Abstract
In the present study the antiviral properties of the bacteriocin subtilosin against Herpes simplex virus type 1 (HSV-1) and the safety and efficacy of a subtilosin-based nanofiber formulation were determined. High concentrations of subtilosin, the cyclical antimicrobial peptide produced by Bacillus amyloliquefaciens, were virucidal against HSV-1. Interestingly, at non-virucidal concentrations, subtilosin inhibited wild type HSV-1 and aciclovir-resistant mutants in a dose-dependent manner. Although the exact antiviral mechanism is not fully understood, time of addition experiments and western blot analysis suggest that subtilosin does not affect viral multiplication steps prior to protein synthesis.
Poly(vinyl alcohol) (PVOH)-based subtilosin nanofibers with a width of 278 nm were produced by the electrospinning process. The retained antimicrobial activity of the subtilosin-based fibers was determined via an agar well diffusion assay. The loading capacity of the fibers was 2.4 mg subtilosin/g fiber, and loading efficiency was 31.6%. Furthermore, the nanofibers with and without incorporated subtilosin were shown to be nontoxic to human epidermal tissues using an in vitro human tissue model.
Taking together these results subtilosin-based nanofibers should be further studied as a novel alternative method for treatment and/or control of HSV-1 infection.
Keywords: subtilosin, bacteriocin, antiviral, nanofiber
Introduction
Herpes simplex virus type 1 and 2 (HSV-1 and HSV-2, respectively), are serious human pathogens. HSV-1 is often associated with orofacial infections and encephalitis, whereas HSV-2 has been found to be the most frequent cause of genital herpes, the major cause of genital ulcers worldwide [9, 40, 52]. However, in the past decade several studies have identified HSV-1 as the causative agent of at least half of the new genital herpes episodes [4, 21]. HSV infections persist during the lifetime of the host, generally establishing latent infections [17, 18]. In addition, it has been shown that genital herpes represents a risk factor for the transmission of human immunodeficiency virus (HIV), increasing the risk of contagion by two- to threefold [26, 27, 31].
Nowadays, the agents most commonly used for the management of herpetic infections are the nucleoside analogues acyclovir (ACV), penciclovir (PCV) and their respective prodrugs valacyclovir and famciclovir. The triphosphate forms of these analogues selectively inhibit the viral DNA polymerase (DNA pol) activity. After more than three decades of ACV administration, drug-resistant HSV isolates are rarely found in immunocompetent subjects (0.1–0.7%) but more frequently recovered from immunocompromised patients (4–14%) [12, 34, 36, 47]. HSV resistance to ACV generally arises as a result of mutations in viral thymidine kinase and in a lesser number from mutations in the viral DNA pool [19, 38]. The pyrophosphate analogue foscarnet (FOS) is often used in the management of resistant HSV infections. However, FOS resistance is rising rapidly and its use is reserved to the cases where other drugs fail because it is more toxic and less bioavailable [11, 33]. Considering the incidence of HSV infections, the reported emergence of ACV, PCV and FOS resistant mutants and that it has been recently shown that antiviral treatment of herpetic infection fail to reduce HSV and HIV transmission [10, 29] there is a need for new antiherpetic compounds with different mechanisms of action. Antimicrobial peptides are produced by a variety of organisms including insects, fungi, Gram-positive and Gram-negative bacteria, and many of them have been reported as viral inhibitors [1, 28]. For example, cationic peptides like -defensins, hecate, and synthetic derivatives of magainins are active against HSV in vitro replication [6, 22] while melittin, a 26 amino acid amphipathic peptide isolated from the venom of the European honeybee Apis melliphera, inhibits the replication of both HIV and HSV [2, 6, 51]. Several antimicrobial peptides ribosomally produced by bacteria, collectively called bacteriocins, have been described for the members of the genus Enterococcus [7]. Wachsman et al. were the first to describe an antiviral peptide produced by E. faecium CRL35. The peptide, 3.5 kDa in size, inhibited late stages of the HSV-1 and HSV-2 multiplication cycle [51]. In addition, Serkedjieva et al. described a 5.0 kDa-peptide, produced by Lactobacillus delbrueckii subsp. bulgaricus with activity against influenza virus [37].
Based on the documented history of bacterially-produced peptides, we chose to investigate the novel bacteriocin subtilosin, for such characteristics. Subtilosin is a 3.4 kDa, cyclical peptide [32] produced by both Bacillus subtilis [5] and B. amyloliquefaciens [42] with proven antimicrobial activity against a variety of human pathogens, including the bacterial vaginosis-associated Gardnerella vaginalis. Sutyak et al. further established that subtilosin has potent spermicidal activity and is nontoxic to human vaginal tissues [41]. Due to its range of activities and its safety for human use, subtilosin was an obvious target for investigation into its antiviral properties. Furthermore, we have chosen to examine the feasibility of subtilosin’s incorporation into a nanofiber-based delivery system, considering the successful use of this method with other bacteriocins [16, 24, 25]. Here, we report for the first time the antiviral activity of the anionic antimicrobial peptide subtilosin, and its safety for use in a novel nanofiber delivery system.
Materials and Methods
Compounds
Subtilosin was isolated and purified from cultures of the producer strain, Bacillus amyloliquefaciens KATMIRA1933, according to the protocols previously described by Sutyak et al. [41]. All subtilosin solutions were prepared in sterile ddH2O and maintained at 4°C until use. Acyclovir and foscarnet were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Drug stock solutions were prepared in dimethyl-sulfoxide (DMSO) with a final concentration of 0.1% and diluted with maintenance medium (MM) consisting of minimum essential medium (MEM) (Gibco, Carlsbad, CA, USA) with 2% inactivated fetal bovine serum. Poly(vinyl alcohol) (PVOH, Mw=61 kDa, Sigma-Aldrich) was chosen as a carrier polymer due to its high biocompatibility [16]. Trypticase™ soy broth (TSB), trypticase™ soy agar (TSA), agar and yeast extract were purchased from Becton, Dickinson and Company (Sparks, MD, USA). Yeast extract was added into TSB and TSA in the amount of 0.6% as a nutritional supplement to improve microbial growth. The indicator organism Micrococcus luteus ATCC 10240 was chosen due to its high sensitivity to bacteriocins and its widespread used as a bacteriocin-sensitive indicator strain by academia and industry [35].
Cells and viruses
African green monkey kidney (Vero) cells were grown as monolayers in MEM (Gibco) supplemented with 5% inactivated fetal bovine serum and 50 µg/mL of gentamycin. HSV-1 strain F (tk+) was obtained from the American Type Culture Collection (Rockville, MD, USA). HSV-1 strain Field (tk deficient) was kindly provided by Dr. G. Andrei (Rega Insititue, Leuven, Belgium). Virus stocks were prepared in Vero cells.
Cell cytotoxicity assays
To determine the cytotoxic concentrations of the compounds, confluent monolayers of Vero cells were grown in tissue culture plates for 48 h and exposed to various concentrations of the compounds. After 48 h of incubation, cell viability was examined by the ability of the cells to cleave the tetrazolium salt MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide) (Sigma-Aldrich) with the mitochondrial enzyme succinate dehydrogenase, yielding a quantifiable blue product (formazan) [15]. The precise MTT procedure has been previously described [43, 50]. The CC50 was defined as the compound concentration that reduced Vero cells viability by 50%, calculated by regression analysis.
Virucidal assay
HSV-1 tk+ and tk deficient preparations were incubated with 200 µg/mL of subtilosin or with MM (control) for 0, 15, 45 and 90 min at 37°C. Then, aliquots were taken and diluted in a serial fashion in MM in order to infect Vero cell monolayers grown in 24-well culture plates to test virus survival using a plaque assay. Plaques were counted exclusively in wells where subtilosin concentration was less than 0.2 µg/mL, a concentration 50 fold lower than the concentration needed to inhibit virus multiplication in 50% compared to untreated infected cultures). To assay the effect of subtilosin over the viral particles at antiviral concentrations, HSV-1 was incubated with subtilosin at concentrations ranging from 6 to 100 µg/mL or MM for 60 min at 37°C. Then, aliquots were taken and Vero cells were infected to test virus survival using a plaque assay.
Virus yield reduction assay
Antiviral activity was evaluated by the virus yield reduction assay. For that purpose, Vero cells grown in 24-well culture plates for 48 h were individually infected with the two strains of HSV-1 at a multiplicity of infection (m.o.i.) of 1 PFU/cell. After 1 h of adsorption at 37°C, the infective viral particles were removed and cells were covered with MM (control) or MM containing various concentrations of subtilosin. After 24 h of incubation at 37°C, the supernatants were harvested and cell-free virus yields were determined by a plaque assay on Vero cell monolayers. The antiviral activity was expressed as the EC50 (50% effective concentration), i.e. the compound concentration required to reduce plaque formation by 50% after 72 h compared with the untreated infected cultures. The EC50 values were calculated by plotting percentages of inhibition versus different concentrations of each compound.
Effect of time of addition of subtilosin on HSV-1 production
Subtilosin at a concentration of 50 µg/mL was added to confluent monolayers of Vero cells either at 1, 3, 5 or 8 h post-infection (p.i.) with HSV-1 (m.o.i. = 1). Cultures were further incubated at 37°C until 24 h p.i. and extracellular virus infectivity was quantified by plaque assay on Vero cells.
Western-blotting
Monolayers of confluent Vero cells were infected with HSV-1 (m.o.i. = 1) or left non-infected (control), and cultures were incubated at 37°C for 1 h to allow virus internalization. After removing the inoculum, monolayers were covered with MM alone or containing different concentrations of subtilosin, and incubated at 37°C for 24 h. Immediately after, cells in duplicate wells were lysed with Laemmli sample buffer (Bio-Rad, CA, USA) with 5% β-mercaptoethanol. Samples were heated for 2 min in boiling water before loading onto 10% acrylamide gels. Following electrophoresis, the resolved proteins were transferred to a PVDF membrane (Perkin Elmer Life Sciences, Inc., Waltham, MA, USA) in a dry system (LKB Multiphor II, Pharmacia, Sweden). Glycoprotein gD was revealed with mouse anti-gD (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and a peroxidase anti-mouse immunoglobulin G (Promega, Madison, WI, USA) as secondary antibody. ERK1 used as a loading control was revealed with rabbit anti-ERK1 (Santa Cruz Biotechnology Inc.) and a peroxidase anti-rabbit immunoglobulin G (Promega) as secondary antibody. Chemiluminescence was visualized using a chemiluminescence kit (Perkin Elmer Life Sciences).
Preparation of antimicrobial fibers
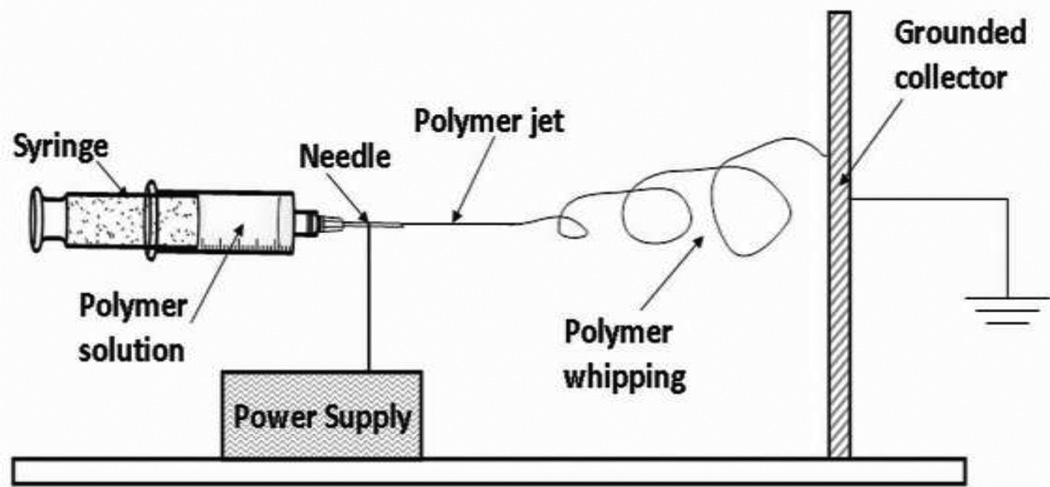
PVOH (1.5 g) was dissolved in 10 mL Millipore water and heated at 80°C for 8 h. After cooling, the PVOH solution was blended with a purified 4.6 mg/mL subtilosin solution for a final concentration of 0.12 g/mL PVOH and 0.9 mg/mL subtilosin. The PVOH+subtilosin solution was then transferred into a 10 mL plastic syringe with a blunt-end metal needle (O.D. 1.27 mm). The syringe filled with stock solution was mounted onto a syringe pump (New Era Pump Systems Inc., Farmingdale, NY, USA). Figure 1 displays the schematic diagram of the electrospinning facility. A high-voltage external electric field was applied to the polymer solution under which a Taylor cone [53] formed at the needle end. As electrostatic repulsion on the liquid surface surpassed the surface tension, a polymer liquid jet was then erupted from the Taylor cone. After experiencing jet whipping [39] and solidification, polymeric fibers were deposited onto the grounded aluminum foil collector. The whole process was maintained at the condition of 15 kV for voltage supply, 0.2 mL/h for feeding rate, and 10 cm for needle-to-collector distance. As a negative control, 0.12 g/mL PVOH solution without subtilosin was electrospun by the same procedure as above. After 5 h of processing, the fibrous mat was directly peeled off from the grounded collector covered with aluminum foil. Fibers were then dried under vacuum for 4 h prior to further use.
Figure 1. Schematic diagram of nanofiber formation through the process of electrospinning.
A PVOH and subtilosin mixed solution (final concentrations: 0.12 g/mL PVOH and 0.9 mg/mL subtilosin) was loaded into a plastic syringe, which was then mounted onto a syringe pump. During the electrospinning process, a liquid polymer jet is ejected at the end of the needle when a high-voltage electric field is applied to the polymer solution. The liquid polymer undergoes jet whipping and solidification, and the resultant polymer fibers are then deposited onto the grounded aluminum collector surface. The whole process was maintained at the condition of 15 kV for voltage supply, 0.2 mL/h for feeding rate, and 10 cm for needle-to-collector distance. As a negative control, a 0.12 g/mL PVOH solution without subtilosin was electrospun by the same procedure as above. Fibers were collected from the grounded surface after 5 h processing.
Morphology characterization of antimicrobial nanofibers
Surface images of the antimicrobial PVOH fibers with and without subtilosin were collected with a commercial Nanoscope IIIa Multi-Mode AFM (Veeco Instruments, Plainview, NY, USA) equipped with a J scanner, which was operated in tapping mode using a silicon cantilever. The scanned images were obtained at the scan size of 5×5 µm and 50×50 µm. The scan frequency was set at 0.1 Hz. The section analysis embedded in the software Nanoscope 3.0 was utilized to calculate the fiber diameter distribution.
Assessment of subtilosin loading capacity and efficiency via well diffusion assay
The evaluation of subtilosin loading capacity and efficiency was conducted by agar plate well diffusion inhibition assays [13]. All nanofiber mats were sterilized by direct exposure to UV light (257 nm) in a biosafety cabinet (Forma Class II, A2 Biological Safety Cabinet, Thermo Fisher Scientific, Pittsburgh, PA, USA) for 10 min per side prior to use. Twenty mg of PVOH+subtilosin fibers were cut with sterile tweezers, immersed in 200 µL sterile ddH2O, and kept at 4°C for 24 h. Twenty mg of PVOH fibers were also dissolved as a negative control to eliminate the possibility of PVOH inhibition. An overnight culture of M. luteus in TSB (c. 109 CFU/mL) was diluted 100-fold by blending with 55°C soft agar (TSB supplemented with 7 g/L agar). Then, 4 mL of the soft agar containing M. luteus was transferred onto the surface of a TSA plate. After 30 min of solidification, a sterile glass pipette (approx. 5 mm diameter) was used to create wells in the agar plate. 50 µL of purified subtilosin, PVOH+subtilosin fibers (24 h water solution), negative control and their double dilutions were injected into the wells in duplicate. After 24 h incubation at 37°C, minimum inhibitory concentration (MIC) was defined as the lowest concentration to form a visible inhibition circle on the M. luteus growth layer. Tests were repeated three times.
Skin cytotoxicity assays
The safety of nanofiber mats with and without incorporated subtilosin for use on human skinwas tested on a cultured, reconstituted human epidermal model. The EpiDerm (NHEK) tissue model (MatTek Corporation, Ashland, MA, USA) consists of normal, human-derived epidermal keratinocytes that are cultured to form a highly differentiated model of the epidermis, and allows for in vitro testing of a compound’s irritancy and cytotoxicity. All EpiDerm tissues were maintained at 4°C upon receiving and prior to use. The standard method developed by MatTek, and later outlined by Frasch et al. [20], was followed. Briefly, all tissues were preincubated in room temperature culture medium at 37°C with 5% CO2 for 1 h prior to use. Nanofiber mats containing 12% PVOH or 12% PVOH+subtilosin were sterilized by direct exposure to UV light in a biosafety cabinet for 10 min per side, and then cut to size using a sterilized size 5 cork borer (Thermo Fisher Scientific). These nanofiber “discs” were applied onto the surface of the pre-conditioned tissues with light pressure to ensure that the disc came in full contact with the tissue. Positive (50 µl Triton-X) and negative (50 µl sterile ddH2O) controls and all test compounds were assayed in triplicate. The tissues were exposed to the test compounds for 24 h at 37°C with 5% CO2. After exposure, the tissues were washed with PBS, transferred into MTT assay buffer, and further incubated for 3 h at 37°C with 5% CO2. After a second wash with PBS, the tissues were submerged in isopropanol and held at room temperature overnight to allow for extraction of the formazan dye from the tissues. The following day, the optical density of the formazan-isopropanol mixture from each tissue was measured in triplicate at 570 nm. Cell viability was then calculated as a percentage of the average negative control tissues. Tests were performed at least twice.
Results
Antiviral activity: subtilosin inhibits HSV-1 multiplication
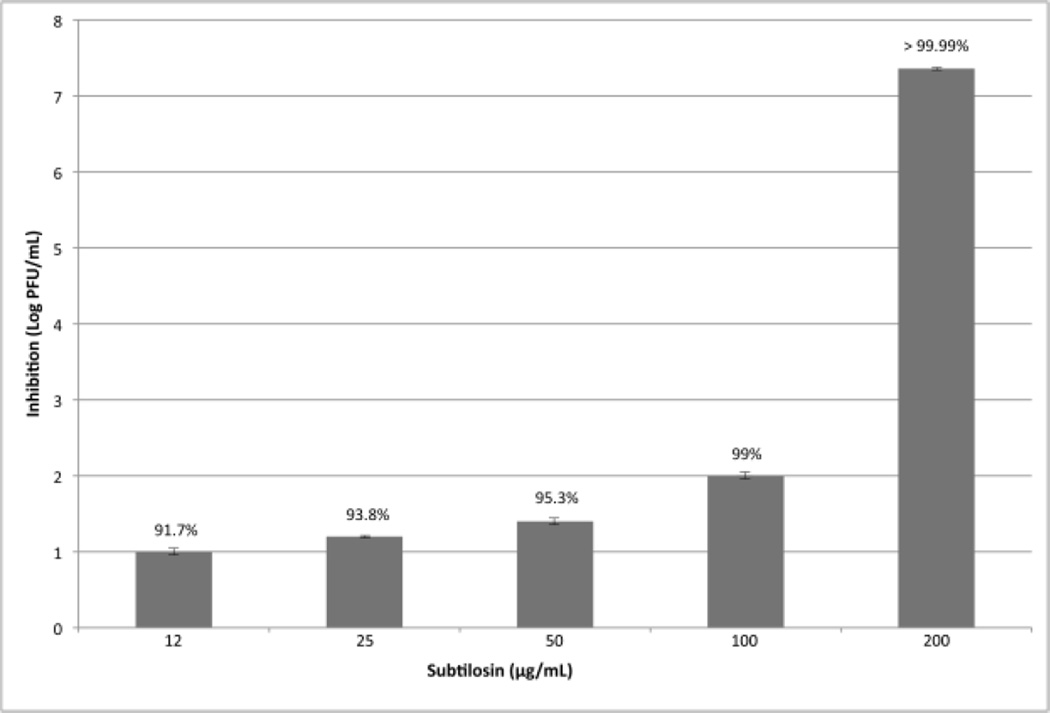
To assess the antiviral activity of subtilosin, we first determined the subtilosin’s concentration that reduced cell viability to 50% as compared to the control (CC50) by using the MTT method. A virus yield inhibition assay was then performed to determine cell-free infectivity and the selectivity index (SI), i.e. the relationship between CC50 and EC50 values. For comparative purposes, the inhibitory effect of ACV and FOS was also assayed. Between 12 and 100 µg/mL, subtilosin inhibited HSV-1 replication in a dose-dependent manner (Figure 2) and a remarkably strong inhibition of virus replication was obtained with 200 µg/mL subtilosin. At a concentration of 100 µg/mL, subtilosin inhibited replication in a 99% and at 12 µg/mL, a concentration 26 fold lower than the CC50, subtilosin still inhibited approximately 90% of HSV-1. A 50% of inhibition was found with subtilosin at a concentration of 9.6 µg/mL. Lower concentrations of the compound did not display considerable antiviral activity. The SI value for subtilosin against HSV-1 tk+ was 33, while the values for ACV and FOS were 957 and 14, respectively. On the other hand, subtilosin rendered a SI value of 31 against tk-deficient HSV-1, while the SI value for ACV was only 10.3 (Table 1).
Figure 2. Subtilosin inhibits HSV-1 replication in a dose-dependent manner.
Vero cells infected with HSV-1 tk+ (m.o.i.= 1) were incubated with different concentrations of subtilosin for 24 h. Then, supernatants were harvested and cell free virus yields were determined by a plaque assay. Values are means ± SD of the log reduction in HSV-1 titers for each concentration compared to no drug. Data are from three independent experiments, where each titration was carried out in duplicate. Percentages of inhibition of the different treatments are expressed in each column.
Table 1.
Cytotoxicity and antiviral activity of Subtilosin, Aciclovir (ACV) and Foscarnet (FOS) against HSV-1.
| Compound (MW) |
Structural Formula |
IUPAC name | Virus | CC50 (µg/ mL) |
EC50(µg/ mL) |
SI |
|---|---|---|---|---|---|---|
| Subtilosin * (3398.9) |
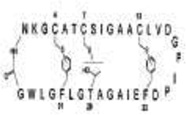 |
- | HSV-1 tk+ | 314 | 9.6 | 33 |
| HSV-1 tk deficient | 314 | 10 | 31 | |||
| FOS (300.04) |
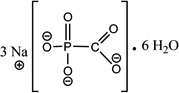 |
phosphonoformic acid trisodium salt hexahydrate |
HSV-1 tk+ | 210 | 15 | 14 |
| HSV-1 tk deficient | - | - | - | |||
| ACV (225.2) |
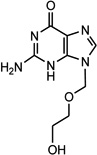 |
9-(2- hydroxyetoxymetyl) guanine |
HSV-1 tk+ | 67 | 0.07 | 957 |
| HSV-1 tk deficient | 67 | 6.4 | 10.4 | |||
CC50 compound concentration required to reduce cell viability by 50%, as determined by the MTT method.
EC50: compound concentration required to reduce virus yield by 50%.
SI (selectivity index): ratio CC50 / EC50.
Structural formula of subtilosin reproduced from Marx et al. [32].
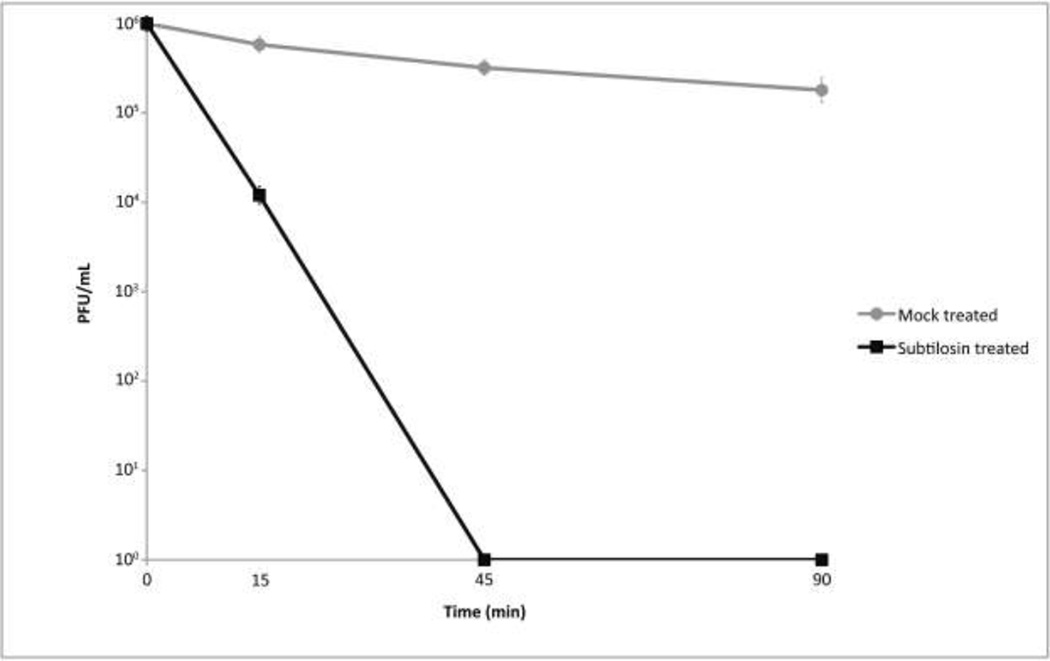
Since it has been reported that microbicidal peptides such as defensins may inactivate HSV on direct contact [30] and because we found an unusually high inhibition at 200 µg/mL, a virucidal assay was performed to determine if subtilosin at this concentration produces a direct effect on the viral particle. To that purpose, viral suspensions were incubated with subtilosin 200 µg/mL for 15, 45 and 90 min. Then, aliquots were taken, diluted in MM and used to infect Vero cell monolayers to test virus survival using a plaque assay. The incubation of viral suspension with subtilosin for 15 min at 37°C resulted in an inhibition of the viral titer of 99%. After 45 min of treatment, the reduction of viral infectivity was higher than 99.99%, indicating that subtilosin is a potent virucidal agent active against tk-positive and tk-deficient (data not shown) HSV-1 (Figure 3).
Figure 3. Subtilosin inactivate HSV-1 particles at a concentration of 200 µg/mL.
HSV-1 tk+ and tk deficient (data not shown) were incubated with subtilosin at a concentration of 200 µg/mL or with MM for 0, 15, 45 and 90 min at 37°C. Then, aliquots were taken and Vero cells were infected to test virus survival using a plaque assay. Values are means of HSV-1 tk+ infectivity ± SD from three independent experiments, where each titration was carried out in duplicate.
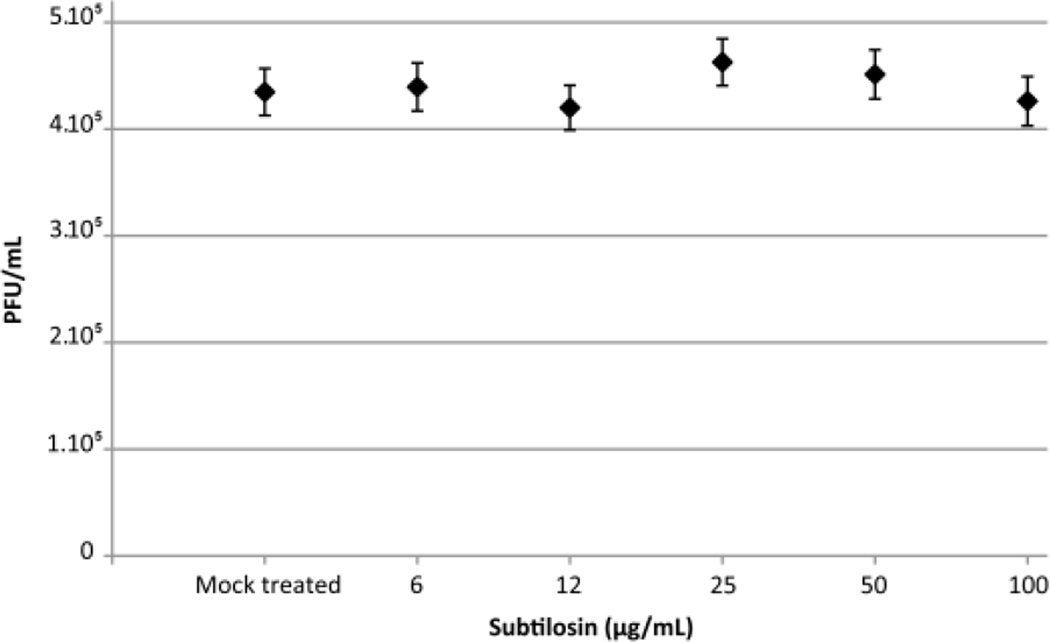
To establish if subtilosin’s inhibitory activity at low concentration was due to its direct effect on the viral particle, we performed a similar experiment at concentrations below 200 µg/mL. Treatment of viral suspensions for 1 h at 37°C with 6 to 100 µg/mL of subtilosin did not reduce viral infectivity (Figure 4), indicating that the reduction of the viral titers at these concentrations (as seen in Figure 2) were only due to antiviral activity.
Figure 4. Low concentrations of subtilosin do not produce a virucidal effect against HSV-1.
HSV-1 tk+ was incubated with subtilosin at concentrations ranging from 6 to 100 µg/mL or MM for 60 min at 37°C to assay virucidal activity of subtilosin at concentrations that display antiviral activity. Then, aliquots were taken and Vero cells were infected to test virus survival using a plaque assay. Values are means ± SD from three independent experiments, where each titration was carried out in duplicate.
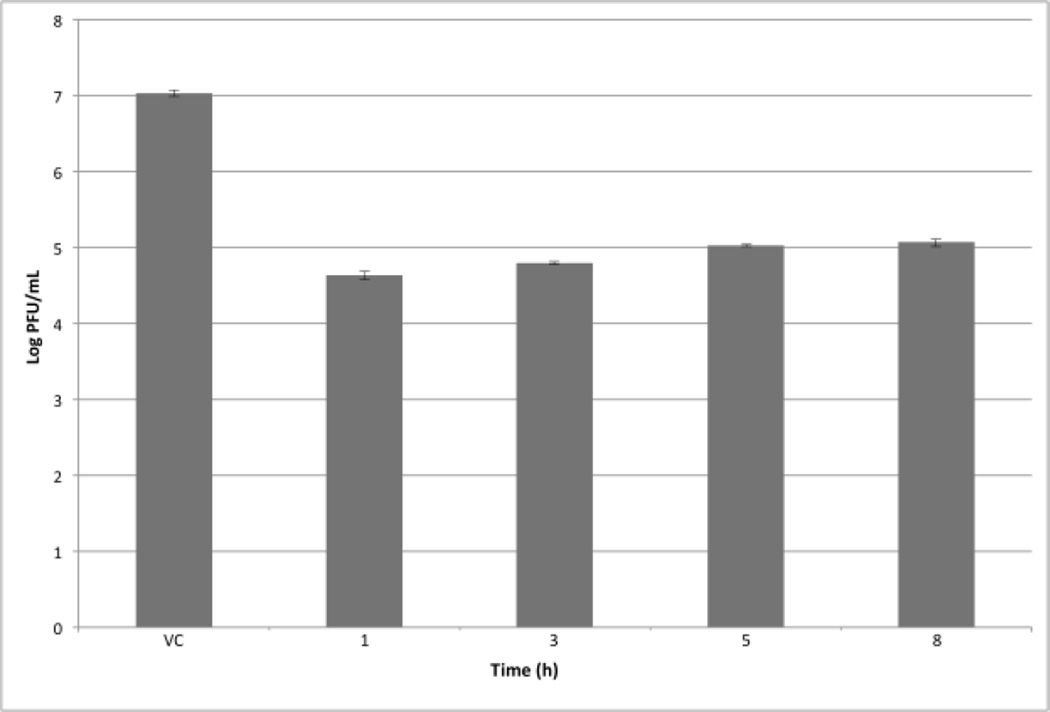
In order to determine if subtilosin interferes with the initial steps of the viral multiplication cycle at a non-virucidal concentration, a time of addition experiment was performed. For that purpose, 50 µg/mL of subtilosin was added to HSV-1 infected Vero cells at different times after infection and at 24 h p.i., cell free infectivity was determined. As it depicted in Figure 5, when subtilosin was present starting from 1 h, 3 h, 5 h or 8 h p.i., virus yields were reduced by approximately 2 log, suggesting that subtilosin’s antiviral activity is not due to an inhibition of early stages of viral multiplication.
Figure 5. Effect of time of subtilosin addition on HSV-1 production.
Vero cells infected with HSV-1 (m.o.i. = 1) were incubated with MM (VC) or MM containing subtilosin 50 µg/mL added either at 1, 3, 5 or 8 h p.i. Cultures were further incubated at 37°C until 24 h p.i Then, cell free virus yields were determined by plaque assay. Values are means ± SD from three independent experiments, where each titration was carried out in duplicate.
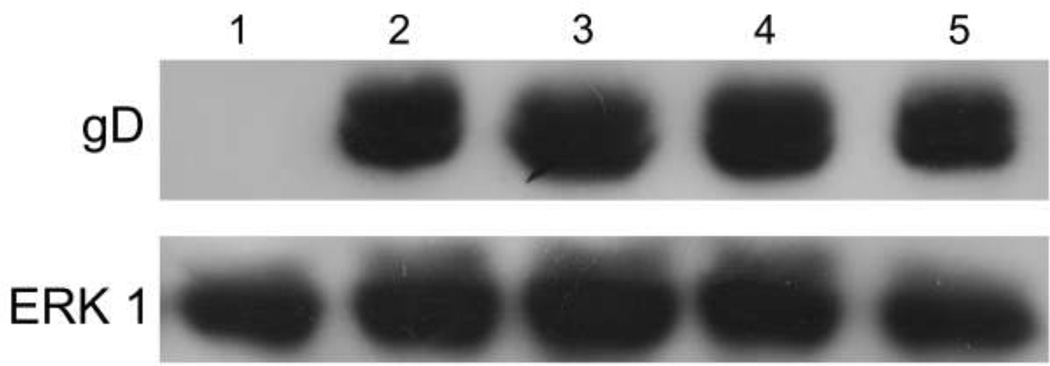
To further characterize the inhibitory action of subtilosin, Vero cells were infected with HSV-1 and treated with different concentrations of the peptide ranging from 25 to 100 µg/mL. Afterwards, a polyacrylamide gel electrophoresis followed by a Western blot analysis was performed to determine if subtilosin reduces viral protein synthesis. As shown in Figure 6, viral protein gD synthesis was not affected by the different concentrations used in this experiment, suggesting that the antiviral mechanism of subtilosin does not interfere with this or previous steps in the viral multiplication cycle.
Figure 6. Viral protein gD synthesis is not affected by subtilosin treatment.
Vero cells infected (Lanes 2–5) or not (Lane 1) with HSV-1 tk+ at a m.o.i. of 1 were incubated at 37°C for 1 h to allow internalization. Afterwards, the inoculum was discarded and the monolayers were covered with MM or MM containing subtilosin at different concentrations (Lane 2: virus control; lane 3: 25 µg/mL; lane 4: 50 µg/mL; lane 5: 100 µg/mL) and incubated at 37°C. At 24 h p.i. cell lysates were collected and subjected to Western blotting and glycoprotein gD was analyzed with an antibody against gD. To verify equal loading, Western blotting was performed with an antibody to ERK1. Data are from one of two different experiments.
Formulation
Nanofiber morphology
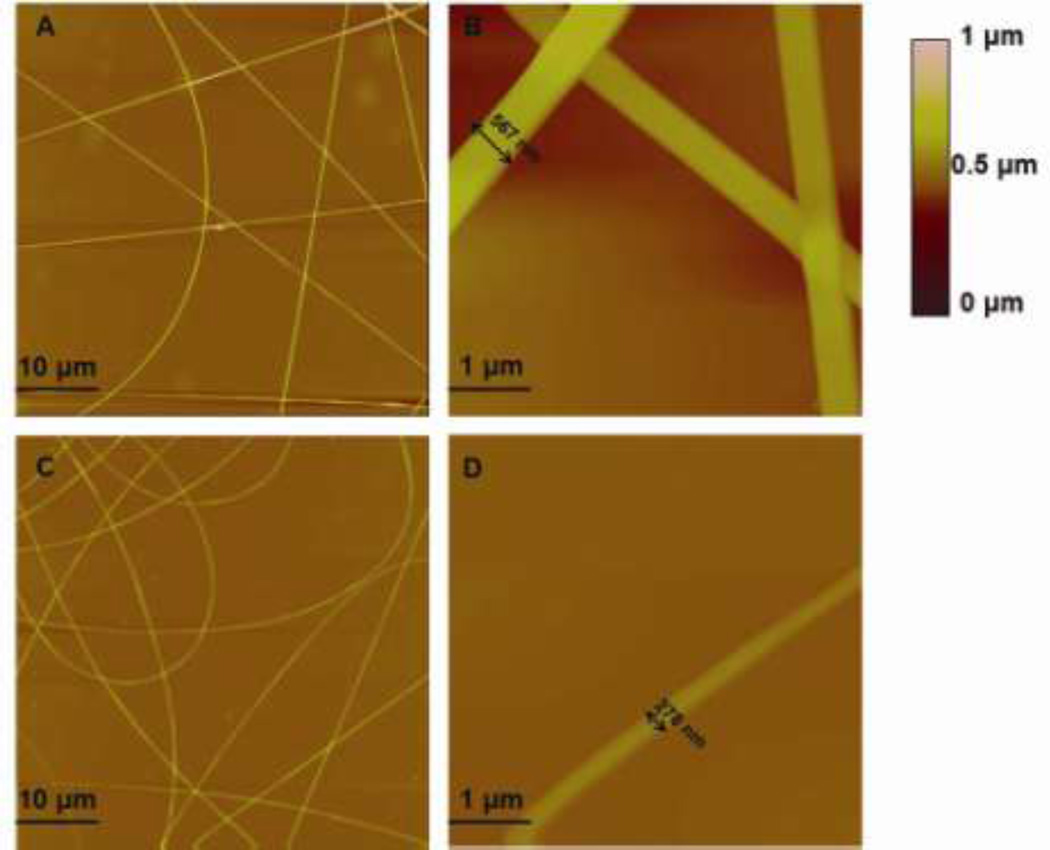
Figure 7 shows the tapping mode-atomic force microscopy (TP-AFM) images of antimicrobial PVOH fibers. Relatively straight fibers were formed from the pure PVOH solution (Figure 7A), and an individual PVOH fiber’s diameter was 567 nm (Figure 7B) as calculated by the section analysis embedded in the Nanoscope software. Addition of subtilosin affected the fiber’s morphology, resulting in thinner fibers from the electrospinning process (Figure 7C). An individual PVOH+subtilosin fiber diameter was determined to be 278 nm (Figure 7D). This phenomenon is likely due to subtilosin’s interaction with PVOH, resulting in weakened entanglement between the PVOH polymer chains, shifting the critical entanglement concentration of PVOH solution toward a higher value and subsequently reducing fiber diameter.
Figure 7. Surface morphology of polyvinyl alcohol-based nanofibers with and without incorporated subtilosin.
Tapping mode-atomic force microscopy (AFM) images of PVOH electrospun fibers including (A) 50 µm×50 µm height image of PVOH electrospun fibers; (B) 5 µm×5 µm height image of PVOH electrospun fibers (C) 50 µm×50 µm height image of PVOH+subtilosin electrospun fibers; (D) 5 µm×5 µm height image of PVOH+subtilosin electrospun fiber.
Loading capacity and loading efficiency
Loading capacity was defined as the amount of subtilosin released from a gram of subtilosin+PVOH nanofiber. Loading efficiency was defined as the ratio of loading capacity to the amount of subtilosin used in the blended solution to produce 1 g of nanofiber. First, the MIC of subtilosin against M. luteus was determined by well diffusion assay as 60 mg/L. The 4-fold dilution of a 20 mg nanofiber per 200 µL ddH2O solution, equivalent to 25 mg fiber/mL ddH2O, was the lowest dilution that retained antimicrobial activity, establishing it as the MIC. The concentration of subtilosin released from 25 mg of fiber in 1 mL of sterile ddH2O was equal to 60 µg/mL. Thus, the loading capacity was calculated to be 2.4 mg subtilosin per gram of fiber.
Since the water acting as a solvent in the subtilosin+PVOH solution was evaporated and dried during the electrospinning and vacuum processes, 1 g of nanofiber was assumed to be primarily composed of PVOH. Since 8.3 mL of 0.12 g/mL PVOH solution was required to form 1 g of fiber, the amount of subtilosin used in the blended solution was 7.6 mg. Thus, loading efficiency was calculated to be 31.6%. The loss of subtilosin may be the result of the high voltage applied [23] or the difficulty of subtilosin release from encapsulation.
Nanofibers cytotoxicity
The safety of subtilosin-based nanofibers for use on human skin tissues was tested using the in vitro EpiDerm model. After 24 h of exposure, the tissues exposed to the negative control (ddH2O) and PVOH fibers retained 100% viability. Tissues exposed to the PVOH+subtilosin fibers retained an average of 98.5% viability in comparison to the negative control. There was zero viability for the tissues exposed to the positive control, Triton-X.
Discussion
Antimicrobial peptides have shown to act against viruses in many different ways. Most of them exert their activity by directly inactivating the viral particle or inhibiting early events in virus multiplication cycle [28]. For example, the peptides MCP-1 and MCP-2, the human neutrophil peptide (HNP)-1 and brevinin-1 inactivate viral particles [14, 30, 54]. On the other hand, dermaseptins, lactoferricin and the polyphemusin analogue T22 seem to interfere at the level of virus-cell interface, thus blocking viral entry [3, 8, 44]. However, other antimicrobial peptides exert their antiviral activity by preventing virus multiplication, as melittin and cecropin which were shown to inhibit the HIV replication by suppressing viral gene expression [48]. Other bacteriocins such as CRL35 and ST4V, isolated from Enterococcus faecium and Enterococcus mundii respectively, inhibited the HSV-1 and HSV-2 replication in a dose-dependent manner [46, 49, 51]. In this work, we studied subtilosin’s activity against HSV-1. We found that subtilosin at a non-cytotoxic concentration of 200 µg/mL inactivates HSV-1 viral particles. The incubation of viral suspension with 200 µg/mL of subtilosin for 15 min was enough to reduce infectivity by 99% and after 45 min of treatment the reduction of viral infectivity was higher than 99.99%. Interestingly, at lower non-virucidal doses, subtilosin inhibits HSV-1 multiplication cycle in a dose dependent manner. At subtilosin’s concentration of 100 µg/mL, 99% inhibition of HSV-1 multiplication was achieved. Remarkably, 12 µg/mL subtilosin, a concentration 26 fold lower than the CC50, caused more than 90% inhibition of HSV-1. In addition, subtilosin was equally active against HSV-1 tk+ and tk-deficient strains and rendered higher SI values than FOS, raising the possibility for using subtilosin against ACV-resistant mutants. It was previously mentioned that antimicrobial peptides generally display virucidal activity or interfere with early events in viral multiplication cycle. Interestingly, subtilosin is able to inactivate viral particles and to inhibit viral multiplication, although in different concentrations. Furthermore, it does not seem to affect any viral multiplication step up to viral protein synthesis as shown by the time of addition of subtilosin experiment and Western blot analysis, suggesting that it may interfere with late stages such as viral formation and release. Taking into account that some studies suggest that subtilosin binds to lipid membranes, that high concentrations of the compound may result in membrane permeabilization [45] and that viral formation and release are viral steps particularly sensible to membrane structure, future analysis will be conducted to elucidate the precise mechanism underlying the antiviral activity of subtilosin.
Considering the incidence of HSV infections and the issues regarding the clinical approved antiherpetic drugs, such as the reported emergence of ACV, PCV, and FOS resistant mutants and that it has been recently shown that the available antiviral treatment of herpetic infections fail to reduce HSV and HIV transmission because they do not prevent subclinical reactivations of the virus [10, 29], novel treatment methods are of critical importance. One possible alternative is the use of subtilosin in a nanofiber-based formulation. As we demonstrated, subtilosin is readily encapsulated into PVOH fiber mats, which may be directly applied onto areas of infection. Moreover, nanofibers both with and without incorporated subtilosin were demonstrated to be fully safe for human use. Heunis and Dicks [25] have demonstrated the feasibility of incorporating a variety of different types of antimicrobials into nanofibers, and proven that the fibers retain antimicrobial activity against bacterial pathogens such as methicillin-resistant Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae.
Conclusion
In this report, the natural, safe antimicrobial peptide subtilosin was shown to have potent virucidal and antiviral activity against HSV-1. Preliminary research on the safety of subtilosin in a novel nanofiber formulation indicates that the formulation has potential for use in human applications. Further research will be conducted to elucidate the molecular mechanism underlying the antiviral activity of subtilosin, to optimize the nanofiber formulation and to assay the activity of subtilosin nanofibers against this and other Herpesviridae family members.
Acknowledgments
This work was supported by the Universidad de Buenos Aires (UBACYT 20020090200271 and UBACYT 20020110100076 to N.I.T. and M.B.W.); the Agencia Nacional de Promoción Científica y Técnica (ANPCYT) PICT (00985/07 to N.I.T. and M.B.W.); the Bill and Melinda Gates Foundation Grand Challenges Exploration (Round 5, Phase I Grant OPP1025200 to M.L.C., S.X., P.J.S., and K.S.N.); the United States Department of Agriculture National Research Initiative (2009-35603-05071 to J.L. and Q.H.), and the National Institutes of Health/National Institute of Allergy and Infectious Diseases (1R01AI084137 to K.S.N., M.L.C., and P.J.S).
Footnotes
Transparency declaration
Nicolás I. Torres, Katia Sutyak Noll and Shiqi Xu equally contributed to the research and to this manuscript. Mónica B. Wachsman, Michael L. Chikindas and Qingrong Huang are the manuscript’s senior authors in the areas of virology, bacteriology and formulation chemistry, correspondingly.
References
- 1.Abriouel H, Franz C, Ben Omar N, Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 2011;35:201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- 2.Albiol Matanic V, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Andersen J, Osbakk S, Vorland L, Traavik T, Gutteberg T. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001;51:141–149. doi: 10.1016/s0166-3542(01)00146-2. [DOI] [PubMed] [Google Scholar]
- 4.Azwa A, Barton S. Aspects of herpes simplex virus: a clinical review. J Fam Plann Reprod Health Care. 2009;35:237–242. doi: 10.1783/147118909789587376. [DOI] [PubMed] [Google Scholar]
- 5.Babasaki K, Takao T, Shimonishi Y, et al. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem. 1985;98:585–603. doi: 10.1093/oxfordjournals.jbchem.a135315. [DOI] [PubMed] [Google Scholar]
- 6.Baghian A, Kousouglas K. Role of the Na+, K+ pump in herpes simplex type 1-induced cell fusion: melittin causes specific reversion of syncytial mutants with the syn 1 mutation to syn+ (wild-type) phenotype. Virology. 1993;196:548–556. doi: 10.1006/viro.1993.1510. [DOI] [PubMed] [Google Scholar]
- 7.Balla E, Dicks L, Du Toit M, et al. Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecium BFE 1071. Appl Environ Microbiol. 2000;66:1298–1304. doi: 10.1128/aem.66.4.1298-1304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belaid A, Aouni M, Khelifa R, Trabelsi A, Jemmali M, Hani K. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J Med Virol. 2002;66:229–234. doi: 10.1002/jmv.2134. [DOI] [PubMed] [Google Scholar]
- 9.Berger J, Houff S. Neurological Complications of Herpes Simplex Virus Type 2 Infection. Arch Neurol. 2008;65:596–600. doi: 10.1001/archneur.65.5.596. [DOI] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa J, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Cli n. 2003;21:311–320. doi: 10.1016/s0733-8635(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 12.Choong K, Walker N, Apel A, Whitby M. Aciclovir-resistant herpes keratitis. Clin Experiment Ophtalmol. 2010;38:309–313. doi: 10.1111/j.1442-9071.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 13.Cintas L, Rodriguez J, Fernandez M, et al. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol. 1995;61:2643–2648. doi: 10.1128/aem.61.7.2643-2648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daher K, Selsted M, Lehrer R. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. J Inmunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 16.Duan B, Yuan X, Zhu Y, et al. A nanofibrous composite membrane of PLGA428 chitosan/PVA prepared by electrospinning. Eur Polymer J. 2006;42:2013–2022. [Google Scholar]
- 17.Efstathiou S, Preston C. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 2005;111:108–119. doi: 10.1016/j.virusres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Fatahzadeh M, Schwartz R. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Field H. Herpes simplex virus antiviral drug resistance-current trends and future prospects. J Clin Virol. 2001;21:261–269. doi: 10.1016/s1386-6532(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 20.Frasch H, Dotson G, Barbero A. In vitro human epidermal penetration of 1- bromopropane. J Toxicol Environ Health A. 2011;74:1249–1260. doi: 10.1080/15287394.2011.595666. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 22.Håvard J. Therapeutic approaches using host defense peptides to tackle herpes virus infections. Viruses. 2009;1:939–964. doi: 10.3390/v1030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heunis T, Botes M, Dicks L. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics & Antimicrob Prot. 2010;2:46–51. doi: 10.1007/s12602-009-9024-9. [DOI] [PubMed] [Google Scholar]
- 24.Heunis T, Bshena O, Klumperman B, et al. Release of bacteriocins from nanofibers prepared with combinations of poly(D,L-lactide) (PDLLA) and poly(ethylene oxide) (PEO) Int J Mol Sci. 2011;12:2158–2173. doi: 10.3390/ijms12042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heunis T, Dicks L. Nanofibers offer alternative ways to the treatment of skin infections. J Biomed Biotechnol. 2010 doi: 10.1155/2010/510682. 2010:pii 510682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill C, McKinney E, Lowndes C, et al. Epidemiology of herpes simplex virus types 2 and 1 amongst men who have sex with men attending sexual health clinics in England and Wales: implications for HIV prevention and Management. Euro Surveill. 2009;14 doi: 10.2807/ese.14.47.19418-en. article 19418. [DOI] [PubMed] [Google Scholar]
- 27.Hook E, Cannon R, Nahmias A, et al. Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection. J Infect Dis. 1992;165:251–255. doi: 10.1093/infdis/165.2.251. [DOI] [PubMed] [Google Scholar]
- 28.Jenssen H, Hamill P, Hancock R. Peptide antimicrobial agents. Clinical Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012;379:641–647. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehrer R, Daher K, Ganz T, Selsted M. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Looker K, Garnett G, Schmid G. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx R, Stein T, Entian K, et al. Structure of the Bacillus subtillis peptide antibiotic subtilosin A determined by 1H NMR and matrix assisted laser desorption/ionization time-of-flight mass spectrometry. J Protein Chem. 2001;20:501–506. doi: 10.1023/a:1012562631268. [DOI] [PubMed] [Google Scholar]
- 33.Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 34.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pongtharangkul T, Demirci A. Evaluation of agar diffusion bioassay for nisin quantification. Appl Microbiol Biotechnol. 2004;65:268–272. doi: 10.1007/s00253-004-1579-5. [DOI] [PubMed] [Google Scholar]
- 36.Schulte E, Sauerbrei A, Hoffmann D, Zimmer C, Hemmer B, Mühlau M. Acyclovir resistance in herpes simplex encephalitis. Ann Neurol. 2010;67:830–833. doi: 10.1002/ana.21979. [DOI] [PubMed] [Google Scholar]
- 37.Serkedjieva J, Danova S, Ivanova I. Antiinfluenza virus activity of a bacteriocin produced by Lactobacillus delbrueckii. Appl Biochem Biotechnol. 2000;88:285–295. [Google Scholar]
- 38.Shin J, Cai G, Weinberg A, et al. Frequency of acyclovir-resistant herpes simplex virus in clinical specimens and laboratory isolates. J Clin Microbiol. 2001;39:913–917. doi: 10.1128/JCM.39.3.913-917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin Y, Hohman M, Brenner M, et al. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl Phys Lett. 2001;78:1149–1151. [Google Scholar]
- 40.Steiner I, Kennedy P, Pachner A. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 41.Sutyak K, Anderson R, Dover S, et al. Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol. 2008;2008:540758. doi: 10.1155/2008/540758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutyak K, Wirawan R, Aroutcheva A, et al. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol. 2008;104:1067–1074. doi: 10.1111/j.1365-2672.2007.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talarico L, Castilla V, Rámirez J, et al. Synergistic in vitro interactions between (22S,23S)-3β-bromo-5α,22,23-trihydroxystigmastan-6-one and foscarnet against herpes simples virus type 1. Chemotherapy. 2006;52:38–42. doi: 10.1159/000090242. [DOI] [PubMed] [Google Scholar]
- 44.Tamamura H, Otaka A, Murakami T, et al. Interaction of an anti-HIV peptide, T22, with gp120 and CD4. Biochem Biophys Res Commun. 1996;219:555–559. doi: 10.1006/bbrc.1996.0272. [DOI] [PubMed] [Google Scholar]
- 45.Thennarasu S, Lee D, Poon A, et al. Membrane permeabilization, orientation, and antimicrobial mechanism of subtilosin A. Chem Phys Lipids. 2005;137:38–51. doi: 10.1016/j.chemphyslip.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Todorov S, Wachsman M, Knoetze H, et al. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soybeans. Int J Antimicrob Agents. 2005;25:508–513. doi: 10.1016/j.ijantimicag.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 47.van Velzen M, van Loenen F, Meesters R, et al. Latent acyclovir-resistant herpes simplex virus type 1 in trigeminal ganglia of immunocompetent individuals. J Infect Dis. 2012;205:1539–1543. doi: 10.1093/infdis/jis237. [DOI] [PubMed] [Google Scholar]
- 48.Wachinger M, Kleinschmidt A, Winder D, et al. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 49.Wachsman M, Castilla V, De Ruiz Holgado A, et al. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir Res. 2003;58:17–24. doi: 10.1016/s0166-3542(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 50.Wachsman M, Farías M, Takeda E, et al. Antiviral activity of enterocin CRL35 against herpesviruses. Int J Antimicrob Agents. 1999;12:293–299. doi: 10.1016/s0924-8579(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 51.Wachsman M, López E, Ramírez J, et al. Antiviral effect of brassinosteroids against herpes virus and arenaviruses. Antiviral Chem Chemother. 2000;11:71–77. doi: 10.1177/095632020001100107. [DOI] [PubMed] [Google Scholar]
- 52.Whitley R, Roizman B. Herpes simplex virus infections. The Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 53.Yarin A, Koombhongse S, Reneker D. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J Appl Phys. 2001;90:4836–4846. [Google Scholar]
- 54.Yasin B, Pang M, Turner J, et al. Evaluation of the Inactivation of Infectious Herpes Simplex Virus by Host-Defense Peptides. Eur J Clin Microbiol Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]