Abstract
Adolescent experiences of social deprivation result in profound and enduring perturbations in adult behavior, including impaired sensorimotor gating. The behavioral deficits induced by adolescent social isolation in rats can be ameliorated by antipsychotic drugs blocking dopamine D2 receptors in the prefrontal cortex (PFC) or by chronic administration of a cannabinoid CB1 receptor antagonist. The patterning and abundance of D2 receptors in the PFC evolves concurrently with CB1 receptors through the period of adolescence. This evidence suggests that mature expression and/or surface distribution of D2 and CB1 receptors may be influenced by the adolescent social environment. We tested this hypothesis using electron microscopic immunolabeling to compare the distribution of CB1 and D2 receptors in the PFC of adult male Sprague-Dawley rats that were isolated or socially reared throughout the adolescent transition period. Prepulse inhibition (PPI) of acoustic startle was assessed as a measure of sensorimotor gating. Social isolation reduced PPI and selectively decreased dendritic D2 immunogold labeling in the PFC. However, the decrease was only evident in dendrites that were not contacted by axon terminals containing CB1. There was no apparent change in the expression of CB1 or D2 receptors in presynaptic terminals. The D2 deficit therefore may be tempered by local CB1-mediated retrograde signaling. This suggests a biological mechanism whereby the adolescent social environment can persistently influence cortical dopaminergic activity and resultant behavior.
Keywords: Prefrontal cortex (PFC), schizophrenia, electron microscopy (EM), dopamine (DA), adolescence, isolation rearing
1. INTRODUCTION
Social isolation throughout adolescence in male rats induces a unique behavioral phenotype characterized by deficits in sensorimotor gating, locomotor hyperactivity, increased aggression, and impaired novel object recognition in adulthood (Fone and Porkess 2008; Geyer et al. 1993). The emergence and persistence of these behavioral deficiencies are contingent upon the period of isolation encompassing peripubertal development (Wilkinson et al. 1994), implicating a still-plastic neurocircuitry in their etiology. The prefrontal cortex (PFC), a late-maturing brain region, mediates many of these behaviors (Akirav and Maroun 2006; Bubser and Koch 1994; de Bruin et al. 1983). Postnatal development of the PFC continues throughout adolescence, during which time its immature circuitry remains vulnerable to potent inputs from subcortical and limbic regions that are heavily responsive to psychological stress and trauma (Casey et al. 2010). The prelimbic (PL) area of the PFC is a convergent target of glutamatergic projections from limbic brain regions such as the amygdala and hippocampus as well as dopaminergic efferents of the ventral tegmental area (VTA) (Brinley-Reed et al. 1995; Carr et al. 1999; Maurice et al. 1998). The permanent behavioral deficits observed in isolated rats may therefore be induced by a failure of these circuits to receive critical input during adolescence, a developmental stage when social play is most rewarding (Vanderschuren et al. 1997).
Interestingly, many of the behaviors manifested in adulthood by adolescently isolated rats mimic rodent models of schizophrenia as well as psychomotor symptoms of schizophrenia, a psychiatric disorder that typically manifests at the close of adolescent development. In particular, the sensorimotor gating deficit measured through prepulse inhibition (PPI) of the acoustic startle response is considered a behavioral hallmark of rodent models of schizophrenia (Braff and Geyer 1990) and is impaired in isolation-reared rats (Geyer et al. 1993). Furthermore emphasizing the relevance of isolation rearing to psychiatric disease, isolation-induced behavioral deficits can be ameliorated or eliminated through the administration of antipsychotic drugs targeting dopamine D2 receptors, the same pharmaceutical agents effective at treating schizophrenia (Geyer et al. 1993).
The efficacy of antipsychotic medication depends upon cortical D2 receptors (Takahashi et al. 2006). The PFC D2 receptor enables dopamine to modulate excitatory and inhibitory neural circuits whose activation in turn influences dopamine release from the VTA (Doherty and Gratton 1999; Tseng and O’Donnell 2004). D2 receptor signaling can be further mediated by co-activation of cannabinoid-1 receptors (CB1) (Glass and Felder 1997; Kearn et al. 2005). Like the D2 receptor, its activation results in inhibition of adenylyl cyclase and subsequent decrease in cAMP and protein kinase A activity (Kearn et al. 2005), and can reduce excitatory transmission in the PFC (Heng et al. 2011).
Both D2 and CB1 receptor agonists induce sensorimotor gating deficits (Peng et al. 1990; Schneider and Koch 2003). Significant maturational changes in the endocannabinoid system occur over postnatal development, and adolescents may be uniquely vulnerable to the effects of CB1 receptor activation (Viveros et al. 2005). CB1 receptor agonist administration throughout the pubertal period can induce a long lasting sensorimotor gating deficiency yet does not do so if the same dose is given to adult rats. This gating deficit can be reversed by D2 receptor antagonists (Schneider and Koch 2003). Moreover, administration of a CB1 receptor agonist to isolation-reared rats during adolescence exacerbates the PPI deficit (Malone and Taylor 2006), while administration of a CB1 receptor antagonist over the same period ameliorates the behavioral effects of social isolation (Zamberletti et al. 2012).
Interestingly, adolescent abuse of marijuana, which exerts its psychoactive effects through the CB1 receptor, is considered a significant risk factor for schizophrenia (Semple et al. 2005). This may implicate a complex interaction of adolescent PFC cannabinoid and dopamine circuitry in the development of schizophrenic psychopathology (Caspi et al. 2005). The pharmacological and clinical evidence supporting the relevance of CB1 and D2 receptors to psychiatric disease and animal models thereof underscores the need to understand effects of environmental factors on the in vivo subcellular relationship between these two critical receptors in the PFC. As in schizophrenia, behavioral deficits induced by isolation rearing are aggravated by CB1 agonist administration and ameliorated by D2 receptor antagonists. This may indicate a disruption of CB1 and/or D2 receptor signaling created by the mental state induced through isolation rearing as well as a formative effect of the adolescent social environment on the normal distribution of these receptors.
We tested the hypothesis that the sensorimotor gating dysfunction observed in isolated rats is underwritten by altered distribution of D2 and/or CB1 in neurons of the PFC. This was achieved using dual electron microscopic immunolabeling in conjunction with measuring PPI of acoustic startle in adult rats that were socially isolated from postnatal day 23 through 77 (throughout the adolescent transition period: postweaning into early adulthood). Immuno-electron microcopy allows observation of subtle isolation-induced changes in subcellular D2 receptor distribution specific to post-synaptic dendrites receiving input from CB1-containing or other axon terminals.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
Experimental protocols involving animals in this study followed NIH guidelines concerning the Care and Use of Laboratory Animals in Research and were approved by the Animal Care Committee at Weill Cornell Medical College. All efforts were made to reduce suffering and to minimize the number of animals needed for experiments. Male Sprague-Dawley rats were obtained from a commercial vendor (Taconic Farms, Hudson, NY) immediately after weaning at postnatal day 21. These animals were housed at 70 – 74°F and 30–70% humidity in plastic cages of 12″ X 12″ X 6.5″ and maintained with HEPA-filtered air. All were given nesting and bedding materials and maintained on a 12-hour light/dark cycle (lights on from 7AM to 7PM) in a common animal facility. Food and water were available ad libitum.
2.2 Social Isolation and Prepulse Inhibition Testing
At postnatal day 23, nine of eighteen rat pups were segregated and reared in isolation while the remaining nine were reared in cohorts of three per cage for eight weeks. To minimize handling-induced effects on isolated rats, cages were cleaned once per week for isolation-reared rats and two times per week for group-reared rats. The level of cleanliness was monitored by two independent observers and found to be similar in both sets of cages, as the isolates generated less waste than three group-reared rats combined in a standard housing unit. Both isolation and group-reared animals were kept in a common room so that isolates maintained auditory, visual, and olfactory input from other rats.
Prepulse inhibition was assessed after 8 weeks of either isolation or group-rearing by testing acoustic startle using a single-chambered SR-LAB startle apparatus with digitized signal output (San Diego Instruments, San Diego, CA). Each subject was placed in a clear acrylic cylindrical tube (8.2 cm in diameter) within the chamber for testing. This tube had ventilation slots and afforded enough room for movement and rotation so that restraint stress was not a confounding factor. A Radioshack Supertweeter (Radioshack Corporation, Fort Worth, TX) was mounted inside the testing chamber and generated background noise and acoustic stimuli. The subject’s startle magnitude was transduced through a piezoelectric device mounted under the acrylic tube. Sound levels were calibrated with a Radioshack digital sound level meter.
The testing session began with an acclimation period of 5 min in the chamber with the light and fan on with 65 dB of background noise. The first six acoustic stimuli were startle-eliciting pulses consisting of a 40 msec burst of 120 dB noise without a prepulse. Prepulses were presented as 20 msec stimuli 3, 6, or 12 dB above the 65 dB background noise prior to the startle-inducing pulse. Following the first six pulse-alone stimuli, the three different prepulse + pulse sequences were presented five times each in a pseudorandom sequence with ten pulse-alone stimuli. Inter-stimuli intervals ranged between 8 to 23 seconds, averaging 15 seconds. Acoustic startle testing concluded with five additional pulse-alone stimuli. Rats were then returned to their home cage.
Percent prepulse inhibition (% PPI) was calculated for each subject using the formula: %PPI = (Average startle magnitude to XdB prepulse+pulse stimulus)/(Average startle magnitude to pulse-alone trials) * 100, where X = 3, 6, or 9, depending on the prepulse volume. Responses to the first six pulse-alone stimuli were not included in the calculation of averages in order to minimize inter-animal variability due to exaggerated initial startle responses. All other pulse-alone and prepulse+pulse trials were included in the calculation of average % PPI for each animal, unless the rat was noticed to be mobile or moving in the chamber when the startle-eliciting stimulus sounded, in which case the animal’s movement may have disrupted an accurate piezoelectric transfer of startle. On average, less than one of the total trials per animal and never more than two trials per animal were discarded for this reason.
2.3 Tissue Preparation
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused through the aortic arch with sequential delivery of (1) 10 ml of heparin-saline (1000 U/ml), (2) 60 ml of 3.75% acrolein and 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4), and (3) 150 ml of 2% PFA in 0.1 M PB. Brains were excised and post-fixed in 2% PFA in 0.1 M PB for 30 min. Brains were then transferred to cold 0.1 M PB and cut into 40 μm coronal sections on a Vibratome (Leica, Deerfield IL). Coronal sections through the prelimbic prefrontal cortex (PL) were selected at 2.70 mm anterior to bregma (Paxinos 2004).
2.4 Immunohistochemistry
Sections through the frontal cortex from isolated and socially reared rats were marked with identifying punches and pooled in the same solutions throughout to ensure identical labeling conditions between groups. These floating sections were processed for immunoperoxidase CB1 labeling and immunogold D2 labeling as described previously (Fitzgerald et al. 2012a). Sections were placed in 1% sodium borohydride in 0.1 M PB for 30 min and then washed in successive rinses of 0.1 M PB. Sections were then incubated in cryoprotectant solution (25% sucrose with 3.5% glycerol in 0.05 M PB) for 15 min and then freeze-thawed by sequential immersion in liquid chlorodifluoromethane (Freon, Refron Inc, NY), liquid nitrogen, and room temperature 0.1 M PB.
Sections were then immunoblocked in a solution of 0.5% BSA in 0.1 M Tris-buffered saline (TBS; pH 7.6) for 30 min prior to incubation in the primary antibody solution, which consisted of the rabbit anti-D2R (1:250) and guinea pig anti-CB1R (1:3000) with 0.1% BSA in 0.1 M TBS. Tissue sections were left in this primary antibody cocktail overnight at room temperature for 36 hours at 4°C.
2.5 CB1R immunoperoxidase labeling
Free-floating sections were rinsed in 0.1 M TBS and incubated for 30 min in a 1:400 dilution of biotinylated goat anti-guinea pig immunoglobulin G (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Sections were then washed in 0.1 M TBS and placed in avidin-biotin peroxidase complex (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA) for 30 min. The peroxidase reaction product was produced in 0.022% 3,3′-diaminobenzidine (DAB, Aldrich, Milwaukee, WI) with 0.003% hydrogen peroxide in 0.1 M TBS for 7 min.
2.6 D2R immunogold labeling
Immunogold-silver labeling followed the DAB reaction. Sections were rinsed in 0.01 M phosphate-buffered saline (PBS; pH 7.4), blocked in a solution of 0.8% BSA with 0.1% gelatin in 0.01 M PBS for 10 min, and incubated in a 1:50 dilution of goat anti-rabbit IgG conjugated with 1 nm colloidal gold (Amersham, Arlington Heights, IL, USA) for 2 hours. Gold particles were fixed in 2% glutaraldehyde in 0.01 M PBS. Sections were washed in 0.2 M citrate buffer (pH 7.4) prior to silver enhancement using the IntenS-EM kit (Amersham) for 7 min. Brain sections were post-fixed in 2% osmium tetroxide in 0.1 M PB for 1 hour. Post-fixed sections were washed in 0.1 M PB followed by dehydration through a graded series of ethanols and propylene oxide then incubated overnight in a 1:1 mixture of propylene oxide and Epon (Embed 812; Electron Microscopy Sciences, Fort Washington, PA). Sections were flat embedded between two sheets of Aclar plastic after incubation in 100% Epon for 2 hours.
The flat-embedded brain region containing the PL was trimmed into a trapezoid and cut into ultra-thin 60 nm sections with a diamond knife (Diatome, Fort Washington, PA) on an ultratome, collected on 400-mesh copper grids (Electron Microscopy Sciences), and counterstained with uranyl acetate and lead citrate.
2.7 Antisera
The CB1R was labeled with an affinity-purified polyclonal antibody raised in guinea pig against its C-terminus. This antibody has been shown to have no immunoreactivity in the cortex, striatum, or hippocampus of mutant mice lacking the CB1R (Fitzgerald et al. 2012a; Katona et al. 2006; Lane et al. 2011).
The D2R was identified using an affinity-purified antiserum generated in rabbit against amino acids 216–311 of the human D2R long isoform (Brana et al. 1997). Antiserum selectivity was demonstrated by positive immunolabeling in human embryonic kidney (HEK) cells transfected with pcDNA-FLAG-D2L plasmid (Kearn et al. 2005). A single band immunolabeled for the D2R at the predicted weight of 50kDa was shown in Western blots of rat whole-brain homogenate and was eliminated in a preadsorption control (Pickel et al. 2006). Antibody specificity in the mouse PL was further confirmed using a preadsorption control in PFA-fixed vibratome sections (Fitzgerald et al. 2012a).
2.8 Electron Microscopy
Electron microscopy images were collected by an investigator blind to rearing condition. Ultra-thin sections were examined at 60 kV with a Philips CM10 transmission electron microscope (FEI, Hillsboro, OR) interfaced with an AMT Advantage HR/HR-B CCD camera (Advantage Microscopy Techniques, Danvers, MA) that was used to capture digital images. Micrographs were collected from the Epon-tissue interface where there is optimal penetration of primary and secondary antisera.
833 total images for quantification were collected from the PL of isolated and group-reared rats. 40–50 micrographs were collected from each subject, which resulted in a total of 441 micrographs from socially-reared rats and 392 micrographs from isolated rats. In each subject, micrographs were collected from Layer III of the PL wherever both CB1 immunoperoxidase and D2 immunogold labels were present in the same field of view. Micrographs were obtained from adjacent but not touching grid squares from each copper grid as described by Hara and Pickel (2008).
Neuronal profiles containing CB1 or D2 immunoreactivity were identified according to the criteria set forth by Peters et al., 1990. Axon terminals were identified by the abundant presence of synaptic vesicles. Dendrites frequently contained organelles such as mitochondria or ER and were often contacted by vesicle-containing axon terminals, while dendritic spines did not contain mitochondria, were small in diameter (less than 0.5 μm), had bulbous heads, and were likely to contain electron-dense postsynaptic specializations underneath axon terminal contacts. Synapses were defined as symmetric (GABAergic) or asymmetric (glutamatergic) by the presence of a thin or thick post-synaptic specialization, respectively. Immunogold (D2R) labeling appeared as black puncta approximately 40 nm in diameter, while immunoperoxidase labeling (CB1R) was identified by a diffuse electron-dense reaction product that was not evident in similar profiles throughout the surrounding neuropil.
Micrographs were collected at either 13,500X on a CM-10 transmission electron microscope (FEI, Hillsboro, OR) or at 18,500X on a Technai 12 BioTwin transmission electron microscope (FEI, Hillsboro, OR) interfaced with a digital camera (Advanced Microscopy Techniques, Danvers, MA). D2R immunogold particles were counted and categorized as to whether they were in axon terminals, dendrites, or dendritic spines, in contact with the plasma membrane, or present in profiles containing the CB1R immunoperoxidase reaction product or adjacent to profiles containing CB1R immunoperoxidase. Two or more gold particles touching but with defined boundaries were counted as separate particles.
2.9 Data Analysis
MCID Elite software Version 6.0 (Imaging Research Inc., Ontario, Canada) was used to measure the diameter, major axis and minor axis length, perimeter, area, and form factor of immunolabeled neuronal profiles. Measures and counts were made by hand by an investigator blind to rearing condition. Adobe Photoshop CS4 (Version 11.0.2) and Microsoft PowerPoint 2008 for Mac (Version 12.3.2) were used to compile and edit the micrographs shown here.
In the electron microscopic analysis of CB1 and D2 receptor distribution, only cross-sections of neuronal profiles that could entirely fit within the frame of the micrograph were counted (i.e., dendrites, dendritic spines, axon terminals). Therefore, labeled cell somata were not included. However, numerous neuronal somata contained D2 receptor immunogold in Layer III of the PL, and two of these were dual labeled with CB1 immunoperoxidase. Furthermore, glia were not included in counts. Because of the highly irregular shape of glial processes, it was not possible to determine in a single cross-section whether numerous labeled glial processes represented one or many labeled glia. Thus, utilizing this type of count to compare a treatment group side-by-side with a control group was considered inappropriate. The D2 receptor was, however, observed within glia of the PL, consistent with our past report (Duffy et al. 2011). Differences in postsynaptic D2 receptor distribution between isolated and group-reared rats are reported in the results. Statistical significance was determined at p < 0.05.
3. RESULTS
3.1 Isolation rearing and prepulse inhibition
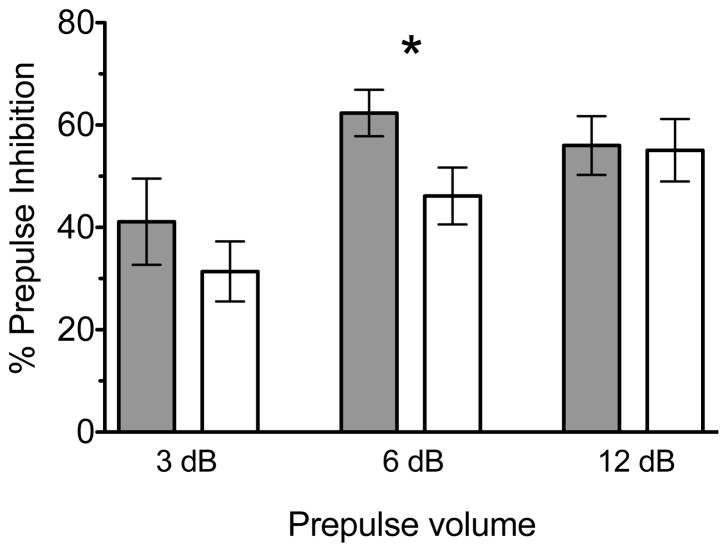
Prepulse inhibition (PPI) of acoustic startle was assessed in isolated rats as a measure of sensorimotor gating. All nine rats from each condition were tested after eight weeks of either isolation or social rearing conditions (at PD 79, in adulthood). The amplitude of the startle response to the pulse-alone stimulus did not differ between groups (t16 = 1.213, p = 0.25), indicating no baseline difference in acoustic startle in adult isolation-reared rats. However, PPI was deficient in isolated rats relative to socially housed animals (Figure 1), consistent with past reports of isolation-induced PPI disruption (Fone and Porkess 2008). Between-group planned comparisons at each level of prepulse volume show a significant difference in % PPI at the 6 decibel (dB) prepulse level (t16 = −2.220, p < 0.05), indicating a significantly lower PPI in isolated rats than in socially-reared controls.
Figure 1.
Between-group planned comparisons at each level of prepulse volume show a significant difference in % PPI at the 6 decibel (dB) prepulse level (t = −2.220, p < 0.05), indicating a significantly lower PPI in isolated rats than in socially-reared controls (* p < 0.05, n = 9 per group, data is represented as mean ± S.E.M). Prepulse volume denotes the decibel level of the prepulse above background noise. Group-reared animals are represented by grey bars while isolates are represented by white in the histogram shown.
3.2 Pre- and post-synaptic distribution of CB1 and D2 receptors in the PFC
Global distribution of pre- and post-synaptic CB1 and D2 receptors was similar in both groups of animals (Table 1). CB1 was located presynaptically, observed in axons and axon terminals but not in dendrites or spines. D2 immunogold was rarely contained within CB1-immunoreactive terminals. D2 receptors were observed within dendrites, dendritic spines, and axon terminals. D2-labeled dendrites were frequently contacted by CB1-containing axon terminals as well as by unlabeled axon terminals.
Table 1.
Overall distribution of CB1 and D2 receptors is similar in neuronal profiles of isolated and socially reared rats. A total number of 3312 profiles were measured and compared between groups.
| CB1R terminal | D2R terminal | CB1R + D2R terminal | D2R dendrite | D2R spine | |
|---|---|---|---|---|---|
| Social | 29% | 25% | 1% | 41% | 4% |
| Isolated | 30% | 26% | 1% | 39% | 4% |
Parameters that were measured, compared, and found not to differ between groups include D2 immunogold distribution in axon terminals and immunolabeled axon terminal size (assessed by minimum axis length, average diameter, area, form factor, and perimeter). Likewise, no significant difference was found in the size of unlabeled dendritic profiles (n=169). A total number of 3312 immunolabeled neuronal profiles were measured in Layer III of the PL in isolated and socially reared rats: 971 CB1 axon terminals, 861 D2 axon terminals, 20 dual-labeled terminals, 1330 D2 dendrites (342 of which were contacted by CB1 axon terminals), and 130 D2 dendritic spines.
3.3 Isolation-induced changes in postsynaptic D2 receptor distribution
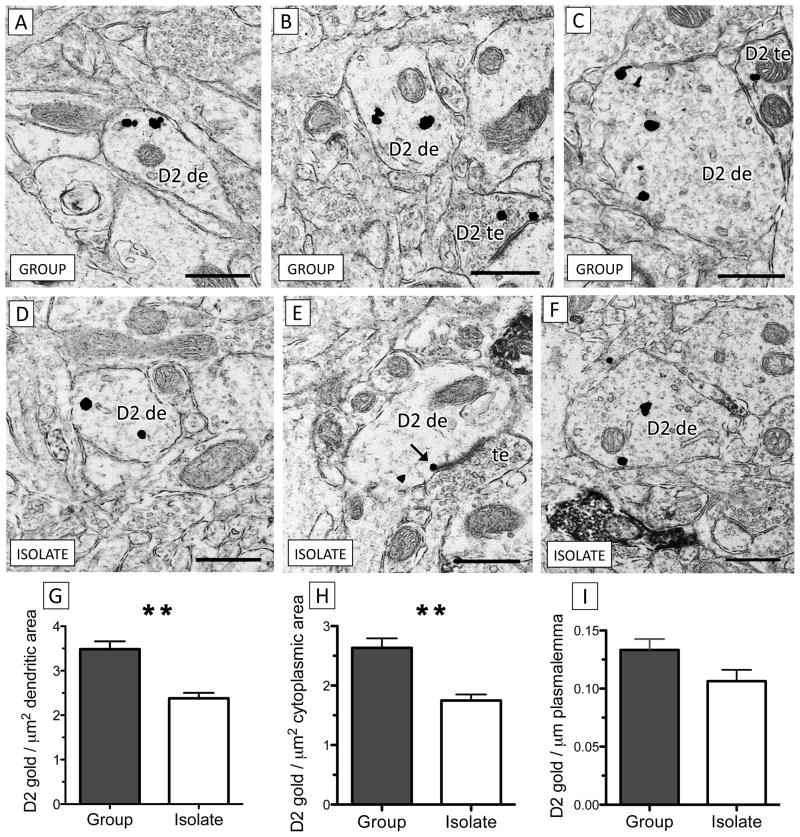
D2 receptor expression in dendrites of the PL in isolated rats was significantly lower than in animals reared in social groups. However, this difference was only significant in dendrites that were not contacted by CB1-labeled terminals (Figure 2). In this population, isolation rearing induced a decrease in total dendritic D2 immunogold density (the number of particles per μm dendritic area; t16 = −3.66, p < 0.005), which was further analyzed as to the positioning of cytoplasmic and plasmalemmal D2 immunogold particles. Cytoplasmic D2 significantly decreased in isolated animals (t16 = −3.67, p < 0.005) while there was a trend towards decreased plasmalemmal D2 (t16 = −1.92, p = 0.07), measured as D2 immunogold per μm dendritic perimeter.
Figure 2.
There is a significant decrease in dendritic D2 receptor expression in the PL of isolated rats relative to group-housed controls. (A–C) Dendrites of various sizes from socially-reared rats contain D2 immunogold (D2 de). In (C), a dendrite containing D2 immunogold is contacted by a D2 terminal (D2 te). (D–) Dendrites from the PL of isolated rats contain qualitatively less D2 immunogold than group-housed controls. In (E), a D2 immunogold particle (black arrow) contacts the outer edge of the postsynaptic density of an asymmetric excitatory-type synapse formed by an unlabeled axon terminal (te). (G–I) Dendrites of isolation-reared rats contain significantly less total D2 (G) and cytoplasmic (H) immunogold per μm2 area relative to group-reared controls but the density of plasmalemmal D2 immunogold particles per μm membrane (I) does not significantly differ between groups (** p < 0.005, n = 9 per group, data is represented as mean ± S.E.M). Scale bars = 500 nm.
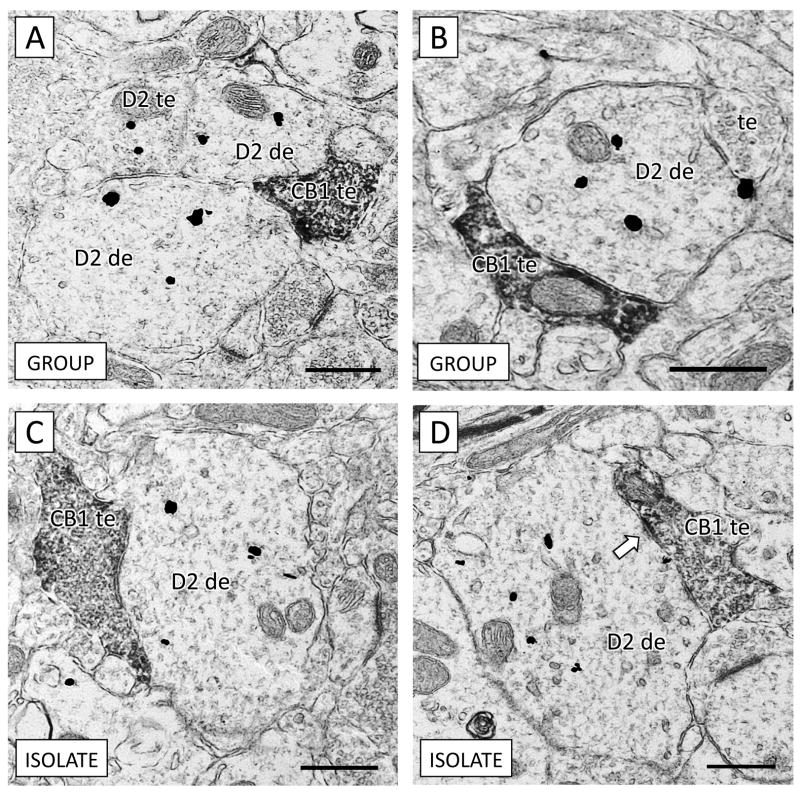
In dendrites apposing CB1-containing terminals, no significant difference was observed in D2 content in isolated rats, either in total D2 (t16 = −1.71, p = 0.11), plasmalemmal (t16 = −1.18, p = 0.25), or cytoplasmic (t16 = −0.78, p = 0.45). These CB1 terminals apposing D2 dendrites occasionally contained synaptic specializations within the plane of section (Figure 3). In isolated rats, 30% of D2 dendrites apposed CB1-containing axon terminals relative to 23% of D2 immunoreactive dendrites contacting CB1 terminals in socially reared animals. This difference was not statistically significant (λ2 = 2.767, p = 0.1).
Figure 3.
There is no significant difference between group-reared (A–B) and isolated (C–D) rats in D2 receptor density in dendrites contacted by CB1-containing axon terminals. (A) An axon terminal containing CB1 immunoperoxidase reaction product (CB1 te) apposes two D2-immunogold labeled dendrites (D2 de). An axon terminal containing D2 immunogold (D2 te) also contacts both dendrites. (B) Both an unlabeled axon terminal (te) and a CB1 terminal contact a dendrite containing D2 immunogold. (C) A large CB1 axon terminal targets a D2-containing dendrite in the PL of an isolation-reared rat. (D) A CB1 immunoreactive axon terminal forms a symmetric inhibitory-type synaptic contact (white arrow) with a dendrite containing D2 immunogold. Scale bars = 500 nm.
A trend towards increased size of dendrites containing D2, as measured by minimum axis diameter, was observed in isolated rats (t16 = 1.80, p = 0.08). While this was not statistically significant, any increase in dendritic size could contribute to an apparent decrease in immunogold particles per unit area. Therefore, a cluster analysis was undertaken to determine the influence of dendritic size on immunogold density. D2-containing dendrites were clustered by minimum axis diameter using k-means analysis into small, medium, and large-sized dendrites. D2 immunogold was significantly decreased in all diameter dendrites from isolated animals, indicating that the decreased D2 observed was not a function of increased dendritic size in isolates. This does not, however, exclude the possibility that isolation rearing could induce a slight morphological change in D2-containing dendrites that may be consistent with reduced dendritic branching and complexity in pyramidal neurons of the PFC in adult isolated animals (Wang et al. 2011).
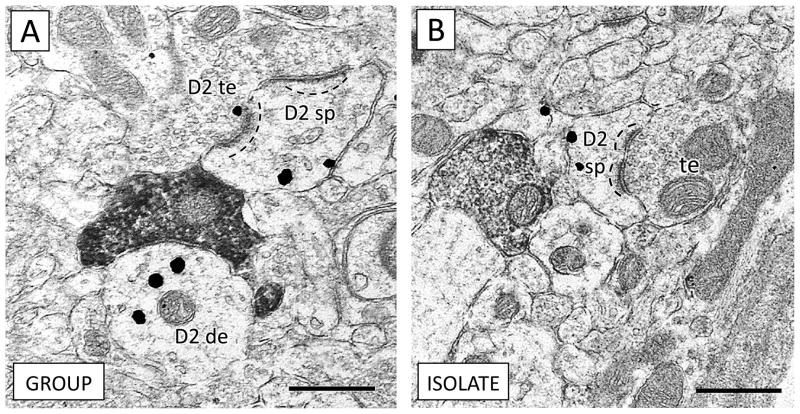
Differences in dendritic dopamine D2 receptor distribution in the prPFC of isolated rats were limited to the dendritic shaft. D2 receptor expression in dendritic spines was similar in group-reared and isolation-housed rats (t16 = −1.71, p = 0.11; Figure 4). There were furthermore no morphological differences (such as cross-sectional area, perimeter, average diameter, or ratio of major to minor axes) in D2-containing spines of isolated rats.
Figure 4.
Expression of dopamine D2 receptors is similar in dendritic spines of group-reared (A) and isolated (B) rats. (A) A D2-containing terminal (D2 te) forms an synapse with a dendritic spine expressing D2 (D2 sp). An apposing CB1 terminal contacts a D2 dendrite (D2 de). (B) An unlabeled terminal (te) forms an asymmetric excitatory-type contact with a spine containing D2 immunogold (D2 sp). In both animals, note the positioning of D2 immunogold opposite the postsynaptic density of the dendritic spines (dashed lines), consistent with dopaminergic input onto spine necks (Freund et al. 1984). Scale bars = 500 nm.
3.4 Presynaptic D2 and CB1 receptor distribution is similar in isolation and group-reared rats
Isolation and group-reared rats did not show significant differences in presynaptic D2 distribution in singly labeled terminals or in terminals dually labeled for the D2 and CB1 receptors (Figure 5). There was also no observed difference in any morphological measurements of terminals solely containing CB1. In both isolated and group-reared rats, the vast majority of CB1 terminals that formed synaptic contacts in the plane of section formed symmetric inhibitory-type membrane specializations indicative of GABAergic axon terminals. In group-reared rats, 97% of membrane specializations formed by CB1 axon terminals were symmetric inhibitory-type, and in isolated animals all discernable membrane specializations formed by CB1 terminals were symmetric.
Figure 5.
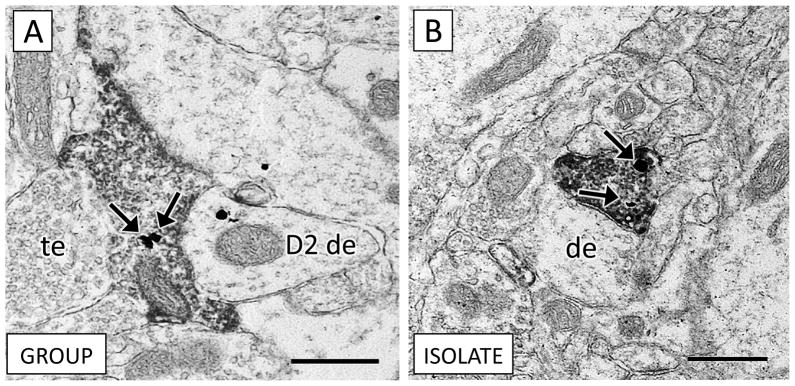
Axon terminals containing CB1 immunoperoxidase were rarely dual labeled for D2 immunogold in both socially-reared (A) and isolation-reared (B) rats. (A) An axon terminal characterized by the abundant presence of synaptic vesicles contains both CB1 immunoperoxidase and D2 immunogold labeling (arrows). This axon terminal is adjacent to an unlabeled terminal (te) and a D2-dendrite (D2 de). (B) A CB1 axon terminal from an isolation-reared rat holds two D2-immunogold particles (arrows) and contacts an unlabeled dendrite (de). Scale bars = 500 nm.
4. DISCUSSION
A PPI deficit and decreased dendritic D2 in the PL were observed in isolation-reared rats compared to socially-reared controls. This decrease in D2, however, only occurred in dendrites that did not receive local input from CB1-containing axon terminals. There was no apparent effect of isolation rearing on the presynaptic density of D2 receptors or in CB1-containing axon terminals. These results are discussed along with their implications for adolescent neuronal vulnerability to social isolation.
4.1 Prepulse inhibition deficit in isolation-reared rats
Isolated rats showed a PPI deficit similar to previous reports. This finding has been replicated by many laboratories (for review, see Fone and Porkess 2008), and demonstrates the experimental validity of our isolation-housing paradigm, as development of PPI can be disrupted by numerous factors such as housing environment and handling effects (Krebs-Thomson et al. 2001; Weiss et al., 1999). We observed a statistically significant PPI deficit only at the 6 dB prepulse volume. At 3 dB, it is possible that group-reared animals cannot reliably discern the prepulse while at 12 dB, the PPI response of both groups of animals may have already reached maximal levels.
4.2 Decreased postsynaptic D2 receptors in the PFC of isolated rats
Our observation of decreased dendritic D2 receptor density in the PFC of isolated rats is consistent with altered dopamine neurotransmission observed in the PFC of adult rats following isolation rearing (Fabricius et al. 2010; Fabricius et al. 2011; Han et al. 2011; Peters and O’Donnell 2005). Dopaminergic innervation of the PFC is shaped during adolescence when tyrosine-hydroxylase (TH) axonal processes increase through puberty before decreasing to adult levels (Kalsbeek et al. 1988). Social isolation during this time induces aberrant firing activity of dopaminergic neurons in the ventral tegmental area (VTA), including increased spontaneous burst firing and irregular activation patterns (Fabricius et al. 2010). Stimulation of the VTA in adult rats isolated as adolescents induces erratic responses of pyramidal neurons in the PFC compared to the plateau depolarization observed in group-reared controls (Peters and O’Donnell 2005). Increased basal levels of dopamine have been inconsistently reported in the PFC of isolated rats (Bortolato et al. 2011; Han et al. 2011).
Cortical dopaminergic signaling irregularities have also been observed in other rodent models of schizophrenia characterized in part by PPI deficits. In the neonatal hippocampal lesion model of schizophrenia, pyramidal neurons in the PFC of lesioned adult rats display abnormal responses to dopamine (Tseng et al. 2009). In the lesioned rats relative to controls, normal inhibitory firing responses to the D2 agonist quinpirole were dampened (Tseng et al. 2008) while excitatory responses to NMDA and dopamine D1 receptor agonists were exaggerated (Tseng et al. 2007). This may be indicative of a hyperexcitable cortical state. Indeed, in adult rats inoculated with lipopolysaccharide (LPS) in the hippocampus as neonates, early neuronal cytokine-mediated inflammation eliminates the response of fast-spiking interneurons to quinpirole in the PFC and reduces PPI (Feleder et al. 2010). The unresponsiveness to D2 agonists observed in these studies is consistent with decreased postsynaptic D2 availability in the PFC, as we observed in isolated rats.
4.3 Social isolation does not alter D2 receptor density in dendrites contacted by CB1-containing terminals
While isolation rearing induces a significant decrease in postsynaptic D2 receptors, dendrites locally contacted by axon terminals containing CB1 are protected from this change. D2 immunogold density in this dendritic population is comparable between isolates and group-housed rats. The selective decrease in D2 levels in dendrites without visible CB1 axonal input may either reflect a local effect of CB1 modulation on postsynaptic D2 receptor availability in isolated rats, a differential influence of isolation rearing on two separate populations of D2-containing dendrites (i.e. interneurons versus pyramidal cells), or a combination of these factors.
Social isolation as well as other stressors may in fact alter CB1 signaling in the PFC through effects on the endogenous CB1 receptor agonists, anandamide and 2-arachidonoylglycerol (2-AG). Isolation rearing increases the content of endocannabinoid 2-AG in the PFC (Sciolino et al. 2010). A similar increase in 2-AG occurs in the PFC of adult male rats in response to chronic stress, and correlates with termination of the hypothalamic-pituitary-adrenal (HPA) stress response (Hill et al. 2010). Endocannabinoids therefore may offer a neuronal mechanism towards adaptive habituation to intense psychological stress. Alterations in endocannabinoid signaling have furthermore been implicated in psychiatric diseases such as schizophrenia (Fernandez-Espejo et al. 2009).
Within the PFC, isolation-induced changes in endocannabinoid tone could provide a modulatory mechanism that influences the local dendritic environment. Presynaptic CB1 receptor involvement is crucial to postsynaptic D2-mediated plasticity (Shen et al. 2008) although CB1 receptors are not contained within dopaminergic axon terminals in the PFC (Fitzgerald et al. 2012b). Endocannabinoids fine-tune excitation and inhibition within neuronal circuitry through their activation of presynaptic CB1 receptors on GABAergic and glutamatergic terminals (Freund et al. 2003). Altered endocannabinoid signaling in isolated rats could therefore conceivably alter excitatory and/or inhibitory input onto D2-containing dendrites. A stress-induced adaptation of CB1 signaling might obviate the significant decrease in D2 receptor density observed in dendrites distal to input from axon terminals containing the CB1 receptor.
The CB1 receptor is indeed implicated in the targeting of postsynaptic D2 receptors in the PFC (Fitzgerald et al. 2012a) as well as the striatum (Lane et al. 2011). Dendritic D2 receptors are expressed by pyramidal neurons as well as interneurons of the PFC. Axon terminals containing the CB1 receptor most frequently target pyramidal neurons (Hill et al. 2011). Surprisingly, altered D2 receptor trafficking in mice lacking CB1 is only evident in parvalbumin-containing dendrites (Fitzgerald et al. 2012a) although this population of fast-firing interneurons is infrequently contacted by CB1-containing terminals and is far less abundant than pyramidal neurons in the PFC (Beaulieu 1993; DeFelipe et al. 2002; Gulledge and Jaffe 1998). Furthermore, expression of parvalbumin in the PFC is decreased in CB1 knockout mice (Fitzgerald et al. 2011), similar to alterations observed in fast-firing interneurons in isolation-reared rats (Schiavone et al. 2009) and schizophrenic subjects (Lewis et al. 2001). While CB1-containing terminals only rarely contact PFC parvalbumin interneurons, nearly all of them contain the D2 receptor (Fitzgerald et al. 2012a). It is therefore worth considering that the majority of dendrites receptive to CB1 inputs may belong to one cell population (pyramidal neurons) whereas dendrites that are not contacted by CB1 afferents may belong to another (parvalbumin interneurons), and that isolation rearing has a differential effect on D2 expression in these two cell types.
However, whether the isolation-induced dendritic decrease in D2 occurs in interneurons, pyramidal neurons, or both, the net effect would be disinhibition of the PFC in isolated rats. D2 receptor activation becomes excitatory in fast-spiking PFC interneurons during but not before the adolescent transition period (Tseng and O’Donnell 2007), a period encompassed by the isolation rearing protocol. In contrast, D2 receptor activation is largely inhibitory in pyramidal cells (Beazely et al. 2006; Gulledge and Jaffe 1998; Zheng et al. 1999). Our observation of decreased dendritic D2 receptors therefore is consistent with evidence of a disinhibited PFC in schizophrenic patients and in rodent models of the disorder (Gruber et al. 2010; Lewis et al. 2005; Spencer et al. 2003). Because the decrease in D2 receptors may occur at least in part in dendrites of interneurons, it should also be considered that altered D2 expression therein could be either a cause or a consequence of alterations observed in GABAergic interneurons in isolated rats (Bloomfield et al. 2008; Harte et al. 2007), other rodent models of schizophrenia (Lodge et al. 2009; Wang et al. 2008), and schizophrenic subjects (Hashimoto et al. 2003).
4.4 Conclusions
Isolated rats are genetic equivalents of their socially-housed peers yet suffer a somatosensory gating deficit and concomitant alterations to the critical target of antipsychotic medications-- the cortical D2 receptor (Takahashi et al. 2006). While a genetic predisposition almost certainly underscores schizophrenic etiology, accumulating evidence shows genes do not tell the whole tale (Tsuang 2000). The subcellular changes in expression patterns of dopamine receptors induced solely by isolation from peers during the vulnerable time of adolescent development elucidate one way that an adverse environment effectuates neuronal changes, which may facilitate the development of schizophrenic neuropathology in genetically susceptible individuals.
Highlights.
Isolation-reared adult rats show a reduction in PFC dendritic dopamine D2 receptors.
Cannabinoid CB1 receptor signaling may locally mitigate this D2 receptor decrease.
A sensorimotor gating deficit is also evident in isolation-reared adult rats.
Acknowledgments
The authors thank Dr. Jerylin Gang for valuable discussions regarding the interpretation of data, and June Chan for technical support. The authors also gratefully acknowledge funding from the National Institutes of Health (MH40342 and DA004600 to VMP; DA021696 and DA011322 to KM; T32 DA7274 to MLF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–65. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain research. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Tong A, Wei W-L, Van Tol H, Sidhu B, Macdonald JF. D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptor-dependent. Journal of neurochemistry. 2006;98:1657–1663. doi: 10.1111/j.1471-4159.2006.04064.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield C, French SJ, Jones DNC, Reavill C, Southam E, Cilia J, Totterdell S. Chandelier cartridges in the prefrontal cortex are reduced in isolation reared rats. Synapse. 2008;62:628–631. doi: 10.1002/syn.20521. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5α-reductase expression: Relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiat. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Brana C, Aubert I, Charron G, Pellevoisin C, Bloch B. Ontogeny of the striatal neurons expressing the D2 dopamine receptor in humans: an in situ hybridization and receptor-binding study. Brain Res Mol Brain Res. 1997;48:389–400. doi: 10.1016/s0169-328x(97)00114-9. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neuroscience letters. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Bubser M, Koch M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology. 1994;113:487–492. doi: 10.1007/BF02245228. [DOI] [PubMed] [Google Scholar]
- Carr DB, O’Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19:11049–11060. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Soliman F, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biological Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, van Oyen HG, Van de Poll N. Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behavioural Brain Research. 1983;10:209–232. doi: 10.1016/0166-4328(83)90032-3. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Effects of medial prefrontal cortical injections of GABA receptor agonists and antagonists on the local and nucleus accumbens dopamine responses to stress. Synapse. 1999;32:288–300. doi: 10.1002/(SICI)1098-2396(19990615)32:4<288::AID-SYN5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Fitzgerald ML, Chan J, Robinson DC, Milner TA, Mackie K, Pickel VM. Acetylcholine α7 nicotinic and dopamine D(2) receptors are targeted to many of the same postsynaptic dendrites and astrocytes in the rodent prefrontal cortex. Synapse. 2011;65:1350–1367. doi: 10.1002/syn.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, Sotty F. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neuroscience letters. 2010;482:117–122. doi: 10.1016/j.neulet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Steiniger-Brach B, Helboe L, Fink-Jensen A, Wörtwein G. Socially isolated rats exhibit changes in dopamine homeostasis pertinent to schizophrenia. Int J Dev Neurosci. 2011;29:347–350. doi: 10.1016/j.ijdevneu.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Feleder C, Tseng KY, Calhoon GG, O’Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biological Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Viveros M-P, Núñez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology. 2009;206:531–549. doi: 10.1007/s00213-009-1612-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Chan J, Mackie K, Lupica CR, Pickel VM. Altered dendritic distribution of dopamine D2 receptors and reduction in mitochondrial number in parvalbumin-containing interneurons in the medial prefrontal cortex of cannabinoid-1 (CB1) receptor knockout mice. The Journal of Comparative Neurology. 2012a;520:4013–4031. doi: 10.1002/cne.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Lupica CR, Pickel VM. Decreased parvalbumin immunoreactivity in the cortex and striatum of mice lacking the CB1 receptor. Synapse. 2011;65:827–831. doi: 10.1002/syn.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Shobin E, Pickel VM. Cannabinoid modulation of the dopaminergic circuitry: Implications for limbic and striatal output. Prog Neuropsychopharmacol Biol Psychiatry. 2012b;38:21–29. doi: 10.1016/j.pnpbp.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neuroscience and biobehavioral reviews. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang W, Shao F, Li N. Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain research. 2011;1385:175–181. doi: 10.1016/j.brainres.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Hara Y, Pickel VM. Preferential relocation of the N-methyl-D-aspartate receptor NR1 subunit in nucleus accumbens neurons that contain dopamine D1 receptors in rats showing an apomorphine-induced sensorimotor gating deficit. Neuroscience. 2008;154:965–977. doi: 10.1016/j.neuroscience.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT-Y, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu Q-S, Gorzalka BB, Hillard CJ. Recruitment of Prefrontal Cortical Endocannabinoid Signaling by Glucocorticoids Contributes to Termination of the Stress Response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TY, Gray M, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Nat Acad Sci. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung K-M, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. Journal of Neuroscience. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn C, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Molecular pharmacology. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Giracello D, Solis A, Geyer MA. Post-weaning handling attenuates isolation-rearing induced disruptions of prepulse inhibition in rats. Behavioural Brain Research. 2001;120:221–224. doi: 10.1016/s0166-4328(00)00374-0. [DOI] [PubMed] [Google Scholar]
- Lane DA, Chan J, Fitzgerald ML, Kearn CS, Mackie K, Pickel VM. Quinpirole elicits differential in vivo changes in the pre- and postsynaptic distributions of dopamine D2 receptors in mouse striatum: relation to cannabinoid-1 (CB1) receptor targeting. Psychopharmacology. 2011;221:101–113. doi: 10.1007/s00213-011-2553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. The American journal of psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. The effect of Delta9-tetrahydrocannabinol on sensorimotor gating in socially isolated rats. Behavioural Brain Research. 2006;166:101–109. doi: 10.1016/j.bbr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. 3. Elsevier Academic Press; San Diego, CA, USA: 2004. [Google Scholar]
- Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats. Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- Peters A, Palay LS, de F, Webster H. The Fine Structure of the Nervous System: Neurons and their Supporting Cells. Oxford University Press; New York, NY, USA: 1991. [Google Scholar]
- Peters YM, O’Donnell P. Social isolation rearing affects prefrontal cortical response to ventral tegmental area stimulation. Biological Psychiatry. 2005;57:1205–1208. doi: 10.1016/j.biopsych.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. The Journal of Comparative Neurology. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause K-H. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biological Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Bortolato M, Eisenstein SA, Fu J, Oveisi F, Hohmann AG, Piomelli D. Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience. 2010;168:371–386. doi: 10.1016/j.neuroscience.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biological Psychiatry. 2006;59:919–928. doi: 10.1016/j.biopsych.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biological Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng K-Y, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural Brain Research. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biological Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral reviews. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behavioural pharmacology. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J, Domeney AM. Isolation rearing-induced disruption of prepulse inhibition: further evidence for fragility of the response. Behavioural Pharmacology. 1999;10:139–149. doi: 10.1097/00008877-199903000-00003. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Viganò D, Guidali C, Rubino T, Parolaro D. Long-lasting recovery of psychotic-like symptoms in isolation-reared rats after chronic but not acute treatment with the cannabinoid antagonist AM251. Int J Neuropsychopharmacol. 2012;15:267–280. doi: 10.1017/S1461145710001185. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]