes TC, Antunes FD, Teixeira-Silva F. Trait and state anxiety in animal models: Is there correlation? Neurosci Lett. 2009;450:266–269. doi: 10.1016/j.neulet.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Gurguis GNM, Vitton BJ, Uhde TW. Behavioral, sympathetic and adrenocortical responses to yohimbine in panic disorder patients and normal controls. Psychiatry Research. 1997;71:27–39. doi: 10.1016/s0165-1781(97)00041-3. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacology Biochemistry and Behavior. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Ippoliti R, Lendaro E, Bellelli A, Brunori M. A ribosomal protein is specifically recognized by saporin, a plant toxin which inhibits protein synthesis. FEBS Letters. 1992;298:145–148. doi: 10.1016/0014-5793(92)80042-f. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbH saporin. American Journal of Physiology. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla neurons. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2006;291:R751–R759. doi: 10.1152/ajpregu.00800.2005. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformldehyde fixative. A new fixative for immunoelectron microscopy. Journal of Histochemistry and Cytochemistry. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Progress in Neuro-Pschopharmacology and Biological Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sciences. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. The Journal of Comparative Neurology. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiology and Behavior. 2002;77:723–729. doi: 10.1016/s0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L. Trimethylthiazoline (TMT) supports conditioned flavor avoidance and activates viscerosensory, hypothalamic, and limbic circuits in rats. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2005;288:R1716–R1726. doi: 10.1152/ajpregu.00479.2004. [DOI] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Research. 1998;788:287–293. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Pacák K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Research. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Baffi JS, Pacák K. The role of ascending neuronal pathways in stress-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. Journal of Neuroendocrinology. 1999;11:529–539. doi: 10.1046/j.1365-2826.1999.00365.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, Johnston AL, File SE. Selective agonists and antagonists for 5-hydroxytryptamine receptor subtypes, and interactions with yohimbine and FG 7142 using the elevated plus-maze test in the rat. Journal of Pharm Pharmacol. 1987;39:917–928. doi: 10.1111/j.2042-7158.1987.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. The Journal of Comparative Neurology. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. European Journal of Pharmacology. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, III, CAM, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JC. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biological Psychiatry. 1999a;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biological Psychiatry. 1999b;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav. 1993;44:463–469. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology. 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand ST, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Hrycyshyn AW, Flumerfelt BA. Collateral axonal projections from the A1 noradrenergic cell group to the paraventricular nucleus and bed nucleus of the stria terminalis in the rat. Experimental Neurology. 1988;102:121–124. doi: 10.1016/0014-4886(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Research. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- Zhu L, Onaka T. Involvement of medullary A2 noradrenergic neurons in the activation of oxytocin neurons after conditioned fear stimuli. European Journal of Neuroscience. 2002;16:2186–2198. doi: 10.1046/j.1460-9568.2002.02285.x. [DOI] [PubMed] [Google Scholar]