Abstract
Background
Little is known about the cost to Medicare of breast cancer screening or whether regional-level screening expenditures are associated with cancer stage at diagnosis or treatment costs, particularly as newer breast cancer screening technologies like digital mammography and computer aided detection (CAD) have diffused into the care of older women.
Methods
Using the linked Surveillance, Epidemiology and End Results-Medicare database, we identified 137,274 women aged 66–100 who had not had breast cancer, and assessed the cost to fee-for-service Medicare of breast cancer screening and work-up during 2006–07. For women who developed cancer, we calculated initial treatment cost. We then assessed screening-related cost at the Hospital Referral Region level, and evaluated the association between regional expenditures and work-up test utilization, cancer incidence, and treatment costs.
Results
In the United States, the annual costs to fee-for-service Medicare for breast cancer screening-related (comprised of screening plus work-up) and treatment were $1.08 billion, and $1.36 billion, respectively. For women age 75 years and over, annual screening-related expenditures exceeded $410 million. Age-standardized screening-related cost per beneficiary varied more than two-fold across regions (from $42 to $107 per beneficiary); digital screening mammography and CAD accounted for 65% of the difference in screening-related cost between HRRs in the highest and lowest quartile of cost. Women residing in high-screening-cost HRRs were more likely to be diagnosed with early stage cancer (incidence rate ratio, 1.78; 95% CI, 1.40–2.26). There was no significant difference in the cost of initial cancer treatment per beneficiary between highest and lowest screening-cost HRRs ($151 v 115, p=.20).
Conclusions
The cost to Medicare of breast cancer screening exceeds $1 billion annually in the fee-for-service program. Regional variation is substantial and driven by the use of newer and more expensive technologies; it is unclear whether higher screening expenditures are achieving better breast cancer outcomes.
Background
As the spectrum of cancer care includes screening as well as treatment, a comprehensive understanding of breast cancer cost must incorporate cost of screening and assoicated work-up. While the body of evidence concerning Medicare expenditures for cancer treatment has grown, relatively little is known about cost associated with screening Medicare beneficiaries for breast cancer.1–3 This is especially important among older women, as recent guidelines have concluded that there is insufficient evidence to assess the benefits and harms of screening mammography in women 75 years or older. 4,5
It is particularly timely to consider the cost implications of breast cancer screening, as newer breast cancer screening technologies such as digital mammography and computer aided detection (CAD) have expanded the options available to clinicians and are diffusing into clinical practice.6,7 The adoption of these new technologies can increase costs directly, through reimbursement for the tests, and also lead to higher rates of supplementary imaging, biopsy, or cancer detection.8,9 It is critical to assess the relation between screening expenditures and population outcomes, since newer modalities can increase cancer detection rates, but may not improve patient outcomes, particularly among older women.10–14
Ideally, higher breast cancer screening expenditures at the population level should correspond to earlier stage at diagnosis, lower treatment cost, or both. This can be evaluated by comparing differences in screening cost and cancer outcomes across geographic regions, hypothesizing that women living in regions that “invest” more in screening services may be less likely to be diagnosed at a later stage. However, in actual practice, it is unclear whether higher screening costs are associated with earlier stage at diagnosis or lower cancer treatment costs at the population level.
To address these knowledge gaps, we estimated national breast cancer screening and treatment costs in the Medicare fee for service program. Our second objective was to assess regional variation in breast cancer screening cost, while our third objective was to determine the association between regional screening cost and breast cancer incidence and treatment costs. These data will inform clinicians and policy makers, in a context of debate about Medicare reimbursement for various cancer screening modalities and concerns about growth in cancer expenditures.
Methods
Study Overview
We conducted a retrospective cohort study of female Medicare beneficiaries who were free of breast cancer as of December 31, 2005 and followed them for two years to assess breast cancer screening, work-up of suspicious lesions, and incident breast cancer. For the subgroup of women who developed breast cancer during the study period, we estimated treatment cost during the initial 12 months after diagnosis.3 At the regional level, we then evaluated the association between screening cost and outcomes (overall and stage-specific breast cancer incidence and treatment cost). The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Data Source and Study Sample
We used the Surveillance, Epidemiology, and End Reults (SEER)-Medicare database, which links patient-level information on all incident cancer patients reported to SEER registries with Medicare claims and also maintains a file of 5% sample of Medicare beneficiaries without cancer who live in SEER registries.15 The population-based SEER registries account for approximately 28% of the U.S. population. 16 To create a cohort eligible for breast cancer screening in whom we could measure cancer incidence, we selected female beneficiaries from Medicare’s 5% sample (cancer and non-cancer) who lived in a SEER region during 2006–7 and had not had breast cancer as of December 31, 2005 documented through either SEER or Medicare ICD-9 billing codes To be included, a woman had to be 66–100 years old on January 1, 2006, have a valid zip code, and have continuous fee-for-service Medicare Part A and B coverage throughout the study period. Among women who developed breast cancer in 2006–2007, we excluded those women whose cancer was reported only on death certificate or autopsy or had missing diagnosis date.
We categorized race as white, black, other and median household income at zip code level according to quintiles. We used a modified list of Elixhauser comorbidity conditions to assess comorbidity (Appendix Table 1).17–19
Screening and work-up cost
We assessed the use and cost of breast cancer screening-related (comprised of screening plus work-up) procedures using ICD-9 procedure and diagnosis codes and Healthcare Common Procedure Coding System codes (HCPCS; Appendix Table 2).20–25 Screening includes screening mammography (digital and film) and CAD billed with screening mammography, while work-up includes diagnostic mammography (digital and film), CAD billed with diagnostic mammography, other breast imaging and biopsy. We used a validated algorithm with updated procedure codes to identify whether a mammography was screening or diagnostic.26 We calculated the cost of these procedures according to the actual reimbursement by Medicare.27–30 Costs were adjusted to 2009 USD accounting for temporal and geographic variation.30,31
Treatment cost
To assess cancer treatment costs, we used a matched control group approach to determine the difference in Medicare expenditures between women diagnosed with breast cancer and women without a cancer diagnosis. This enabled us to account for “background” medical expenditures, with the difference in costs between the cancer and non-cancer groups attributable to cancer-related costs. Women diagnosed with their first breast cancer in 2006 or 2007 were matched 1:1 with women from Medicare’s 5% sample who never had breast cancer based on SEER region, age quartile, comorbidity (0 vs. 1+ condition), and regional quartile Medicare expenditures in the 12 months before diagnosis. After assigning a matched pair, Medicare payments from the month of diagnosis through 12 months later were summed for both cases and controls.3,30 We subtracted Medicare payments for the control and for screening during the month of diagnosis from the total payments for the case and refer to the difference as treatment cost3,30. To test the stability of treatment cost in the 5% sample, we calculated treatment cost as described above for all women reported to SEER as having been diagnosed with their first breast cancer in 2006 or 2007; the difference in national treatment cost estimates was <2%.
Extrapolating to national cost
Using Medicare enrollment tables for the study period, we calculated the number of female fee-for-service Medicare beneficiaries age 65–74, 75–84 and 85 or older.32 For each age group, we multiplied the number of beneficiaries by our calculated screening cost per beneficiary and summed to estimate total Medicare breast cancer screening cost. Similarly, we applied age-specific SEER cancer incidence to the number of beneficiaries and multiplied by treatment cost per diagnosed beneficiary to estimate total Medicare expenditures for the first year of breast cancer treatment.33
Regional Variation
We used Hospital Referral Regions (HRRs) to examine geographic variation.34 We assigned women to HRR based on zip code of residence, and a priori restricted the sample to HRRs with 50 or more women who were diagnosed with breast cancer in the full SEER-Medicare data.35 The age-standardized cost estimates for each HRR were generated by applying age-specific costs in each HRR to the national age distribution (described above) of female fee-for-service Medicare beneficiaries.
Statistical Analysis
We assigned women to the quartile of her HRR screening-related cost and assessed use of screening and work-up procedures according to screening-related cost using Cochrane-Armitage test of trend, as well as chi-square tests. We then used Poisson regression to assess the relation between quartile of screening-related cost and breast cancer incidence rate, adjusting for age, race, comorbidity and income. Women contributed person-time from January 1, 2006 until the earliest of breast cancer diagnosis, death, or December 31, 2007. At the HRR level, we used linear regression to determine whether higher quartile of screening-related cost was associated with increased treatment cost adjusting for mean age, income and percent white at HRR level, as well as average annual Medicare expenditures for all care per capita within that HRR. We used SAS version 9.2(SAS Institute, Inc., Cary, NC) to conduct all analyses.
Results
There were 137,274 women in the study cohort; 41.8% were less than 75 years of age, 83.8% were white and half had one or more comorbid conditions (Table 1). During the study period, 43.5% of women had at least one screening mammogram with women 66–74 years being much more likely than women 85–100 years to receive a mammogram (57.2% v 15.2% respectively, p<.001). A higher percentage of women with highest quintile of median household income received a screening mammogram, while a lower percentage of those with increased number of comorbid conditions had screening mammogram (p<.001 for both). During that time, the average annual breast screening-related cost per beneficiary was $63. There was a strong inverse relation between age and cost, with breast cancer screening-related costs decreasing from $84 per beneficiary age 66–74 years to $60 (age 75–84 years) and $21 for women 85–100 years of age (p<.001, Table 2). During the same time period, the mean cost of initial treatment per diagnosed patient was approximately $16,600 for all ages and $21,300, $12,800, and $11,500 for women diagnosed at ages 66–74, 75–84, and 85–100, respectively (p<.001).
Table 1.
Demographic Characteristics of Study Sample
| Total Sample | N | % | % Patients Receiving Screening Mammography |
|---|---|---|---|
| 137,274 | |||
|
| |||
| Age | |||
| 66–74 | 57,417 | 41.8 | 57.2 |
| 75–84 | 55,176 | 40.2 | 42.0 |
| 85–100 | 24,681 | 18.0 | 15.2 |
|
| |||
| Race | |||
| White | 114,989 | 83.8 | 44.7 |
| Black | 9,952 | 7.3 | 38.8 |
| Other | 12,333 | 9.0 | 36.4 |
|
| |||
| Median Household Income | |||
| < $33,000 | 26,036 | 19.0 | 38.7 |
| $33,000–$39,999 | 22,325 | 16.3 | 43.2 |
| $40,000–$49,999 | 29,312 | 21.4 | 43.8 |
| $50,000–$62,999 | 27,348 | 19.9 | 44.9 |
| ≥$63,000 | 28,157 | 20.5 | 47.2 |
| unknown | 4,096 | 3.0 | 40.2 |
|
| |||
| Comorbid Conditions | |||
| 0 | 68,727 | 50.1 | 47.4 |
| 1 to 2 | 49,132 | 35.8 | 44.8 |
| 3+ | 19,415 | 14.1 | 26.6 |
Chi-sqare p-value for receipt of screening mammography and demographic characteristics <.001 for all comparisons in table.
Table 2.
Average Annual Cost to Medicare for Breast Screening and Treatment ($2009 USD) during 2006–7
| Age Group (number beneficiariesa) | |||||
|---|---|---|---|---|---|
| 66–74 8.04 M |
75–84 5.84 M |
85–100 2.87 M |
All Ages | ||
| Screening Costb | Cost Per Beneficiary | $56 | $40 | $13 | $ 724 M |
| Total Cost | $ 448 M | $ 236 M | $ 39.2 M | ||
| Work-Up Costc | Cost Per Beneficiary | $28 | $20 | $8 | $ 359 M |
| Total Cost | $ 223 M | $ 114 M | $ 22.2 M | ||
| Screening-Related = Screening + Work-Up Cost | Cost Per Beneficiary | $84 | $60 | $21 | $ 1.08 B |
| Total Cost | $ 671 M | $ 350 M | $ 60 M | ||
| Treatment Cost | Number Diagnosed with Breast Cancer | 40,607 | 29,388 | 10,617 | $ 1.36 B |
| Total Cost | $ 864 M | $ 377 M | $ 122 M | ||
Number of national fee for service Medicare beneficiaries
Includes cost for screening mammography (digital and film) and screening computer aided detection.
Includes cost for diagnostic mammography (digital and film), diagnostic computer aided detection, breast and related imaging and biopsy.
Extrapolating these costs to the US fee-for-service Medicare population, the annual costs to Medicare for breast cancer screening and workup of suspicious lesions were $723.1 million and $359.0 million, respectively, with a screening-related total of $1.08 billion. This compares with an annual cost to Medicare of $1.36 billion for treatment. In the subgroup of women who were older than 75 years, total screening-related and treatment costs were $410.6 million and $498.5 million, respectively.
Regional Variation
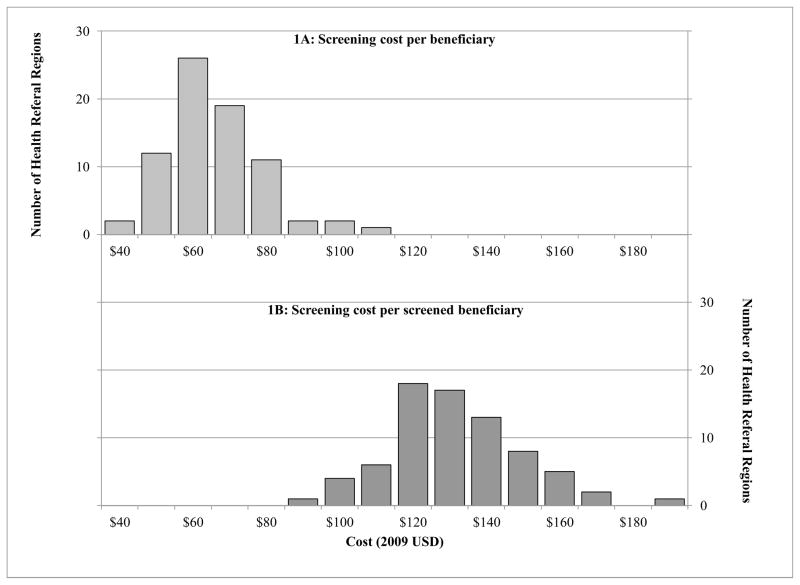
There was substantial regional variation in screening-related cost per beneficiary among the 75 HRRs in this analysis (Figure 1). At the HRR level, the mean screening-related cost per beneficiary ranged from approximately $40 to $110, with a median of $64 (interquartile range [IQR], $57, 72). Similarly, there was nearly a two-fold difference in cost per screened beneficiary between the highest and lowest HRRs, with a median HRR-level cost of $129 (IQR, $120–143).
Figure 1.
Regional variation in breast cancer screening costs
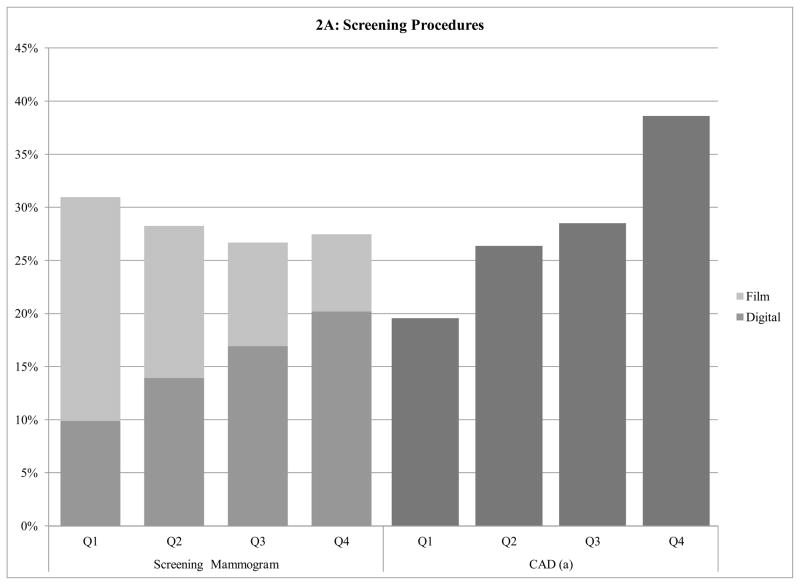
At the regional level, the use of specific screening tests was correlated with regional-level screening-related cost. The use of screening mammography increased from 40.9% of women in the lowest quartile of HRR screening-related expenditures, to 47.6% in the highest quartile (p<.001; Figure 2A). Although there was no significant relation between quartile of regional screening-related costs and the use of film screening mammography (p-value for trend: .92), women living in higher-cost regions were significantly more likely to undergo digital screening mammography. Approximately 10% of women who lived in the lowest quartile region received at least one digital screening mammogram, increasing to 13.9%, 16.9%, and 20.2% in the second, third, and fourth quartile of screening-related costs, respectively (p<.001). There was a two-fold difference in the screening CAD use between women in the highest and lowest quartiles of HRR screening-related cost, 38.6% vs. 19.6%, respectively (p<.001).
Figure 2.
Figure 2A: Screening mammography and Computer Aided Detection use according to Health Referral Region screening-related cost
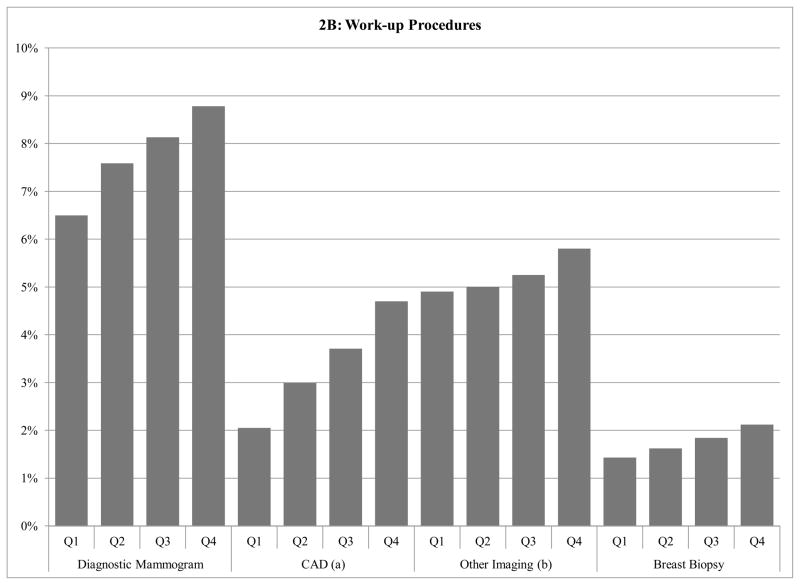
Figure 2B: Work-up breast imaging and biopsy use according to Health Referral Region screening-related cost
a CAD=Computer Aided Detection
b Other Imaging Includes: Breast Ultrasound, Breast MRI, positron emitting tomography imaging; computed tomography of head, brain, thorax, abdomen; MRI of brain, brainstem; radiologic examination, consultation, report; three-dimensional rendering; bone and joint imaging; radiopharmaceutical localization of tumor
c 1st quartile screening + work-up cost per beneficiary: <$57, 2nd quartile: $57–64, 3rd quartile: $65–70, 4th quartile: >$70
Patients in HRRs with the highest screening-related cost also tended to have higher utilization of work-up procedures such as diagnostic mammography, other breast imaging or biopsy when compared with lower cost regions (Figures 2B). For instance, while 2.1% of all women who lived in the highest quartile HRRs underwent biopsy, 1.4% of women in the lowest cost regions underwent biopsies during the study period (p<.001).
Digital screening mammography accounted for 47% of the difference in costs between women living in HRRs with the highest and lowest quartiles of screening related cost. Breast biopsy (21%) and CAD (18%) were also major contributors to regional cost difference, while utilization of film screening mammography (5%), diagnostic mammography (7%), or other imaging modalities (2%) did not contribute substantively.
Cancer Incidence and Treatment Cost
Women residing in areas with higher screening-related cost per beneficiary were significantly more likely to be diagnosed with breast cancer (adjusted Incidence Rate Ratio (IRR), 1.54 for highest vs. lowest quartile; 95% CI, 1.29–1.84; Table 3). The increase in breast cancer diagnoses was attributable to early stage cancers, as women residing in HRRs with higher screening-related cost tended to have a significantly higher incidence rate of in situ or stage I cancers. When we assessed in situ and stage I cancer incidence separately, those in the second, third and fourth quartiles had, significantly increased risk of being diagnosed with stage I breast cancer compared to those in the first quartile:40%, 49% and 65%, respectively. A similar trend was found for incidence of in situ disease, but only those in the fourth quartile of screening cost had significantly higher incidence of in situ disease than those in the first quartile (IRR, 2.12; 95% CI, 1.36–3.30). There was no significant association between quartile of screening-related cost and the incidence of stage IV breast cancer. The incidence of stage IV cancer was 0.19 and 0.18 per 1,000 in the highest and lowest quartiles, respectively. The results were similar when we defined HRRs by quartile of screening-related cost per screened beneficiary.
Table 3.
The association of regional screening-related cost and breast cancer incidence and treatment cost
| Overall Incidence | In Situ or Stage 1 Incidence | Stage IV Incidence | Treatment Coste (per beneficiary) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile | Annual Incidencec | IRRd (95% CI) | Annual Incidence | IRR (95% CI) | Annual Incidence | IRR (95% CI) | Cost | P-value | |
| Screening + Work-Up Costa (per beneficiary) | 1 | 3.3 | REF | 1.8 | REF | 0.2 | REF | $115 | REF |
| 2 | 4.7 | 1.34 (1.13–1.60) | 2.5 | 1.41 (1.11–1.80) | 0.2 | 0.87 (0.45–1.71) | $157 | .37 | |
| 3 | 5.0 | 1.40 (1.17–1.68) | 2.7 | 1.48 (1.16–1.90) | 0.3 | 1.11 (0.57–2.17) | $136 | .50 | |
| 4 | 4.6 | 1.54 (1.29–1.84) | 2.8 | 1.78 (1.40–2.26) | 0.2 | 0.84 (0.40–1.75) | $151 | .20 | |
| Screening + Work-Up Costb (per screened beneficiary) | 1 | 3.6 | REF | 1.8 | REF | 0.2 | REF | $148 | REF |
| 2 | 4.1 | 1.12 (0.94–1.34) | 2.3 | 1.35 (1.06–1.71) | 0.3 | 0.68 (0.34–1.36) | $138 | .96 | |
| 3 | 5.1 | 1.29 (1.09–1.52) | 2.8 | 1.44 (1.14–1.82) | 0.3 | (00.98.53–1.83) | $116 | .55 | |
| 4 | 4.8 | 1.35 (1.14–1.60) | 2.9 | 1.66 (1.32–2.09) | 0.2 | 0.70 (0.35–1.43) | $161 | .73 | |
1st quartile screening + work-up cost per beneficiary: <$57, 2nd quartile: $57–64, 3rd quartile: $65–70, 4th quartile: >$70
1st quartile screening + work-up cost per screened beneficiary: <$121, 2nd quartile $121–131, 3rd quartile: $131–143, 4th quartile: > $143
Annual Incidence per 1,000 person-years
Incidence Rate Ratio (IRR) adjusted for age, race, comorbidity, and income
Treatment cost p-value adjusted for mean age, income, percent white, and average annual total Medicare expenditures per capita of health referral region.
There was no significant relation between screening and treatment costs at the regional level. Even after adjustment for mean age, income, percent white within HRR and average annual total Medicare expenditures per capita, the difference in cost between highest and lowest quartile of screening-related cost per beneficiary was not significant ($115 v $151, p=.20). Findings were similar when HRRs were classified according to screening-related cost per screened beneficiary.
Conclusions
We found that the Medicare fee-for-service program is spending over $1 billion per year on breast cancer screening and work-up of suspicious lesions. This accounted for over 45% of the $2.42 billion total spent by Medicare on screening and the initial treatment phase of breast cancer, suggesting that analyses which focus exclusively on treatment have overlooked a significant contributor to cancer costs. Moreover, over $410.6 million annually is being spent on screening and work-up of women age 75 years and older, although there is insufficient evidence to assess the benefits and harms of screening mammography in this age group.5 This reinforces the need to develop evidence to guide both clinical decision making and coverage decisions.
There was substantial variation in screening-related cost across regions, which was largely attributable to digital screening mammography and CAD, rather than to differential use of screening mammography. Although breast biopsy was rarely performed, the 50% relative difference in biopsy rates across regions was notable and may represent work-up effect of CAD or digital mammography in high cost regions. Data suggests these modalities can increase recall rate, including biopsy, but results have been mixed.36–38
Women residing in high screening-cost regions were as much as 78% more likely to be diagnosed with early stage or in situ breast cancer as women in lower cost regions. The difference in crude incidence of overall and early stage cancers between high and low cost areas was approximately 1 per 1,000 women, which was statistically significant. Notably, the difference in the incidence of diagnosed stage IV cancer was not significant, and the absolute difference between highest and lowest cost areas was approximately 1 in 100,000 women. Taken together, these findings suggest overdiagnosis of breast cancer in the higher cost regions. This is consistent with recent findings from the Norwegian Screening Trial, which found that women residing in areas that implemented a mammography screening program were significantly more likely to be diagnosed with breast cancer, and that 15–25% of cases were overdiagnosed.39
Although our study did not set out to assess the effectiveness of CAD or digital mammography, our findings suggest limited effectiveness of these modalities in older average-risk women. For example, the Digital Mammographic Imaging Screening Trial (DMIST) found that digital mammography does not perform better than film mammography in women 65 years or older.40 As the field of radiology moves towards digital technology, it is important to note that digital will frequently be the only option available. Our results suggest that the cost and effectiveness of such evolutions of technology should be promptly and rigorously evaluated; higher costs associated with adoption of newer modaliteis may not necessarily yield superior outcomes.
We found that higher screening-related cost did not translate into lower treatment cost at the population level. There was a non-significant trend towards the higher screening-related cost areas having higher treatment costs, which may be associated with the higher rate of diagnosing early stage cancer in the higher screening expenditure areas. Because cancer diagnosis was a rare event, there were not enough incident cancer patients to affect treatment costs at the population level. Additionally, the costs associated with higher detection rates for early stage cancer could have been partially balanced out by the slightly (and non-significantly) lower rates of metastatic cancer detected in the higher cost regions.
There are several important considerations. We only have two years of follow-up to measure cancer incidence, which may not be long is not long enough assess advanced stage incident cancer or determine causality between increased spending and breast cancer incidence. Future work should explore this relation over a longer follow-up period, including longer assessment of cost and outcomes. It is also important to note that this study relied on administrative claims and may not capture all procedures performed, but our results are similar to a prior large registry study.41 The SEER-Medicare database relies on physician report and an algorithm to identify hispanic ethnicity of subjects which has been questioned, so we chose to categorize race as white, black, other without respect to ethnicity.42 Our estimates of cancer incidence (4.40 per 1,000 person-years) were slightly lower than those in the overall SEER program (4.82 per 1,000 person-years), but this is primarily due to SEER’s inclusion of first and subsequent breast cancers, while our study sample was restricted to women with first breast cancer only.43
In summary, the costs of breast cancer care in the Medicare population, when incorporating screening costs, are substantially higher than previously documented and the adoption of newer screening modalities will likely contribute to further growth. The growth trajectory may be steeper than projected due to Medicare’s reimbursement strategy which supports rapid adoption of newer modalities, frequently without adequate data to support their use.1
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Cancer Institute (5R01CA149045) and the P30 Cancer Center Support Grant (CCSG) at the Yale Comprehensive Cancer Center.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding/support and role of the sponsor: Support for this project was provided by the Yale Cancer Center. The sponsor played no role in the design of the study, analysis or interpretation of findings, or drafting the manuscript and did not review or approve the manuscript prior to submission. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Drs. Ross and Gross receive support from Medtronic, Inc. to develop and implement methods of clinical trial data sharing and patient-level meta-analyses. Dr. Ross is supported by the National Institute on Aging (K08 AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program, by the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, and by the Pew Charitable Trusts to examine regulatory issues at the U.S. Food and Drug Administration.
Footnotes
Data access and responsibility: All authors had full access to all the data in the study and Dr. Grosstakes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest: Drs. Ross and Gross are members of a scientific advisory board for FAIR Health, Inc. Dr. Abu-Khalaf has received research funding and has served as a consultant for Abraxis Oncology (currently Celgene), Merck, Novartis, Glaxo-Smith-Kline, and Genentech/Roche.
Author contributions: Drs. Gross, Ross, Chagpar, Abu-Khalaf, Killelea, Ma, and Ms. Long were responsible for the conception and design of this work. Dr. Gross and Ms. Long drafted the manuscript. Ms. Long conducted the statistical analysis. Dr. Gross was responsible for acquisition of data and obtained funding. Drs. Gross and Ma provided supervision. All authors participated in the analysis and interpretation of the data and critically revised the manuscript for important intellectual content.
References
- 1.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009 Feb 5;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB. Costs of cancer care: a view from the centers for Medicare and Medicaid services. J Clin Oncol. 2007 Jan 10;25(2):187–190. doi: 10.1200/JCO.2006.08.6116. [DOI] [PubMed] [Google Scholar]
- 3.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008 Jun 18;100(12):888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koroukian SM, Bakaki PM, Schluchter MD, Owusu C. Treatment and Survival Patterns in Relation to Multimorbidity in Patients with Locoregional Breast and Colorectal Cancer. J Geriatr Oncol. Jul;2(3):200–208. doi: 10.1016/j.jgo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for Breast Cancer: An Update for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2009;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton JJ, Foote SB, Green P, Baldwin LM. Diffusion of computer-aided mammography after mandated Medicare coverage. Arch Intern Med. Jun 14;170(11):987–989. doi: 10.1001/archinternmed.2010.104. [DOI] [PubMed] [Google Scholar]
- 7.Rao VM, Levin DC, Parker L, Cavanaugh B, Frangos AJ, Sunshine JH. How widely is computer-aided detection used in screening and diagnostic mammography? J Am Coll Radiol. Oct;7(10):802–805. doi: 10.1016/j.jacr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Noble M, Bruening W, Uhl S, Schoelles K. Computer-aided detection mammography for breast cancer screening: systematic review and meta-analysis. Archives of gynecology and obstetrics. 2009 Jun;279(6):881–890. doi: 10.1007/s00404-008-0841-y. [DOI] [PubMed] [Google Scholar]
- 9.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. Jama. Apr 4;307(13):1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. Jama. 2004 Sep 15;292(11):1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 11.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005 May 21–27;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005 Nov 20;23(33):8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 13.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004 Jul 29;351(5):427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 14.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. Jama. 2008 May 14;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potosky A, Riley G, Lubitz J, Mentnech R, Kessler L. Potential for cancer related health services research using a linked Medicare-tumor related database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002 Aug;40(8 Suppl):IV-49–54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: the advantages of the linked medicare and SEER data. Surveillance, Epidemiology and End Results. J Clin Epidemiol. 1999 May;52(5):463–470. doi: 10.1016/s0895-4356(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 22.ICD-9-CM: International Classification of Diseases, 9th Revision, 3rd Edition, Clinical Modification. 1, 2 & 3. Los Angeles (CA): Practice Management Information Corporation; 1991. [Google Scholar]
- 23.Buck CJ. 2002 ICD-9-CM, Volumes 1, 2, and 3 and HCPCS Level II. Philadelphia (PA): W. B. Saunders Company; 2002. [Google Scholar]
- 24.Kirschner CG, Edwards NK, May DM, et al. Physicians’ Current Procedural Terminology. 4. Chicago (IL): American Medical Association; 1991. [Google Scholar]
- 25.Anderson CA, Beebe M, Dalton JA, et al. Current Procedural Terminology CPT 2002. Chicago (IL): American Medical Association; 2002. [Google Scholar]
- 26.Smith-Bindman R, Quale C, Chu PW, Rosenberg R, Kerlikowske K. Can Medicare billing claims data be used to assess mammography utilization among women ages 65 and older? Med Care. 2006 May;44(5):463–470. doi: 10.1097/01.mlr.0000207436.07513.79. [DOI] [PubMed] [Google Scholar]
- 27.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002 Aug;40(8 Suppl):IV-104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 28.Burkhardt JH, Sunshine JH. Core-needle and surgical breast biopsy: comparison of three methods of assessing cost. Radiology. 1999 Jul;212(1):181–188. doi: 10.1148/radiology.212.1.r99jl46181. [DOI] [PubMed] [Google Scholar]
- 29.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care. 1995 Aug;33(8):828–841. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Warren JL, Brown ML, Fay MP, Schussler N, Potosky AL, Riley GF. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002 Jan 1;20(1):307–316. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 31.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008 May 7;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 32.Services OoI. Medicare Enrollment Table 2.2: Total, Fee-for-Service and Managed Care Enrollees, by Demographic Characteristics as of July, 2007. Bethesda, MD: Centers for medicare and medicaid services; 2008. [Google Scholar]
- 33.Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub (2000–2008) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011 (updated 10/28/2011), based on the November 2010 submission.
- 34.Dartmouth Medical School. Center for the Evaluative Clinical Sciences. The Dartmouth atlas of health care. Chicago, Ill: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 35.Trustees of Dartmouth College. Dartmouth Atlas of Health Care. [Accessed September 29, 2011.];Downloads, Crosswalks, Zip code crosswalks 1995–2010. 2011 http://www.dartmouthatlas.org/tools/downloads.aspx.
- 36.Lewin JM, D’Orsi CJ, Hendrick RE, et al. Clinical Comparison of Full-Field Digital Mammography and Screen-Film Mammography for Detection of Breast Cancer. American Journal of Roentgenology. 2002 Sep 1;179(3):671–677. doi: 10.2214/ajr.179.3.1790671. [DOI] [PubMed] [Google Scholar]
- 37.Skaane P, Hofvind S, Skjennald A. Randomized Trial of Screen-Film versus Full-Field Digital Mammography with Soft-Copy Reading in Population-based Screening Program: Follow-up and Final Results of Oslo II Study 1. Radiology. 2007 Sep;244(3):708–717. doi: 10.1148/radiol.2443061478. [DOI] [PubMed] [Google Scholar]
- 38.Taylor P, Potts HWW. Computer aids and human second reading as interventions in screening mammography: Two systematic reviews to compare effects on cancer detection and recall rate. European Journal of Cancer. 2008;44(6):798–807. doi: 10.1016/j.ejca.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Kalager M, Adami H-O, Bretthauer M, Tamimi RM. Overdiagnosis of Invasive Breast Cancer Due to Mammography Screening: Results From the Norwegian Screening Program. Annals of Internal Medicine. 2012 Apr 3;156(7):491–499. doi: 10.7326/0003-4819-156-7-201204030-00005. [DOI] [PubMed] [Google Scholar]
- 40.Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008 Feb;246(2):376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poplack SP, Carney PA, Weiss JE, Titus-Ernstoff L, Goodrich ME, Tosteson AN. Screening mammography: costs and use of screening-related services. Radiology. 2005 Jan;234(1):79–85. doi: 10.1148/radiol.2341040125. [DOI] [PubMed] [Google Scholar]
- 42.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002 Aug;40(8 Suppl):IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 43.Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov);. SEER*Stat Database: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub (2000–2008) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011 (updated 10/28/2011), based on the November 2010 submission.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.