Abstract
Objective
Laws and regulations require many hospitals to implement rapid-response systems. However, the optimal resource intensity for such systems is unknown. We sought to determine whether a rapid-response system that relied on a patient’s usual care providers, not a critical-care–trained rapid-response team, would improve patient outcomes.
Design, Setting, and Patients
An interrupted time-series analysis of over a 59-month period.
Setting
Urban, academic hospital.
Patients
One hundred seven-one thousand, three hundred forty-one consecutive adult admissions.
Intervention
In the intervention period, patients were monitored for predefined, standardized, acute, vital-sign abnormalities or marked nursing concern. If these criteria were met, a team consisting of the patient’s existing care providers was assembled.
Measurements and Main Results
The unadjusted risk of unexpected mortality was 72% lower (95% confidence interval 55%–83%) in the intervention period (absolute risk: 0.02% vs. 0.09%, p < .0001). The unadjusted in-hospital mortality rate was not significantly lower (1.9% vs. 2.1%, p = .07). After adjustment for age, gender, race, season of admission, case mix, Charlson Comorbidity Index, and intensive care unit bed capacity, the intervention period was associated with an 80% reduction (95% confidence interval 63%–89%, p < .0001) in the odds of unexpected death, but no significant change in overall mortality [odds ratio 0.91(95%confidence interval 0.82–1.02), p=.09]. Analyses that also adjusted for secular time trends confirmed these findings (relative risk reduction for unexpected mortality at end of intervention period: 65%, p = .0001; for in-hospital mortality, relative risk reduction = 5%, p = .2).
Conclusions
A primary-team–based implementation of a rapid response system was independently associated with reduced unexpected mortality. This system relied on the patient’s usual care providers, not an intensive care unit based rapid response team, and may offer a more cost-effective approach to rapid response systems, particularly for systems with limited intensivist availability.
Keywords: cardiac arrest, medical emergency team, rapid response system, rapid response team, patient safety
Substantial public (1, 2), improvement organization (3, 4), and regulatory (5) interests have focused on the use of rapid response systems to protect hospital inpatients who suffer an acute clinical decompensation (6–9). In general, these teams seek to improve outcomes among inpatients outside the intensive care unit (ICU) by sending critical-care–trained responders to the bedside of decompensating patients. Although clinical studies of these teams have clearly had mixed results (6, 8, 10–16), regulators and lawmakers have moved ahead. The Joint Commission set a National Patient Safety Goal calling for hospitals to implement a system that “enables health care staff members to directly request additional assistance from a specially trained individual(s) when the patient’s condition appears to be worsening ” (5). Legislators have also passed laws requiring hospitals to implement rapid response teams (17). Most U.S. hospitals, therefore, are either required or under strong pressure to implement a rapid response system.
However, although most rapid response team studies have focused on ICU-based teams (16), the optimal composition of a rapid response team is unknown, as noted in a recent high-profile review (18). Therefore, a less-resource–intense rapid response system would be preferable if it had outcomes similar to other team models. Furthermore, it is not known whether the benefit of rapid response systems results from the early detection of events, the availability of a critical-care-trained response team limb, or some combination. Some have concluded that the afferent limb of the system—how decompensating patients are identified and the team actually triggered—is the weakest link in the chain (19).
We therefore sought to understand whether a system of care that focused on detection and systematic assessment of patients with clinical instability, rather than on a critical-care-trained response team, would reduce unexpected mortality outside the ICU. Specifically, we did not add clinical staff or use an ICU-based rapid response team, but instead focused on systematic detection of decompensations and notification of patients’ usual care providers.
MATERIALS AND METHODS
Study Design, Patients, and Data Sources
We conducted an interrupted time series analysis, enrolling all adult patients at our urban university medical center who were discharged between January 1, 2004 and November 30, 2008. The hospital has approximately 500 inpatient beds and, during the study period, had between 55 and 77 ICU beds. Patients were identified using our administrative data, which are prospectively collected for nonresearch purposes. We selected January 1, 2004 as the beginning of the cohort because do-not-attempt resuscitation (DNR) status was recorded in the electronic systems beginning at that time. To limit selection bias, we included every adult patient admitted to the hospital. We divided the cohort into three temporal cohorts: the baseline period (22 months, from November 1, 2004 until the intervention was implemented), the implementation period (6 months), and the intervention period (31 months).
We obtained demographic and outcome data from the hospital administrative systems. To record team activations, we designed and built an information system that was integrated into clinical workflow. This system created the event’s clinical documentation and simultaneously created a log of all team activations. To adjust for the acuity of an individual admission, we used patient-level Diagnosis-Related Group weights. We ascertained individual comorbid conditions with the Agency for Healthcare Research and Quality’s Comorbidity Software (20), which uses Elixhauser’s method (21), and assessed the cumulative burden of comorbidity using the Charlson Comorbidity Index, calculated from discharge diagnoses using the method by Quan et al (22). This study was approved by our Institutional Review Board, which provided a waiver of informed consent.
Process Analysis and Intervention Design
We mapped the hospital’s usual processes for responding to acutely decompensating inpatients. We identified a minimum of four conceptual steps that link the inciting event (decompensation) to the goal (receipt of appropriate therapy): 1) monitoring for decompensation, 2) recognizing that a monitored value is abnormal, 3) activating responders, and 4) applying appropriate therapy by responders. These steps by their nature must occur in series, not in parallel. For example, if the person measuring blood pressure does not recognize a blood pressure of 75/40 mm Hg as abnormal, then the composition of the response team is irrelevant since the team will never be activated. We therefore chose to focus first on improving reliability of event detection and responder activation, rather than on the level of training of the response team.
Intervention
On October 25, 2005, our intervention was implemented on all inpatient wards except for ICUs. Patients were included regardless of the admitting department. As depicted in Figure 1, patients are monitored for acutely abnormal vital signs and other issues of major concern, using the Bellomo criteria (12). If any one of these criteria is met, the response team is assembled. This team consists of the patient’s primary nurse, primary house officer or licensed independent practitioner, and the floor’s senior nurse (usually a nurse educator or specialist); for respiratory criteria, the team includes the respiratory therapist covering the patient. In our hospital, outside the ICU, respiratory therapists provide basic respiratory care, including nebulizers and suctioning. Mask ventilation for respiratory failure is only provided in ICUs, and respiratory therapists do not provide advanced interventions such as endotracheal intubation or bronchoscopy.
Figure 1.
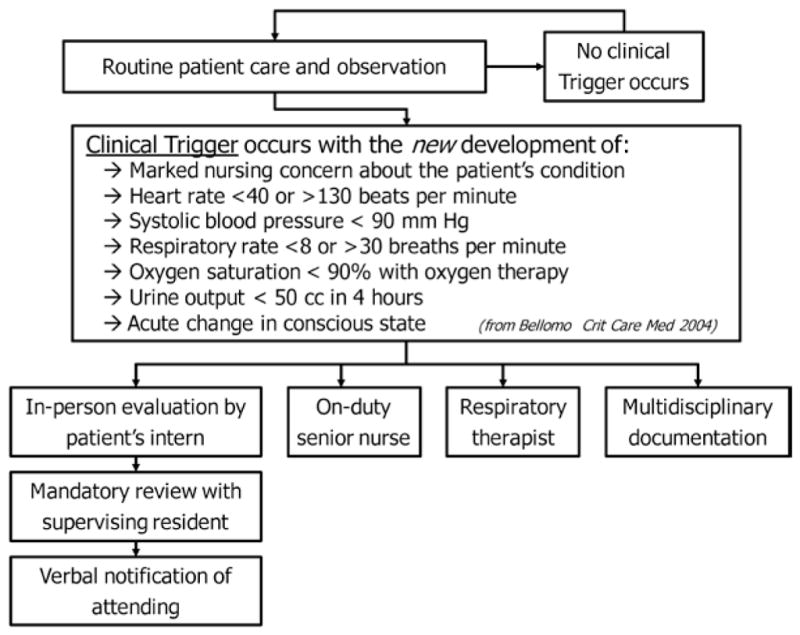
Clinical pathway for monitoring and responding to inpatients with acute decompensations.
These team members respond urgently and in person. In most cases, the patient’s nurse assembles the team by paging the other providers. At our institution, when a primary house officer is unavailable (e.g., postcall), he or she is covered by another house officer who has received a sign-out about the patients. Team composition did not vary based on the time of day. To assist provider decision making at the bedside, a multidisciplinary event note is printed, which retrieves laboratory, medication, DNR order status, and allergy data from the hospital’s information systems in real time. No specific therapies are mandated, but the team must verbally discuss the case with the attending physician within 1 hr of the event. First-year residents must also discuss the case with the supervising resident (Fig. 1).
All providers who participate in the patient’s care in our new system were the same providers responsible for the patient’s care already. However, in the previous system, the floor’s senior nurse, respiratory therapist, and the attending physician participated in care on an as-needed basis when patient acuity was high, but exact criteria were not specified.
Outcome Measures
Unexpected mortality was defined as death outside an ICU in a patient without a DNR order and was our primary outcome. This outcome measure is promoted by the Institute for Healthcare Improvement (23) and was used in the largest randomized rapid response team trial to date (15). In-hospital mortality was defined as any death before hospital discharge.
Statistical Analysis
For unadjusted analyses, the unpaired t test, Wilcoxon rank sum test, or Fisher’s exact test were used depending on the distribution of the variable. Because patients could be admitted to the hospital more than once during the study period, we used a generalized estimating equations model to estimate the independent effect of the intervention on patients’ odds of death or unexpected death, while accounting for patient-level clustering using an autoregressive working correlation structure for within-patient multiple admissions. All models controlled for age, gender, race, season of admission, case mix, Charlson Comorbidity Index, and ICU-to-total bed ratio. We estimated the effect of the intervention under two different scenarios: first, we treated the effect of the intervention as constant throughout the intervention period. Second, because a pre/post analysis may be confounded by time trends unrelated to the intervention under study (7, 24), we evaluated whether time trends might account for the observed effects. There were two stages in this analysis. First, we obtained the probability of death using the generalized estimating equations model described earlier, with an additional variable for time elapsed since the start of the baseline period. This allowed simultaneous adjustment both for time trends and for the multiple covariates described earlier and allowed us to estimate the adjusted probability of death during any admission. We then used a general linear model with the fitted probabilities of death derived from this generalized estimating equations model as the dependent variable. In this model, we also included a three-level time-period indicator (baseline, implementation, and intervention periods) for the cohort, and the interaction between this indicator and number of days since January 1, 2004. Using the estimated coefficient from the main effect of the indicator variable and the interaction term, we obtained the estimated mean difference between the preintervention probability and the intervention probability at the end of the follow-up period. This model could also be used to obtain the estimated mean difference in probabilities at any time point (25).
RESULTS
Patient and Team-Activation Characteristics
We included 171,341 consecutive adult admissions. There were 66,496 admissions in the baseline period, 14,800 in the 6-month implementation period, and 90,045 in the intervention period. Patients in the intervention period were somewhat older (56.0 yrs vs. 55.2 yrs, p < .0001) and more likely to have comorbid conditions (Charlson Comorbidity Index 1.12 vs. 1.00, p < .0001) than patients in the baseline period. Length of stay did not differ significantly between the baseline and intervention periods (4.9 days vs. 4.7 days, p = .1). Further details are presented in Table 1, which for clarity shows only the baseline and intervention periods.
Table 1.
Characteristics of patients
| Baseline Period n = 66,496 | Intervention Period n = 90,045 | |
|---|---|---|
| Demographics | ||
| Age (years) | 55.2 ± 19.8 | 56.0 ± 19.8 |
| Gender (% female) | 59.0% | 58.0% |
| Race/ethnicity | ||
| White | 71% | 71% |
| Black | 10% | 11% |
| Asian | 3% | 4% |
| Hispanic | 4% | 5% |
| Other | 12% | 9% |
| Case mix index | 1.55 ± 1.71 | 1.52 ± 1.58 |
| Comorbidities | ||
| Charlson Comorbidity Index | 1.00 ± 1.12 | 1.12 ± 1.24 |
| Acquired immune deficiency syndrome | 1% | 1% |
| Alcohol abuse | 3% | 3% |
| Chronic blood loss anemia | 2% | 2% |
| Chronic pulmonary disease | 12% | 12% |
| Coagulopthy | 3% | 4% |
| Congestive heart failure | 11% | 12% |
| Deficiency anemias | 11% | 13% |
| Depression | 5% | 6% |
| Diabetes with chronic complications | 5% | 6% |
| Diabetes without chronic complications | 14% | 15% |
| Drug abuse | 2% | 2% |
| Fluid and electrolyte disorders | 12% | 13% |
| Hypertension | 38% | 41% |
| Hypothyroidism | 8% | 9% |
| Liver disease | 4% | 4% |
| Lymphoma | 1% | 2% |
| Metastatic cancer | 4% | 4% |
| Obesity | 2% | 2% |
| Other neurological disorders | 4% | 5% |
| Paralysis | 1% | 1% |
| Peptic ulcer disease | 0% | 0% |
| Peripheral vascular disease | 4% | 5% |
| Psychoses | 2% | 3% |
| Pulmonary circulation disease | 2% | 2% |
| Renal failure | 6% | 12% |
| Rheumatoid arthritis/collagen vascular disease | 2% | 2% |
| Solid tumor without metastasis | 2% | 2% |
| Valvular disease | 7% | 7% |
| Weight loss | 1% | 1% |
| Outcomes | ||
| Unexpected mortality outside the intensive care unit (%) | 0.09% | 0.02% |
| Mortality (%) | 2.08% | 1.95% |
| Length of stay (days) | 4.9 ± 6.9 | 4.7 ± 6.2 |
| Intensive care unit length of stay (days) | 0.6 ± 3.3 | 0.6 ± 2.7 |
The response system was activated 5809 times, an average of 157 times per month or 53 (95% CI 51–54) activations per 1000 discharges. Criteria causing team activation included: systolic blood pressure <90 mm Hg: 32%; heart rate <40 or >130: 24%; oxygen saturation <90% in spite of oxygen: 17%; respiratory rate <8 or > 30: 11%; acute change in conscious state: 7%; oliguria: 5%; and marked nursing concern: 38%. Because response calls could be triggered for more than one criterion, the total is >100%. Calls for isolated nursing concern (in the absence of other vital sign criteria) occurred in only 18% of calls. Slightly more than one fifth (20.7% (95% CI 19.8%–21.7%) of team activations resulted in transfer to an ICU within 24 hrs. Team activations were more common on the medical (7.8 % of patients on the service had team activations), neurologic (3.9%), orthopedic (4.8%), and surgical services (4.1%) than in patients on the obstetrical/gynecologic service (0.6%). Of patients with team activations, 8.8% (95% CI 8.1%–9.5%) died before discharge. Patients who had team activations were 5.8 (95% CI 2.9–11.9, p < .0001) times more likely to have an unexpected death, and 5.7 (95% CI 5.2–6.4, p < .0001) times more likely to die, than patients who did not have team activations.
Outcomes Among Hospitalized Patients
The intervention period was associated with a lower risk of unexpected mortality in unadjusted, adjusted, and adjusted interrupted time-series analyses. The unadjusted rate of unexpected mortality was 0.02% (95% CI 0.01%–0.03%) in the intervention period vs. 0.09% (95% CI 0.06%–0.11%) in the baseline period (p < .0001). The rate of unexpected mortality over time is shown in Figure 2a. After adjustment for age, gender, race, season of admission, case mix, Charlson Comorbidity Index, and ICU bed capacity, the intervention period was associated with an 80% reduction in the odds of unexpected mortality (odds ratio [OR] 0.20 [95% CI 0.11–0.37], p < .0001) (Table 2). Because a pre-/postanalysis may be confounded by time trends unrelated to the intervention under study (7, 24), we performed additional interrupted time-series analyses to evaluate whether time trends might account for the observed effects. In addition to the multiple covariates noted earlier, this model also controlled for time trends unrelated to the intervention. This analysis verified that the intervention period was associated with an increased reduction in unexpected mortality rates beyond what would have been expected from time trends alone. The model estimated that, at the end of the intervention period, the rate of unexpected mortality was 65% lower than would have been expected from time trends and other covariates (absolute risk reduction: three unexpected deaths per 10,000 admissions, p < .0001). This difference remained similar throughout the intervention period. These findings are represented graphically in Figure 3a.
Figure 2.
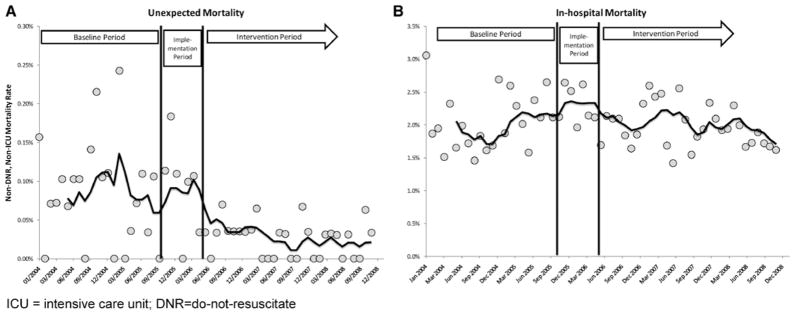
Unadjusted mortality rates. A, The risk of unexpected mortality over time. B, overall in hospital mortality rates over time. Curves are a 6-month moving average.
Table 2.
Adjusted odds ratio for death before and after the intervention for unexpected (non-do-not-attempt resuscitation, non-intensive care unit) mortality
| Adjusted Odds Ratio | p | |
|---|---|---|
| Intervention period | 0.2 (0.11–0.37) | <.0001 |
| Charlson Comorbidity Index (per point increase) | 1.56 (1.37–1.77) | <.0001 |
| Case mix (per point increase) | 1.12 (1.07–1.17) | <.0001 |
| Age (per year increase) | 1.04 (1.02–1.05) | <.0001 |
| Gender (female vs. male) | 0.69 (0.46–1.04) | .08 |
| Race | ||
| Asian | 0.58 (0.08–4.19) | .6 |
| Black | 0.84 (0.39–1.82) | .7 |
| Hispanic | 0.81 (0.2–3.3) | .8 |
| Other | 1.4 (0.78–2.5) | .3 |
| White | 1.0 | — |
| Season | ||
| Spring | 1.3 (0.71–2.4) | .4 |
| Summer | 1.01 (0.56–1.81) | .99 |
| Fall | 0.9 (0.47–1.71) | .7 |
| Winter | 1.0 | — |
| Intensive care unit bed ratio | ||
| Lowest | 0.65 (0.21–2.03) | .5 |
| Second lowest | 0.73 (0.32–1.69) | .5 |
| Third lowest | 0.95 (0.42–2.15) | .9 |
| Highest | 1.0 | — |
Table represents the odds of unexpected mortality (deaths outside of an intensive care unit in patients without do-not-attempt resuscitation orders), adjusted for a number of potential confounders.
Figure 3.
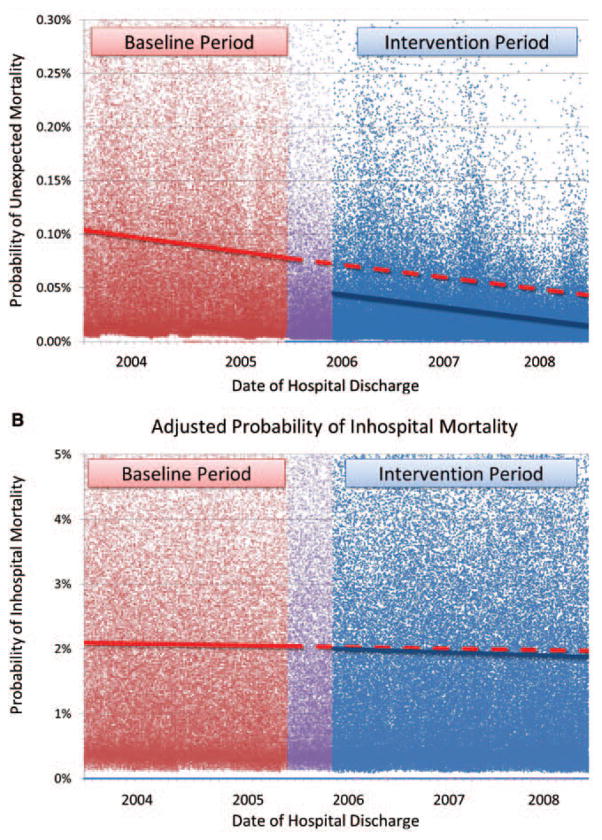
Adjusted effect of intervention, accounting for possible time trends and other confounders. These figures show a graphical representation of a model that adjusts for time trends unrelated to the intervention, as well as multiple other confounders. The dots represent adjusted probabilities of death for each admission in the baseline (red), implementation (purple), and intervention (blue) periods, obtained from a generalized estimating equations model that adjusts for age, gender, race, season, intensive care unit (ICU)-bed capacity, case mix, and Charlson Comorbidity Index (Table 2). The lines represent a daily point estimate of the adjusted probability of mortality, obtained from general linear model that further adjusts for time trends (see Methods section for details). The dashed red line represents what would have been expected in the intervention period based on secular time trends alone. The difference between the dashed red line and the solid blue line represents improvement attributable to the intervention, independent of individual-level factors and time trends. a, Unexpected mortality. This shows that the probability of unexpected mortality was significantly lower in the intervention period compared with the baseline period. At the end of the intervention period, there was a 65% reduction in the probability of unexpected mortality (p < .0001). b, Inhospital mortality. This shows that there was no significant difference in the probability of in hospital mortality after adjustment for individual factors and time trends (p = .2).
The risk of overall in-hospital mortality did not differ significantly between the intervention period and the baseline period. In the intervention period, the mortality rate was 1.95% (95% CI 1.86–2.04), compared with 2.08% (95% CI 1.97–2.19) in the baseline period (p = .07). The risk of death over time is shown in Figure 2b. After adjustment for age, gender, race, season of admission, case mix, Charlson Comorbidity Index, and ICU bed capacity, the intervention period was associated with an OR for overall in hospital mortality of 0.91 (95% CI 0.82–1.02, p = .09) compared with the baseline period (Table 3). Interrupted time-series analyses, adjusted for the factors described earlier as well as time trends, found no significant difference in overall mortality between periods. The point estimate for relative risk reduction was 4.8% at the end of the intervention period; the estimated change in absolute risk was a reduction of nine deaths per 10,000 admissions (95% CI −25 to +5, p = .2).
Table 3.
Adjusted odds ratio for death before and after the intervention for in hospital mortality
| Adjusted Odds Ratio | p | |
|---|---|---|
| Intervention period | 0.91 (0.82–1.02) | .09 |
| Charlson Comorbidity Index (per point increase) | 1.48 (1.44–1.52) | <.0001 |
| Case mix (per point increase) | 1.17 (1.15–1.18) | <.0001 |
| Age (per year increase) | 1.03 (1.03–1.04) | <.0001 |
| Gender (female vs. male) | 0.88 (0.82–0.94) | .0003 |
| Race | ||
| Asian | 1.37 (1.11–1.69) | .003 |
| Black | 0.72 (0.63–0.82) | <.0001 |
| Hispanic | 0.59 (0.45–0.76) | <.0001 |
| Other | 1.38 (1.24–1.54) | <.0001 |
| White | 1.0 | — |
| Season | ||
| Spring | 0.84 (0.76–0.93) | .001 |
| Summer | 0.99 (0.9–1.1) | .9 |
| Fall | 0.91 (0.82–1.01) | .07 |
| Winter | 1.0 | — |
| Intensive care unit bed ratio | ||
| Lowest | 1.11 (0.91–1.35) | .3 |
| Second lowest | 1.05 (0.91–1.21) | .5 |
| Third lowest | 1.02 (0.91–1.14) | .8 |
| Highest | 1.0 | — |
Table represents odds of overall in hospital mortality, adjusted for the same potential confounders
DISCUSSION
We found that a rapid response system that relied on providers already assigned to a patient’s care, rather than on a separate ICU-based rapid response team, was associated with marked reductions in the rate of unexpected mortality, but not with overall in-hospital mortality. This personnel-neutral approach to a rapid response system focused on criteria-based detection of clinical decompensations and reliable activation of responders (the “afferent” limb of the rapid response system (26)), rather than on delivering ICU-based providers to the patient’s bedside.
Our work has two key findings. First, simple team-activation criteria such as those used in this study identify a high-risk group of patients: patients with team activations had a more-than-five-fold higher risk of death compared with those without. Second, our lower-staffing intensity approach produces outcomes comparable with ICU-based approaches. Our results are concordant with those of a large meta-analysis of traditional rapid response teams, which found an overall 34% reduction in adult out-of-ICU cardiac arrests (OR 0.66, 95% CI 0.54–0.80) but no definite effect on overall adult in-hospital mortality (OR 0.96, 95% 0.84–1.09) (16). Our team structure differs significantly from those in most previously reported studies. In each study included in Chan’s meta-analysis, hospitals created and staffed an ICU-based rapid response team. We relied on providers already assigned to the patient to provide the clinical response. To our knowledge, only one published case study describes a system with a similar approach. Moldenhauer et al (27) reported early results after implementing a “clinical triggers” program, which focused on the afferent rather than efferent rapid response system limb. Our study differs from theirs by including all consecutive admitted adults, covering a three-fold longer time span, enrolling more than six times as many patients, and by using multivariable methods to adjust for confounders.
These findings are relevant for clinicians and policymakers. First, we have shown that without a dedicated ICU-based team, organizing the detection of and response to acute clinical decompensations reduces unexpected mortality. This approach requires no additional clinical staffing, preserves provider continuity (which may limit adverse events (28)), and respects traditional tenets of medical education. Our results are also relevant to ongoing discussions about intensivist manpower shortages (29, 30). An important burden on ICU providers is responding to out-of-ICU duties. It may be that intensivists’ time is better spent with the critically ill, in the ICU (31), rather than serving as part of rapid response teams.
Why might our approach result in outcomes comparable with those found with ICU-based teams (16)? One possibility has to do with our team activation rate of 53 activations per 1000 discharges, which is higher than the rates in most prior reports. For example, in three major rapid response system studies, calling rates were 8.7 (15), 15.1 (32), and 39.6 (12) per 1000 discharges. Our higher activation rate may have to do with the structure of our team, which is likely to reduce social barriers to team activation (33), compared with calling a separate, ICU-based team. Another possibility has to do with the types of interventions usually performed by rapid response teams. In most studies, ≥90% of reported rapid response team interventions relate to basic medical care such as fluids, diuretics, and diagnostic tests. For example, in the study by Chan et al (32), only 7% of patients required intubation or central venous access. Bellomo et al (12) found that <10% of team interventions involved advanced airway management or other similar ICU interventions. Moldenhauer et al (27) reported that only 12% of patients required ICU transfer; other interventions involved routine medical interventions. Rothschild et al (34) reported that the most common interventions were fluids, followed by cardiology and other imaging studies.
From an analytic standpoint, our study has several important strengths. It is a very large cohort, with > 170,000 discharges. We enrolled all consecutive patients over a nearly 5-yr period, and we systematically used available information to control for confounding effects at the patient, organizational, and seasonal level. In addition, we have also assessed whether the effect was accounted for by time trends unrelated to the intervention. Accounting for time trends is a key technique to improve the robustness of results from nonrandomized trial designs (24, 35). Even after controlling for time trends, our results indicate an unexpected mortality reduction that remains clinically and statistically significant.
Despite these strengths, any nonrandomized study has important limitations. There are likely to be unmeasured confounders that influence the risk of death. For example, other patient-safety programs undoubtedly contributed to the effect, as confirmed by time-related mortality reductions independent of the intervention. Notably, our intervention was the major hospital-wide patient-safety program implemented during the time period under study. We also attempted to control for time trends and other confounders through multivariable methods, and our results suggest an independent effect of the intervention. Observational studies are also at risk for selection bias. However, we included every adult admitted during the study period; selection bias is therefore unlikely to explain our results. We used administrative data to determine comorbidities, a less precise method than using clinical data. However, we used previously validated methods, and our measure of comorbidity is strongly associated with patient outcomes (p < .0001 for mortality), giving it face validity in our patient population. We did not collect data on the appropriateness of team activations, which would be an interesting topic for future study. It should also be emphasized that our work took place in an academic medical center, which has different personnel structures than other types of institutions, and that it occurred in the context of a comparatively generous ICU:floor-bed ratio. The strategy we used may therefore not generalize well to non-academic settings or those with fewer ICU beds. It is also possible that there may be particular patient subgroups to whom our intervention would generalize less well. Finally, any hospital-wide human-system intervention will have variable uptake and penetrance into practice. There will be failures in activating the team when criteria are met, and team activations when criteria are not fulfilled. This is a fundamental aspect of quality improvement practice and of effectiveness (rather than efficacy) trials. However, this issue would bias the study toward the null rather than toward finding an effect, as we report.
The mechanism by which our intervention reduced unexpected mortality is uncertain. It may relate to reducing barriers to calling for help, involving the attending physician or senior nurse, detecting hospital systems in need of improvement, or other reasons. Most likely, multiple factors are involved. Regardless of the mechanism, our data argue convincingly that a system of care that does not rely on a critical-care-trained rapid response team can result in outcomes similar to those previously reported. Our data do not, though, fully exclude incremental benefit that might be found if we had used an ICU-based team. Finally, the reasons why unexpected mortality, but not overall mortality, was reduced remains unclear, although it is similar to others’ findings (16). It is possible that rapid response systems simply result in changes in DNR status. For example, Chen found that DNR orders increased in hospitals randomized to the medical-emergency team arm of the Medical Emergency Response Improvement Team study (36), and other studies have found that response teams are frequently involved in DNR discussions (37, 38). We did not collect DNR status among patients who survived, so our data are unable to fully address this question. However, it is also possible that the effect on overall mortality is small enough that an even larger study would be required to detect it. Our CI for overall in-hospital mortality only narrowly crosses the null (OR 0.91, 95% CI 0.82–1.02). It is conceivable that new meta-analyses including our results would find a significant result.
CONCLUSIONS
We found that a rapid response system that relied on the patient’s usual care providers, rather than on an ICU-based rapid response team, was independently associated with a lower risk of unexpected mortality. This system required no additional staffing, and these results provide support for an alternative, less-resource intense approach to rapid response systems. In the context of nationwide requirements for rapid response system implementation (5, 17), these results may provide a less costly approach for hospitals to protect the particularly vulnerable population of decompensating inpatients.
Acknowledgments
Supported, in part, by the Physician Faculty Scholars Program of the Robert Wood Johnson Foundation (grant No. 66350, to MDH). Dr. Marcantonio is supported by a Midcareer Investigator Award in Patient-Oriented Research from the National Institute on Aging [K24 AG035075]. The work was additionally supported by the Silverman Institute for Healthcare Quality and Safety and the Stoneman Center for Quality Improvement at the Beth Israel Deaconess Medical Center. The authors, not the supporting groups listed earlier, had complete responsibility for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Footnotes
Drs. Moorman and Aronson participated equally as senior authors in the conception, analytic review, and preparation of the manuscript. Dr. Howell participated in all phases of the study, had full access to all of the data, and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Ngo designed and conducted the analysis and meaningfully contributed to the manuscript. Drs. Folcarelli, Yang, Mottley, Marcantonio, and Sands conceived of the intervention, reviewed the analysis, and meaningfully contributed to the manuscript.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Comarow A. Saving lives. US News and World Report. 2005;139:74, 76, 79. [Google Scholar]
- 2.Kowalczyk L. Hospitals try to break a deadly ‘code:’ Rapid response teams helping to save lives. Boston Sunday Globe. 2005 Nov 27;268(50):A1, A20. Section 1. [Google Scholar]
- 3.Institute for Healthcare Improvement: 100,000 Lives Campaign. 2005 Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm. Accessed.
- 4.Institute for Healthcare Improvement. [Accessed January 19, 2007];Getting Started Kit: Rapid Response Teams. 2007 Available at http://www.ihi.org/NR/rdonlyres/6541BE00-00BC-4AD8-A049-CD76EDE5F171/0/RRTHowtoGuide.doc.
- 5. [Accessed September 21, 2007];Joint Commission on Accreditation of Healthcare Organizations (JCAHO): 2008 National Patient Safety Goals: Hospital. 2007 Available at http://www.jointcommission.org/NR/rdonlyres/82B717D8-B16A-4442-AD00-CE3188C2F00A/0/08_HAP_NPSGs_Master.pdf.
- 6.DeVita MA, Braithwaite RS, Mahidhara R, et al. Medical Emergency Response Improvement Team (MERIT): Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13:251–254. doi: 10.1136/qshc.2003.006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winters BD, Pham J, Pronovost PJ. Rapid response teams–walk, don’t run. JAMA. 2006;296:1645–1647. doi: 10.1001/jama.296.13.1645. [DOI] [PubMed] [Google Scholar]
- 8.Sharek PJ, Parast LM, Leong K, et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a Children’s Hospital. JAMA. 2007;298:2267–2274. doi: 10.1001/jama.298.19.2267. [DOI] [PubMed] [Google Scholar]
- 9.Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34:2463–2478. doi: 10.1097/01.CCM.0000235743.38172.6E. [DOI] [PubMed] [Google Scholar]
- 10.Buist MD, Moore GE, Bernard SA, et al. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: Preliminary study. BMJ. 2002;324:387–390. doi: 10.1136/bmj.324.7334.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179:283–287. doi: 10.5694/j.1326-5377.2003.tb05548.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32:916–921. doi: 10.1097/01.ccm.0000119428.02968.9e. [DOI] [PubMed] [Google Scholar]
- 13.Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: The effect of a medical emergency team. Med J Aust. 2000;173:236–240. doi: 10.5694/j.1326-5377.2000.tb125627.x. [DOI] [PubMed] [Google Scholar]
- 14.Kenward G, Castle N, Hodgetts T, et al. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263. doi: 10.1016/j.resuscitation.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Hillman K, Chen J, Cretikos M, et al. MERIT study investigators: Introduction of the medical emergency team (MET) system: A cluster-randomized controlled trial. Lancet. 2005;365:2091–2097. doi: 10.1016/S0140-6736(05)66733-5. [DOI] [PubMed] [Google Scholar]
- 16.Chan PS, Jain R, Nallmothu BK, et al. Rapid Response Teams: A Systematic Review and Meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 17.An act to promote cost containment, transparency and efficiency in the delivery of quality health care, Enacted in the Acts of 2008. [Accessed May 25, 2012];The General Laws of Massachusetts, Section 53F. 2008 Available at: http://www.malegislature.gov/Laws/GeneralLaws/PartI/TitleXVI/Chapter111/Section53F.
- 18.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365:139–146. doi: 10.1056/NEJMra0910926. [DOI] [PubMed] [Google Scholar]
- 19.Edelson DP. A weak link in the rapid response system. Arch Intern Med. 2010;170:12–13. doi: 10.1001/archinternmed.2009.466. [DOI] [PubMed] [Google Scholar]
- 20.Comorbidity Software, version 3.2. Rockville, MD: 2007. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp [computer program]. Version 3.2. Accessed. [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed February 15, 2007];Institute for Healthcare Improvement: Move Your Dot: Measuring, Evaluating, and Reducing Hospital Mortality Rates (Part1) 2003 Available at http://www.ihi.org/NR/rdonlyres/0776FC52-A92F-4441-95FA-61E102E87F2A/0/MoveYourDotWhitePaper2003.pdf.
- 24.Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357:608–613. doi: 10.1056/NEJMsb070738. [DOI] [PubMed] [Google Scholar]
- 25.Mattison ML, Afonso KA, Ngo LH, et al. Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170:1331–1336. doi: 10.1001/archinternmed.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”–a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81:375–382. doi: 10.1016/j.resuscitation.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Moldenhauer K, Sabel A, Chu ES, et al. Clinical triggers: An alternative to a rapid response team. Jt Comm J Qual Patient Saf. 2009;35:164–174. doi: 10.1016/s1553-7250(09)35022-9. [DOI] [PubMed] [Google Scholar]
- 28.Petersen LA, Brennan TA, O’Neil AC, et al. Does house staff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121:866–872. doi: 10.7326/0003-4819-121-11-199412010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Angus DC, Kelley MA, Schmitz RJ, et al. Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS): Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 30.Krell K. Critical care workforce. Crit Care Med. 2008;36:1350–1353. doi: 10.1097/CCM.0b013e318169ecee. [DOI] [PubMed] [Google Scholar]
- 31.Howell MD. Managing ICU throughput and understanding ICU census. Curr Opin Crit Care. 2011;17:626–633. doi: 10.1097/MCC.0b013e32834b3e6e. [DOI] [PubMed] [Google Scholar]
- 32.Chan PS, Khalid A, Longmore LS, et al. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513. doi: 10.1001/jama.2008.715. [DOI] [PubMed] [Google Scholar]
- 33.Hunziker S, Johansson AC, Tschan F, et al. Teamwork and leadership in cardiopulmonary resuscitation. J Am Coll Cardiol. 2011;57:2381–2388. doi: 10.1016/j.jacc.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Rothschild JM, Woolf S, Finn KM, et al. A controlled trial of a rapid response system in an academic medical center. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2008;34:417–425. 365. doi: 10.1016/s1553-7250(08)34052-5. [DOI] [PubMed] [Google Scholar]
- 35.Shojania KG, Grimshaw JM. Evidence-based quality improvement: The state of the science. Health Aff (Millwood) 2005;24:138–150. doi: 10.1377/hlthaff.24.1.138. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Flabouris A, Bellomo R, et al. MERIT Study Investigators for the Simpson Centre and the ANZICS Clinical Trials Group: The Medical Emergency Team System and not-for-resuscitation orders: Results from the MERIT study. Resuscitation. 2008;79:391–397. doi: 10.1016/j.resuscitation.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Jones DA, McIntyre T, Baldwin I, et al. The medical emergency team and end-of-life care: A pilot study. Crit Care Resusc. 2007;9:151–156. [PubMed] [Google Scholar]
- 38.Parr MJ, Hadfield JH, Flabouris A, et al. The Medical Emergency Team: 12 month analysis of reasons for activation, immediate outcome and not-for-resuscitation orders. Resuscitation. 2001;50:39–44. doi: 10.1016/s0300-9572(01)00323-9. [DOI] [PubMed] [Google Scholar]