Abstract
Ménétrier’s disease is a rare, premalignant disorder of the stomach with no proven effective medical therapy. Increased epidermal growth factor (EGF) receptor signaling has been implicated in the pathogenesis of Ménétrier’s disease. We conducted a single-arm clinical trial with cetuximab, a monoclonal antibody that blocks EGF receptor signaling, in 9 individuals with clinically and histologically documented severe Ménétrier’s disease that impaired quality-of-life to the extent that gastrectomy was being considered. Of the 7 patients who completed the one-month course of treatment, all showed statistically significant improvement both clinically (quality-of-life indices) and biochemically (increased parietal cell mass and gastric acidity). Furthermore, all seven patients who completed the one-month trial elected to continue treatment, and four subsequently showed near complete histological remission. Cetuximab should be considered first line therapy for Ménétrier’s disease.
Introduction
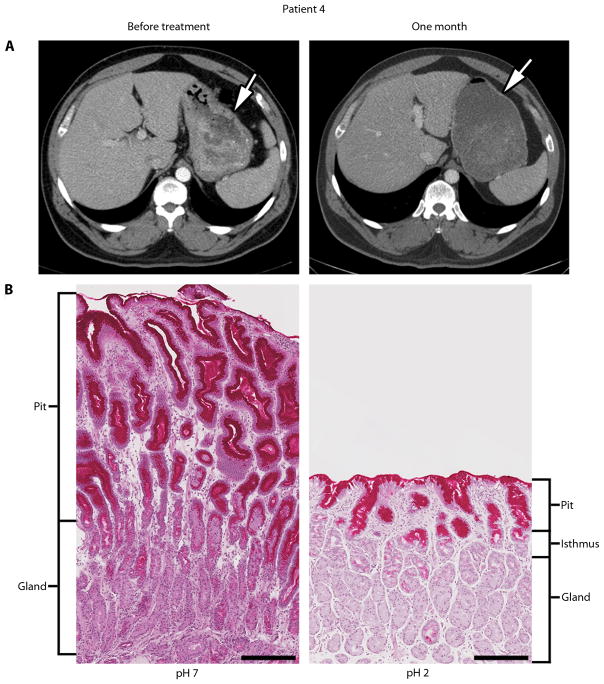
Ménétrier’s disease (hypoproteinemic hypertrophic gastropathy) is a premalignant hyperproliferative disorder of the stomach that is characterized by diffusely enlarged gastric folds, decreased acid production, excess mucus secretion and decreased serum albumin (hypoalbuminemia) due to loss of protein across the gastric mucosa [1]. It usually involves the gastric body (corpus) and spares the antrum. The normal mucosa of the gastric body is made up of relatively short pits and tightly packed specialized glands. The pits are lined by mucus-secreting surface cells called foveolar epithelium that normally occupy ¼ of the mucosal thickness. The specialized glands are composed of acid-secreting parietal cells and mucus-secreting neck cells, which give rise to pepsinogen-secreting zymogenic (chief) cells, as well as scattered endocrine cells. The normal pit to gland ratio is 1:4 (Fig. 1B, right panel). In Ménétrier’s disease, this ratio can be reversed (Fig. 1B, left panel) since the number of surface mucous cells is greatly increased. This histological finding, termed foveolar hyperplasia, is characteristic of, but not pathognomonic for, Ménétrier’s disease. The hyperplasia is driven by expansion of the progenitor cells that are normally confined to the region between the pit and gland designated the isthmus (Fig. 1B, right panel). In Ménétrier’s disease, these progenitor cells are thought to preferentially differentiate into surface mucous cells at the expense of parietal cells and chief cells.
Fig. 1.
Response to one-month course of cetuximab in patient 4. (A) Patient 4 showed a marked reduction in stomach wall thickness by CT scan. An equivalent amount of VoLumen (an oral contrast agent) was administered prior to the scans. Arrows, thickness of gastric wall. (B) Biopsies before and 1 month after treatment show regression of foveolar hyperplasia and restoration of glandular mucosa with return to normal pit to gland ratio of 1:4. Surface mucous cells are strongly positive and mucous neck cells are weakly positive by diastase-resistant periodic acid-Schiff staining. Gastric pH decreased from 7 to 2 after 4 weekly infusions of cetuximab. Scale bar is 250 microns.
The diagnosis of Ménétrier’s disease is based on clinical, endoscopic and histological criteria. To establish the diagnosis, patients must exhibit relevant signs and symptoms that usually include hypoalbuminemia and edema, diffusely enlarged folds in the body of the stomach, prominent foveolar hyperplasia and glandular atrophy with reduced numbers of parietal cells and chief cells. In adults, it is a progressive disorder; there are no reports of spontaneous regression of the disease in patients with symptoms longer than 6 months duration [2–7]. Patients exhibit a constellation of symptoms, which can include abdominal pain, nausea and vomiting, peripheral edema and chronic gastric blood loss [8]. There has been no effective medical therapy, and many patients require gastrectomy as a result of intractable symptoms and concern about gastric cancer.
Evidence from both mice and humans has implicated increased signaling through the EGF receptor in the pathogenesis of Ménétrier’s disease [9, 10]. Transforming growth factor-α (TGFα), one of seven mammalian EGF receptor ligands, increases gastric epithelial cell proliferation, stimulates gastric mucin production and suppresses gastric acidity [11–14]. Transgenic mice that overexpress TGFα in the stomach exhibit all of the histological features of the disorder [9, 11–13]. Patients with Ménétrier’s disease exhibit increased TGFα immunoreactivity in the areas of abnormal gastric mucosa [14].
On the basis of this evidence and the lack of any effective medical therapy, the U.S. Food and Drug Administration (FDA) gave compassionate-use approval to treat a patient with cetuximab, a recombinant, chimeric, IgG1 monoclonal antibody that binds specifically to the extracellular portion of the EGF receptor and inhibits binding of ligands such as TGFα. Treatment of this individual resulted in marked clinical and biochemical improvement [15]. This outcome led us to conduct a single-arm clinical trial to evaluate the effectiveness of cetuximab in the treatment of Ménétrier’s disease. All seven patients who completed the one-month course of cetuximab showed improvement in both quality-of-life and biochemical indices of the disease and elected to continue treatment. Four of the seven patients had near complete histological resolution of the findings of Ménétrier’s disease.
Results
Patient characteristics
Baseline characteristics of patients enrolled in the trial are presented in Table 1. Of the nine patients enrolled, five were men and four were women. The ages of the patients at the time of initial presentation with Ménétrier’s disease ranged from 29 to 79. Of note, four patients (44.4%) also had ulcerative colitis. Of the 4 patients with ulcerative colitis, two (patients 3 and 7) had been treated previously with both immunomodulator therapy (6-mercaptopurine) and a chimeric monoclonal antibody to TNF-α (infliximab). Patient 8 received 6-MP alone and patient 5 received no immunomodulator therapy. Only one patient was maintained on immunomodulator therapy while enrolled in the trial (patient 3). Table 1 also presents the predominant complaint(s) of each patient.
Table 1.
Patient characteristics
| Patient | Age | Sex | Ulcerative colitis | Predominant symptom(s) |
|---|---|---|---|---|
| 1 | 79 | Female | No | Abdominal pain, N/V*, edema |
| 2 | 48 | Female | No | Nausea |
| 3 | 29 | Male | Yes | Abdominal pain, N/V*, edema |
| 4 | 41 | Male | No | Abdominal pain, N/V*, edema |
| 5 | 51 | Female | Yes | Nausea, edema |
| 6 | 57 | Male | No | Gastric blood loss |
| 7 | 33 | Male | Yes | Abdominal pain, N/V* |
| 8 | 57 | Female | Yes | Edema, nausea |
| 9 | 54 | Male | No | Abdominal pain |
N/V, nausea and vomiting
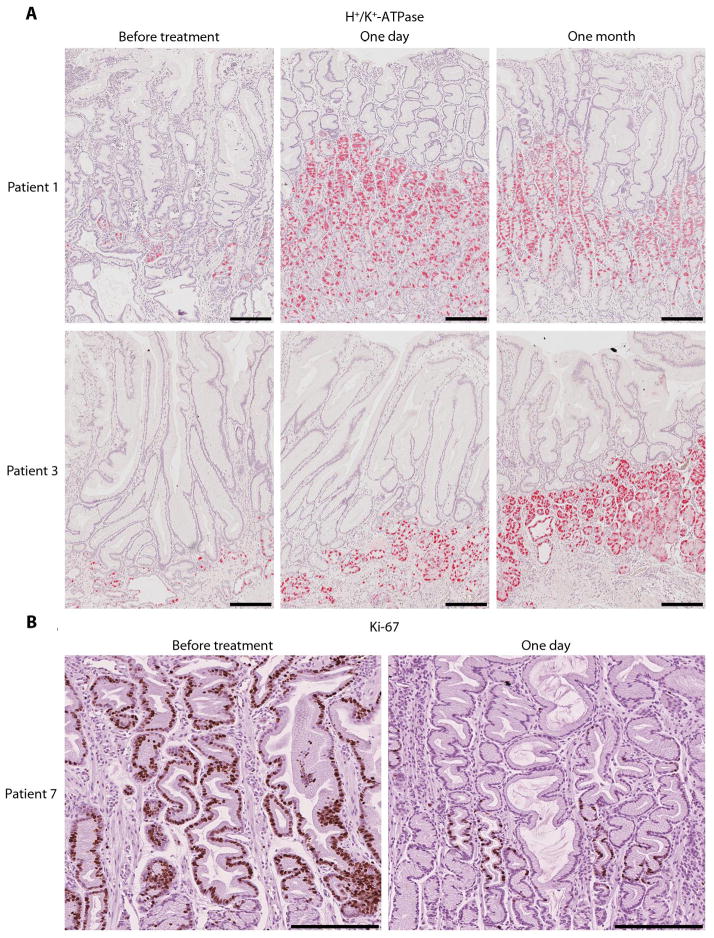
Each participant was treated with a loading dose of intravenous cetuximab (400 mg per square meter of body surface area), followed by three weekly intravenous infusions (250 mg per square meter of body surface area). One patient (patient 8) withdrew from the study after a single dose of cetuximab because of concern over possible side effects from the medication, although there was no overt toxicity at the time of withdrawal. There was, however, a marked reduction in Ki-67 immunostaining, a marker of actively cycling cells, in the involved gastric mucosa 24 hours after the initial dose of cetuximab (Fig. S1). Another patient (patient 9) received two infusions of cetuximab prior to withdrawal. In this case, his major symptom, abdominal pain, persisted. When the baseline gastric biopsies were reviewed after the second dose of cetuximab, a focus of gastric cancer was observed and the patient was removed from the study.
Response to cetuximab treatment
All seven patients who completed the course of treatment reported improvement in their individual predominant symptom(s) within one week of starting treatment, usually within 1 to 2 days. Table 2 presents the primary and secondary outcomes at baseline and after one month of therapy. There was a statistically significant increase in the primary outcome, both the overall quality-of-life index (Ferrans and Powers QLI; P=0.02) and the QLI Health and Functioning subscale (P=0.01). Parietal cell mass, as measured by quantitative immunohistochemistry of the parietal cell marker H+/K+-ATPase (see Materials and Methods), increased three-fold after one month of therapy (P=0.01) and was accompanied by a decrease in mean gastric pH from 6.0 to 4.0 (P=0.05). Mean stomach wall thickness decreased from 13.7 to 9.6 mm, although this was not statistically significantly different (P=0.06). No significant change was detected in other secondary outcomes. Shown as an example in Figure 1, patient 4 exhibited a large reduction in gastric wall thickness (as assessed by CT scan), regression of foveolar hyperplasia and reappearance of parietal cells with restoration of gastric acidity after four weekly doses of cetuximab.
Table 2.
Effects of one-month treatment with cetuximab on patient parameters. N=7 for each parameter shown; data are expressed as mean ± SD.
| Pre-Treatment | 1 Month | Difference (Pre - 1 Month) | P-valueh | |
|---|---|---|---|---|
| QLIa | 15.8 (±6.3) | 20.3 (±5.2) | −4.4 (±3.4) | 0.02 |
| QLI-H&Fb | 12.3 (±7.4) | 19.8 (±6.2) | −7.5 (±4.8) | 0.01 |
| Parietal cell massc | 20.9 (±17.6) | 61.2 (±23.3) | −40.2 (±15.8) | 0.01 |
| Gastric pH | 6.0 (±2.5) | 4.1 (±2.7) | 2.0 (±2.0) | 0.05 |
| Stomach wall thickness (mm)d | 13.7 (±6.4) | 9.6 (±8.0) | 4.1 (±3.4) | 0.06 |
| Ki-67 positivitye | 43.8 (±25.2) | 34.2 (±18.4) | 9.6 (±15.6) | 0.21 |
| Serum gastrin (pg/mL) | 194 (±212) | 245 (±240) | −51.4 (±160.4) | 0.57 |
| Serum albumin (g/dL) | 3.04 (±0.55) | 3.02 (±0.83) | 0.01 (±4.9) | 0.97 |
| Stool α1-antitrypsin (g/mg)f | 0.92 (±0.73) | 0.49 (±0.74) | 0.43 (±5.3) | 0.19 |
| MAPK Ratiog | 1.35 (± 2.62) | 0.95 ± (1.88) | 0.17 (±0.53) | 0.27 |
Ferrans and Power Quality-of-Life Index (QLI). Higher scores indicate a higher QLI. The maximal score possible is 30.
Ferrans and Powers QLI, Health and Functioning subscale. The maximal score possible is 30.
Proportion of cells expressing H+/K+-ATPase, as measured by quantitative immunohistochemistry [45].
Measured along the greater curvature of the stomach on CT scan of the abdomen after administration of intravenous contrast and VoLumen oral contrast.
Mean number of Ki-67 positive cells per gland.
Measured in a single stool sample from each patient at each time point.
Ratio of intensity of phospho-MAPK divided by total-MAPK determined by densitometry on western blot.
mid-P-value (See statistical analysis section of Materials and Methods section)
Although the primary end point (overall QLI) was evaluated after four weekly infusions of cetuximab, we performed gastroscopy 24 hours after the first infusion and obtained grossly involved gastric tissue for analysis. After this short time interval, the parietal cell mass had increased from 20.9 to 41.1 (P=0.02). Figure 2 shows H+/K+-ATPase immunostaining (a selective marker of parietal cells) at baseline, after one day and after one month of cetuximab in patients 1 and 3. To confirm this finding, we pooled pre-treatment samples and one day post-treatment samples from abnormal gastric tissue from 8 patients and performed in-depth shotgun proteomics. This analysis identified a total of 1090 high-confidence protein groups; using conservative filtering and grouping algorithms on the original 3806 protein identifications (See Materials and Methods for details). A total of 47 proteins were statistically significantly different between pre- and post-treatment by at least 2-fold (32 increased and 15 decreased) (Table S1). In shotgun proteomic datasets, proteins are identified by mass spectrometry spectra where higher numbers of observed spectra are interpreted as a measure of protein abundance in the specimen. There was a statistically significant three-fold increase in normalized spectral counts for the α-subunit of H+/K+-ATPase after one day of cetuximab treatment (from 7 to 36 spectral counts, quasi-P=0.049); the β-subunit showed a smaller increase after treatment that did not reach statistical significance (from 15 to 24 spectral counts, quasi-P=0.98). We also identified significant increases in proteins previously identified as markers of parietal cells, mucous neck cells and chief cells. For example, carbonic anhydrase II and calmodulin 2 are expressed by parietal cells [16, 17], mucin 6 is expressed by mucous neck cells [18] and gastric lipase and leucine aminopeptidase 3 are preferentially expressed in chief cells [19, 20] (Table S1).
Fig. 2.
Recovery of parietal cells and decrease in proliferation one day after initiation of cetuximab treatment. (A) Patients 1 and 3 demonstrated rapid (one day) and sustained (one month) increases in H+/K+−ATPase α-subunit immunoreactivity. Scale bar is 250 microns. (B) Patient 7 had a dramatic decrease in Ki-67 staining one day after first dose of cetuximab. Scale bar is 250 microns.
In addition, we assessed two markers of cellular proliferation, Ki-67 and phosphorylated MAPK, after the first dose of cetuximab in all seven patients who completed the trial. The number of Ki-67-positive cells per gland decreased from 43.8 before treatment to 27.2 after 24 hours (P=0.05). Results from patient 7 are shown in Fig. 2B. The ratio of phosphorylated (activated) MAPK to total MAPK by western blotting decreased from 1.35 (± 2.62) before treatment to 0.56 (± 1.78) after 24 hours, although this did not reach statistical significance (P=0.12). Taken together, these results show that monoclonal antibody blockade of the EGF receptor in this hyperproliferative disorder of the stomach caused a rapid response. In addition, these findings support the hypothesis that enhanced EGF receptor signaling directs gastric progenitor cells down a surface mucous cell lineage rather than a parietal cell lineage [21], and underscore the remarkable plasticity of the adult stomach.
Long term follow-up
All seven participants who completed the trial reported that their predominant symptom(s) had improved to the extent that they elected to continue treatment (mean duration of followup 18 months; range 8 to 40 months). Table 3 presents the long-term clinical outcomes for each patient, along with the histological findings and gastric pH at last follow-up or at the time of gastrectomy.
Table 3.
Long term clinical outcomes for those who continued treatment
| Patient | Duration of therapy | Long-term outcome | Gastric histology at last follow-up | Gastric pH at last follow-up |
|---|---|---|---|---|
| 1 | 18 months | Off Treatment | Minimal FH* | 1 |
| 2 | 16 months | Off Treatment | Minimal FH* | 2 |
| 3 | 40 months | On Treatment | Minimal FH* | 3 |
| 4 | 9 months | Gastrectomy | Normal | 2 |
| 5 | 24 months | Gastrectomy | Dysplastic lesion one year after treatment stopped | 7 |
| 6 | 9 months | Gastrectomy | FH* | 2 |
| 7 | 8 months | Gastrectomy | FH* | 3 |
FH, foveolar hyperplasia
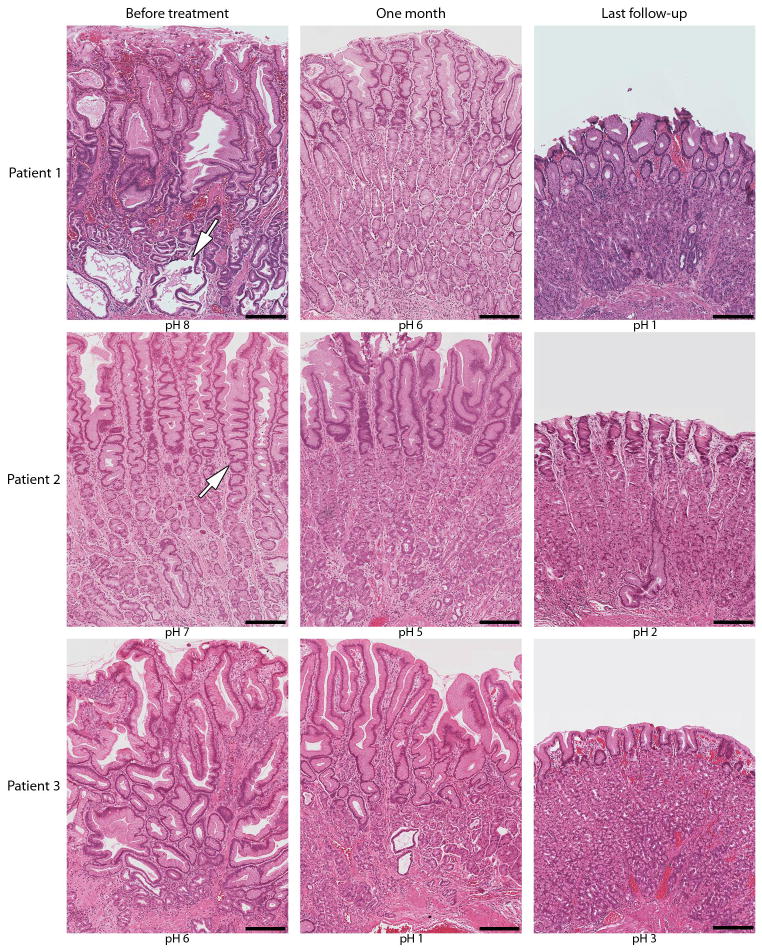
After 18 months of cetuximab, patient 1 had complete resolution of symptoms and a histologically normal stomach (Fig. 3), leading to discontinuation of cetuximab. Nine months later, the stomach was grossly normal with minimal foveolar hyperplasia and a gastric pH of 1. She remains asymptomatic 19 months after discontinuing cetuximab. Patient 2 was treated for 16 months, at which time her gastric mucosa appeared grossly and histologically normal with a gastric pH of 2 (Fig. 3). Cetuximab was stopped and she notes only occasional nausea 4 months later. Patient 3 has been treated with cetuximab for 40 months. Gastric histology at 31 months showed mild to moderate foveolar hyperplasia (Fig. S2); however, 7 months later, gastric histology showed marked improvement with reduced mucosal thickness, minimal foveolar hyperplasia with a gastric pH of 3. He presently experiences occasional nausea and vomiting and has minimal edema.
Fig. 3.
Progressive improvement in gastric histology during cetuximab treatment. Foveolar hyperplasia [with characteristic cysts (arrow in top left panel) and tortuous glands (arrow in middle left panel)] and glandular atrophy are evident the day before treatment by hematoxylin-eosin staining. At last follow-up, patients 1, 2 and 3 showed reduced thickness of gastric mucosa, regression of foveolar hyperplasia and restoration of glandular mucosa and gastric acidity. Cetuximab has been discontinued in patients 1 and 2. Patient 3 had the most distorted architecture at presentation. Progressive histological improvement was noted beginning at 31 months (Fig. S2) and last follow-up at 38 months. Scale bar is 250 microns.
Four of the 7 patients who completed the one-month trial eventually underwent gastrectomy. Patient 4 had complete regression of foveolar hyperplasia after one month of treatment (Fig. 1). He continued on cetuximab for nine months because of continuing abdominal pain. After 9 months of treatment, he underwent gastrectomy due to anxiety about the development of gastric cancer and concern regarding the need for indefinite cetuximab treatment. His stomach was normal histologically at the time of surgery (Fig. S3). Patient 5 underwent gastrectomy when a 4-cm dysplastic lesion was found in the stomach during surveillance gastroscopy more than a year after she electively discontinued treatment. Patient 6 had cessation of gastric bleeding while on cetuximab but elected to undergo gastrectomy because he did not want to continue cetuximab indefinitely. Patient 7 experienced an infusion reaction after 8 months of treatment and elected to undergo gastrectomy. However, each of the patients that ultimately underwent gastrectomy had significant improvement in their predominant symptom(s) during the course of the one-month trial and had elected to continue treatment.
Adverse events in response to cetuximab treatment
There has been no evidence of resistance or diminishing benefit over time in response to long-term treatment with cetuximab. There were no anaphylactic infusion reactions. However, one patient (patient 7) did have an infusion reaction after approximately 8 months of therapy that led him to discontinue therapy. Six of the seven patients who received more than a single infusion developed a rash characteristic of cetuximab therapy [22]. One patient (patient 5) never developed a rash despite an objective response to treatment, a finding at odds with the impression that the severity of the rash correlates with the likelihood of a clinical response to cetuximab in cancer patients [21].
Discussion
This single-arm clinical trial demonstrates efficacy of cetuximab in the treatment of Ménétrier’s disease, a disorder that has been considered refractory to medical therapy. All of the patients had severe disease and would have undergone gastrectomy had they not enrolled in this clinical trial. Despite the small sample size, all seven patients who completed the one-month trial showed significant improvement in quality-of-life indices as well as significantly increased parietal cell mass and gastric acidity. As noted above, Ménétrier’s disease is a rare disorder (www.rarediseases.org); it required 8 years to accrue nine patients, making it unfeasible to include a placebo control arm.
We were able to perform gastroscopy to obtain gastric tissue 24 hours after the initial infusion of cetuximab. Even at this early time, there was already a significant decrease in the number of Ki-67 positive cells in the involved gastric mucosa and a doubling of the parietal cell mass. The finding of increased parietal cell mass within 24 hours of cetuximab treatment is consistent with previous investigations in rodents with acute ablation of parietal cells with DMP-777, a cell-permeant neutrophil elastase inhibitor and parietal cell-specific protonophore [22]. Following cessation of treatment with DMP-777, parietal cells expressing H+/K+-ATPase rapidly return within 24 to 48 hours [22]. Additionally, there was a seven-fold increase in an ultrastructurally defined, immunohistochemically H+/K+-ATPase-negative, pre-parietal cell population in a transgenic mouse model in which parietal cells were ablated by H+/K+-ATPase-promoter-driven expression of an attenuated Diphtheria Toxin A subunit [23, 24]. We have hypothesized that excess EGF receptor stimulation in Ménétrier’s disease results in overproduction of surface mucous cells at the expense of glandular lineages [21]; the results in animals cited above suggest the possibility that in patients with Ménétrier’s disease, there is a relative abundance of H+/K+-ATPase-negative preparietal cells which are held in a state of suspended maturation, but poised to differentiate rapidly upon EGF receptor blockade.
All seven participants who completed the trial elected to continue treatment because of improvements in their predominant symptom(s). There was no evidence that any of these patients treated with prolonged therapy developed resistance to cetuximab. In fact, the interval between infusions was increased to every two to three weeks and most showed continued clinical and histological improvement over time. Patients 1 and 2 were able to stop treatment altogether after remission of both symptoms and histological features of the disease. Patient 3 has been on treatment for over 3 years and continues to show progressive histological improvement after 38 months compared to biopsies after 31 months (Fig. 3 and Fig. S2). This suggests that prolonged therapy may provide continued benefit for some patients.
There have been anecdotal case reports of Ménétrier’s disease patients responding to various pharmacological interventions, including corticosteroids [25]; however, no consistent benefit has been observed. On the basis of the reported high incidence of infusion reactions to cetuximab in patients in the southeastern United States [26], dexamethasone (20 mg orally) was administered to five of the patients 20 minutes prior to cetuximab infusion. Although possible, we consider it highly unlikely that this contributed to the beneficial responses, because the patients that have been treated successfully with corticosteroids such as dexamethasone for Ménétrier’s disease received much higher, continuous dosing. Moreover, five of our patients (Patients 1, 3, 5, 7 and 8) had already been treated with continuous, high-dose corticosteroids for Ménétrier’s disease-associated symptoms without any appreciable benefit prior to enrolling in this trial.
A notable feature of our study population was that four of nine (44%) participants also had ulcerative colitis (Table 1). Seven patients with concurrent Ménétrier’s disease and ulcerative colitis have been reported previously [27, 28]. EGF is involved in healing of the gastrointestinal mucosa, and EGF enemas are effective at reducing disease activity in ulcerative colitis [29]. As a result, there was concern that pharmacological blockade of EGF receptor could exacerbate colitis in these patients. However, none of the four patients with ulcerative colitis had worsening of their colitis symptoms, even with prolonged treatment, and periodic colonic biopsies from these patients did not show evidence of increased histological severity (as an example, sequential images from patient 3 are shown in Fig. S2). The mechanism underlying the association of Ménétrier’s disease and ulcerative colitis is not known [30]. All four patients with ulcerative colitis had pancolitis, although the duration and severity of the disease varied. In all cases, the diagnosis of ulcerative colitis preceded that of Ménétrier’s disease. There was no common therapeutic regimen; two of the patients had received infliximab and 6-MP, one patient received 6-MP alone and one patient received no immunomodulator therapy. Possible underlying factors that may link these two disorders include upregulation of EGF receptor ligands and a “leaky” mucosa that may provide a portal of entry for luminal contents (including commensal bacteria or their products). Whether these or other factors are operative or provide a link between the two disorders remains to be determined. Patient 2 had ankylosing spondylitis, suggesting a possible immunological underpinning to Ménétrier’s disease.
From this trial, we cannot draw any firm conclusions regarding the ability of cetuximab to prevent malignant progression. One of our patients had coexisting gastric cancer that was only recognized after the second dose of cetuximab. The only patient in our study who developed a premalignant lesion during prolonged follow-up had stopped cetuximab therapy approximately 12 months prior to discovery of the lesion.
Although the precise etiology of Ménétrier’s disease remains uncertain, our results suggest that a common feature of all cases of Ménétrier’s disease is enhanced EGF receptor signaling. A form of the disorder, which is seen predominantly in children, has been associated with CMV infection [31–36]. These patients usually have an acute presentation and spontaneous remission of symptoms and histological findings. When examined, increased TGFα immunoreactivity has been observed in the involved gastric mucosa of these cases [37]. Interestingly, the principal envelope glycoprotein of CMV, glycoprotein B, binds EGF receptor, induces EGF receptor-HER3 heterodimers and activates PI3K-AKT signaling [38].
This trial suggests that blocking ligand binding to the EGF receptor with cetuximab is an effective treatment for patients with Ménétrier’s disease. The patients in this study had severe disease, had failed all medical therapy and were considering gastrectomy as the remaining treatment option. Prior to treatment, some were considered poor candidates for surgery due to their hypoalbuminemia and poor nutritional status. The seven patients who completed the one-month trial experienced rapid clinical and biochemical improvement and elected to continue treatment. Four of the patients subsequently had near complete histological remission, and two have remained asymptomatic off treatment for 19 and 4 months, respectively. Five of the patients ultimately required gastrectomy. In the subset of patients with Ménétrier’s disease who are too ill to undergo gastrectomy, cetuximab may be a reasonable bridge to improve their operative risk. Since no other medical therapies have shown to be consistently beneficial, cetuximab should be considered as first line therapy for Ménétrier’s disease. It would be of interest to determine whether an oral EGF receptor tyrosine kinase inhibitor and/or a TACE inhibitor to block cell surface cleavage of TGFα would be as effective in this disorder as intravenous delivery of the EGF receptor monoclonal antibody [39].
Materials and Methods
Overview
This single-arm clinical trial explored the safety, tolerability and clinical and biochemical effect of a four-week course of cetuximab in patients with clinically and histologically confirmed Ménétrier’s disease. Patients were recruited from seven U.S. states and two counties. After recruitment, all participants were enrolled and treated during the one-month trial at the Vanderbilt University Clinical Research Center. This prospective, open-label trial was approved by the Vanderbilt University Medical Center Institutional Review Board, and all participants provided written informed consent.
Patients
Individuals aged 18 to 80 years with symptomatic Ménétrier’s disease that interfered with daily life to the extent that gastrectomy was being considered were eligible for the study. The diagnosis of Ménétrier’s disease was based on clinical and histological criteria. Prior to enrollment, all patients underwent upper endoscopy, which revealed enlarged gastric folds, and histological evaluation that demonstrated foveolar hyperplasia. Two distinct histological patterns of Ménétrier’s disease have been proposed: massive foveolar hyperplasia (MFH) with minimal inflammation and hypertrophic lymphocytic gastritis (HLG) [40]. All of the patients within our study had MFH with little, if any, inflammation. Symptoms in all patients were refractory to other medical therapy such as proton pump inhibitors, anticholinergic medications, or anti-emetics for at least six months. There have been case reports of Ménétrier’s disease linked to H. pylori that were responsive to H. pylori eradication [41–43]. Therefore, patients with active CMV or H. pylori infection were excluded from this study. We also required that patients’ symptoms exceed 6 months in duration prior to entry into the trial to minimize the likelihood of a spontaneous remission. Patients were also excluded if they were pregnant or breastfeeding, had clinically unstable cardiovascular disease, had any unstable coagulation disorder, or had any other active medical condition requiring stabilization, such as malignancy, uncontrolled diabetes, uncontrolled hypertension or infection requiring antibiotics. Patients receiving concurrent monoclonal antibody therapy for other medical conditions were ineligible.
Treatment
Participants received intravenous cetuximab once weekly with an initial loading dose of 400 mg per square meter of body surface area, followed by three weekly doses of 250 mg per square meter of body surface area. To prevent infusion reactions, all patients were premedicated with diphenhydramine (50 mg intravenously) and 5 of the 9 patients also received dexamethasone (20 mg orally). If a participant had subjective improvement in either their predominant symptom(s) or improvement in biochemical or histological parameters, such as hypoalbuminemia, transfusion requirements, histological changes or indices of proliferation, then he or she could elect to continue treatment beyond the one-month trial. For longer term treatment, arrangements were made for patients to receive cetuximab at a medical facility near their homes under a separate IRB-approved protocol, and patients were under the care of their referring physicians. Efforts were made to extend the interval between treatments from weekly to every two to three weeks without dose adjustment. Patients received follow-up gastroscopy every 3 to 6 months.
End points
Outcomes were assessed at three time points during the 4-week trial: at baseline, 24 hours after the loading dose of cetuximab and after the fourth dose. Patients had upper endoscopy performed at each of these three time points with multiple large forceps pinch biopsies taken from the body and antrum of the stomach and a full thickness snare biopsy taken from the body of the stomach. Samples were placed in formalin overnight and then transferred to 70% ethanol for immunohistochemical analysis, or were snap frozen in liquid nitrogen and stored at −80°C. Gastric fluid samples were tested with pH paper. Serum albumin and gastrin were measured at each of these time points through the Vanderbilt University Medical Center diagnostic laboratory. Patients had a CT scan of the abdomen and pelvis after administration of VoLumen and intravenous contrast at baseline and at the end of the trial. Stool samples were collected at baseline and at the end of treatment to assess changes in stool α1-antitrypsin.
The primary outcome was the change from baseline in overall quality-of-life after one month of treatment, as measured by Ferrans and Powers QLI (Generic Version) and the QLI Health and Functioning subscale [44]. The Ferrans and Powers QLI measures quality-of-life across four domains (health and functioning, social and economic, psychological and spiritual, and family dynamics). This scale was chosen because it has been previously validated in patients with various chronic medical conditions, including cancer, chronic kidney disease, and cardiovascular disease, and was also used in our previous report on the use of cetuximab in Ménétrier’s disease [15]. Secondary outcomes were baseline to one-month changes in stomach wall thickness, parietal cell mass, gastric pH, Ki-67 immunohistochemistry, total and activated phospho-MAPK levels in the involved gastric mucosa, serum albumin and gastrin levels and stool α1-antitrypsin. Stomach wall thickness was measured along the greater curvature of the stomach on CT scan of the abdomen after administration of intravenous contrast and VoLumen oral contrast. Ki-67 positivity was expressed as the average number of positive cells per gland based upon counting at least 10 well-oriented glands. All biopsies were reviewed and scored by a single pathologist (MKW). Stool α1-antitrypsin was measured in a single stool sample from each time point. Total and phospho-MAPK were assessed by western blot with rabbit polyclonal antibodies p44/42 MAPK (Erk1/2) and phopho-p44/42 MAPK (Erk1/2) (Cell Signaling Technology), with goat antibody to rabbit horseradish peroxidase (HRP) serving as the secondary antibody. Western blot results were quantified with densitometry. Because the severity of Ménétrier’s disease can vary from region to region within the stomach, three separate biopsies from each patient were pooled from each time point for western blot analysis.
Assessment of parietal cell mass
Parietal cell mass was quantified with an Ariol SL-50 scanner for quantitative immunohistochemistry with an antibody against the α-subunit of the H+/K+-ATPase (clone 12.18, at 1:2000, a gift from Adam Smolka, Medical University of South Carolina, Charleston, SC) followed by alkaline phosphatase–conjugated goat antimouse IgG (with Vector Red substrate detection). Parietal cell mass was calculated based on number and intensity of H+/K+-ATPase immunoreactivity as described previously [45].
Shotgun proteomic analysis
The primary tissues were processed by using a previously described protocol [46]. Briefly, the tissues were solubilized in trifluoroethanol and equal amounts of 100 μg protein from 8 pre- and 8 post-treatment biopsy lysates were pooled for analysis. The homogenates were processed, digested with trypsin and the resulting peptide mixtures were separated into 20 fractions by isoelectric focusing. Each of these fractions was subjected to analysis by LC-MS/MS on a Thermo LTQ instrument. Mass spectrometry data was searched using the MyriMatch version 1.5.2 search algorithm [47] against the human International Protein Index (IPI) database version 3.37 supplemented with 73 potential contaminant sequences in forward and reverse orientation. The search results were filtered and assembled with IDPicker version 2.0 [48]. Peptide identification stringency was set at a maximum of 2.5% reversed peptide identifications (5% overall peptide false discovery rate, FDR) and a minimum of two distinct peptides to identify a given protein within the full dataset. Distinct peptides have dissimilar precursor masses or charge states but may reflect variant peptides of the same primary protein sequence. Parsimony analysis was applied so that IPI database entries that mapped to the identical set of peptide identifications were assigned to ”protein groups” [48]. Such protein groups consist almost exclusively of isoforms or identical proteins resulting from redundancy in the database. We performed statistical analyses by modeling spectral count data using a quasi-likelihood Poisson model [49, 50]. Proteins with at least a two-fold increase in spectral counts and a FDR-corrected quasi P-value of less than 0.05 were considered to be different between the pre- and post-treatment groups. Fold-changes are calculated from the model-derived rates for each group and are normalized for the total number of confident spectral identifications observed in each replicate mass spectrometry analysis.
Statistical analysis
Continuous variables were expressed as means ± SD. Due to the lack of reliability of the asymptotic paired t-test with such a small sample size, paired differences for all measures were examined using nonparametric, exact permutation methods. When the null hypothesis of no change is true, exact tests may be unduly conservative, resulting in P-values larger than the nominal 5% commonly used as a boundary between non-significance and significance. Consequently, we report mid-P-values, which use only ½ (versus all) of the probability of the observed data as part of the extreme values that comprise a P-value [51]. Two patients who withdrew early from the study were not included in the statistical analysis. StatExact version 8.0 was used for all analyses and SAS version 9.2 was used to estimate means and standard deviations.
Supplementary Material
Supplemental Table 1. Proteins identified in involved gastric mucosa of Ménétrier’s disease patients by shotgun proteomic analysis.
Fig. S1. Decreased proliferation in gastric mucosa of patient 8 one day after first dose of cetuximab. Although this patient opted to leave the trial after one treatment, there was a marked decrease in Ki-67 staining in the involved gastric mucosa. Scale bar is 250 microns.
Fig. S2. Long term changes in the stomach and colon of patient 3. (A) Patient 3 demonstrated partial improvement in foveolar hyperplasia after one month of treatment (top panel). Fovelar hyperplasia continued to regress further with long term cetuximab therapy, along with restoration of normal gastric glandular architecture and improved gastric acidity. Scale bar is 250 microns. (B) There was no evidence of increased severity of ulcerative colitis with long term cetuximab as determined by gross and microscopic (data not shown) examination of the colon at sigmoidoscopy.
Fig. S3. Patient 4 had histologically normal stomach at gastrectomy. Scale bar is 250 microns.
Acknowledgments
The work was made possible by the generous support of Vanderbilt University’s Clinical Translational Scientific Award (CTSA) and the Peter Powell Foundation. The authors thank Vivian Siegel, Amy Costa, Catherine B. Meador and Jason Mills for reviewing the manuscript, Frank Revetta for technical assistance and Jeff Franklin and Joe Roland for assistance with preparation of figures. The authors thank Adam Smolka (Medical University of South Carolina, Charleston, SC) for providing the H+/K+−ATPase α-subunit antibody. Cetuximab was provided by ImClone Systems Incorporated (Bristol-Myers Squibb).
Funding: This work was supported by R01 (CA46413 R.J.C.), GI Special Program of Research Excellence (P50 95103 R.J.C.), Mouse Models of Human Cancers Consortium (U01 084239 R.J.C.), Department of Veterans Affairs Merit Review Award (RO1 DK071590 J.R.G.), the Vanderbilt CTSA grant 1UL1RR024975 from NCRR/NIH, and the AGA Funderburg Award in Gastric Biology Related to Cancer (J.R.G.). J.T. and A.M.A. were supported by Vanderbilt’s Medical Scientist Training Program.
Footnotes
Author contributions: W.H.F. maintained the dataset, measured gastric wall thickness, performed statistical analysis, and wrote the paper. J.T. assembled the figures and assisted with analysis. K.T.N. performed quantitative immunohistochemistry for H+/K+ATPase. J.R.G. provided advice on study design. R.J.C.S. and D.C.L. performed shotgun proteomic analysis. A.M.A. assisted with performing western blots for MAPK and phosho-MAPK analysis. B.L. and G.D.A. performed statistical analysis. C.D.L. performed gastroscopy and flexible sigmoidoscopy in order to obtain tissue for analysis. M.K.W. provided pathological interpretation and also quantified K-67 staining. R.J.C. designed the study and wrote the paper.
Competing interests: ImClone Systems Incorporated had no role in the design of the study, in data accrual or analysis, or in preparation of the manuscript.
Accession numbers: NA
References
- 1.Menetrier P. Des polyadenomes gastriques et leur rapport avec le cancer de l’estomac. Arch Physiol Norm Pathol. 1888;1:32–55. 236–262. [Google Scholar]
- 2.Barbosa AJ, Nogueira AM, Leite VH, Lima GF, Junior, Oliveira CA. Parietal cell carcinoma of the stomach and Menetrier’s disease. Arq Gastroenterol. 1987;24:36–40. [PubMed] [Google Scholar]
- 3.Charton-Bain MC, Paraf F, Bruneval P. Superficial gastric carcinoma developed on localized hypertrophic lymphocytic gastritis: a variant of localized Menetrier’s disease? Pathol Res Pract. 2000;196:125–128. doi: 10.1016/S0344-0338(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, Park SS, Oh SY, Kim JH, Kim WB, Lee JH, Choi JW, Kim SJ, Kim CS, Mok YJ. Primary squamous cell carcinoma of the stomach that developed with Menetrier’s disease. Dig Dis Sci. 2007;52:1722–1724. doi: 10.1007/s10620-006-9191-4. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CT, Ito M, Kawase Y, Sekine I, Ohmagari T, Hashimoto S. Early gastric cancer arising from localized Menetrier’s disease. Gastroenterol Jpn. 1991;26:213–217. doi: 10.1007/BF02811083. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MI, Spark JI, Ambrose NS, Wyatt JI. Early gastric cancer in a patient with Menetrier’s disease, lymphocytic gastritis and Helicobacter pylori. Eur J Gastroenterol Hepatol. 1995;7:187–190. [PubMed] [Google Scholar]
- 7.Wood MG, Bates C, Brown RC, Losowsky MS. Intramucosal carcinoma of the gastric antrum complicating Menetrier’s disease. J Clin Pathol. 1983;36:1071–1075. doi: 10.1136/jcp.36.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkerson ML, Meschter SC, Brown RE. Menetrier’s disease presenting with iron deficiency anemia. Ann Clin Lab Sci. 1998;28:14–18. [PubMed] [Google Scholar]
- 9.Dempsey PJ, Goldenring JR, Soroka CJ, Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittelkow MR, Lee DC, Sandgren EP, et al. Possible role of transforming growth factor alpha in the pathogenesis of Menetrier’s disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992;103:1950–1963. doi: 10.1016/0016-5085(92)91455-d. [DOI] [PubMed] [Google Scholar]
- 10.Coffey RJ, Romano M, Goldenring J. Roles for transforming growth factor-alpha in the stomach. J Clin Gastroenterol. 1995;21 (Suppl 1):S36–39. [PubMed] [Google Scholar]
- 11.Coffey RJ, Romano M, Polk WH, Dempsey PJ. Roles for transforming growth factor-alpha in gastric physiology and pathophysiology. Yale J Biol Med. 1992;65:693–704. discussion 621–693. [PMC free article] [PubMed] [Google Scholar]
- 12.Bockman DE, Sharp R, Merlino G. Regulation of terminal differentiation of zymogenic cells by transforming growth factor alpha in transgenic mice. Gastroenterology. 1995;108:447–454. doi: 10.1016/0016-5085(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 13.Joshi V, Ray GS, Goldenring JR. Inhibition of parietal cell acid secretion is mediated by the classical epidermal growth factor receptor. Dig Dis Sci. 1997;42:1194–1198. doi: 10.1023/a:1018845805806. [DOI] [PubMed] [Google Scholar]
- 14.Bluth RF, Carpenter HA, Pittelkow MR, Page DL, Coffey RJ. Immunolocalization of transforming growth factor-alpha in normal and diseased human gastric mucosa. Hum Pathol. 1995;26:1333–1340. doi: 10.1016/0046-8177(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 15.Burdick JS, Chung E, Tanner G, Sun M, Paciga JE, Cheng JQ, Washington K, Goldenring JR, Coffey RJ. Treatment of Menetrier’s disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med. 2000;343:1697–1701. doi: 10.1056/NEJM200012073432305. [DOI] [PubMed] [Google Scholar]
- 16.Lonnerholm G, Selking O, Wistrand PJ. Amount and distribution of carbonic anhydrases CA I and CA II in the gastrointestinal tract. Gastroenterology. 1985;88:1151–1161. doi: 10.1016/s0016-5085(85)80074-3. [DOI] [PubMed] [Google Scholar]
- 17.Mills JC, Syder AJ, Hong CV, Guruge JL, Raaii F, Gordon JI. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci U S A. 2001;98:13687–13692. doi: 10.1073/pnas.231332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 19.Moreau H, Bernadac A, Gargouri Y, Benkouka F, Laugier R, Verger R. Immunocytolocalization of human gastric lipase in chief cells of the fundic mucosa. Histochemistry. 1989;91:419–423. doi: 10.1007/BF00493829. [DOI] [PubMed] [Google Scholar]
- 20.Mills JC, Andersson N, Stappenbeck TS, Chen CC, Gordon JI. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem. 2003;278:46138–46145. doi: 10.1074/jbc.M308385200. [DOI] [PubMed] [Google Scholar]
- 21.Coffey RJ, Washington MK, Corless CL, Heinrich MC. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 23.Karam SM, Leblond CP. Identifying and counting epithelial cell types in the “corpus” of the mouse stomach. Anat Rec. 1992;232:231–246. doi: 10.1002/ar.1092320208. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 25.Davis GE, O’Rourke MC, Metz JR, Kindig WV, Sweeney JG, Kane KN. Hypertrophic gastropathy symptoms responsive to prednisone. A case report and a review of the literature. J Clin Gastroenterol. 1991;13:436–441. doi: 10.1097/00004836-199108000-00014. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncology. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 27.Hatemi I, Caglar E, Aksoy D, Goksel S, Dobrucali A. Menetrier’s disease coexisting with ulcerative colitis and sclerosing cholangitis. Dig Liver Dis. 2008;40:78–79. doi: 10.1016/j.dld.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Hemmings CT. Menetrier’s disease in a patient with ulcerative colitis: a case report and review of the literature. Pathology. 2007;39:282–283. doi: 10.1080/00313020701230732. [DOI] [PubMed] [Google Scholar]
- 29.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 30.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 31.Eisenstat DD, Griffiths AM, Cutz E, Petric M, Drumm B. Acute cytomegalovirus infection in a child with Menetrier’s disease. Gastroenterology. 1995;109:592–595. doi: 10.1016/0016-5085(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 32.Drut RM, Gomez MA, Lojo MM, Drut R. Cytomegalovirus-associated Menetrier’s disease in adults. Demonstration by polymerase chain reaction (PCR) Medicina (B Aires) 1995;55:659–664. [PubMed] [Google Scholar]
- 33.Setakhr V, Muller G, Hoang P, Lambert AS, Geubel A. Cytomegalovirus-associated protein losing gastropathy in an immunocompetent adult: a case report. Acta Gastroenterol Belg. 2007;70:296–299. [PubMed] [Google Scholar]
- 34.Pederiva C, Ruscitto A, Brunetti I, Salvini S, Sala M. Cytomegalovirus-induced protein-losing gastropathy: a case report. Pediatr Med Chir. 2006;28:42–47. [PubMed] [Google Scholar]
- 35.Suter WR, Neuweiler J, Borovicka J, Binek J, Fantin AC, Meyenberger C. Cytomegalovirus-induced transient protein-losing hypertrophic gastropathy in an immunocompetent adult. Digestion. 2000;62:276–279. doi: 10.1159/000007827. [DOI] [PubMed] [Google Scholar]
- 36.Hoffer V, Finkelstein Y, Balter J, Feinmesser M, Garty BZ. Ganciclovir treatment in Menetrier’s disease. Acta Paediatr. 2003;92:983–985. [PubMed] [Google Scholar]
- 37.Xiao SY, Hart J. Marked gastric foveolar hyperplasia associated with active cytomegalovirus infection. Am J Gastroenterol. 2001;96:223–226. doi: 10.1111/j.1572-0241.2001.03480.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 39.Merchant NB, Voskresensky I, Rogers CM, Lafleur B, Dempsey PJ, Graves-Deal R, Revetta F, Foutch AC, Rothenberg ML, Washington MK, Coffey RJ. TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–1191. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfsen HC, Carpenter HA, Talley NJ. Menetrier’s disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993;104:1310–1319. doi: 10.1016/0016-5085(93)90339-e. [DOI] [PubMed] [Google Scholar]
- 41.Lepore MJ, Smith FB, Bonanno CA. Campylobacter-like organisms in patient with Menetrier’s disease. Lancet. 1988;1:466. doi: 10.1016/s0140-6736(88)91251-2. [DOI] [PubMed] [Google Scholar]
- 42.Craanen ME, Blok P, Dekker W, Tytgat GN. Helicobacter pylori and early gastric cancer. Gut. 1994;35:1372–1374. doi: 10.1136/gut.35.10.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badov D, Lambert JR, Finlay M, Balazs ND. Helicobacter pylori as a pathogenic factor in Menetrier’s disease. Am J Gastroenterol. 1998;93:1976–1979. doi: 10.1111/j.1572-0241.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv Nurs Sci. 1985;8:15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804–1819. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 46.Slebos RJ, Brock JW, Winters NF, Stuart SR, Martinez MA, Chambers MC, Zimmerman LJ, Ham AJ, Tabb DL, Liebler DC. Evaluation of Strong Cation Exchange versus Isoelectric Focusing of Peptides for Multidimensional Liquid Chromatography-Tandem Mass Spectrometry. J Proteome Res. 2008;119:1531–1537. doi: 10.1021/pr8004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma ZQ, Chambers SD, Litton MC, Sobecki MD, Zimmerman SM, Halvey LJ, Drake PJBS, Gibson PM, Tabb BW, DL IDPicker 2.0: Improved protein assemby with high discrimination peptide identification filtering. J Proteome Res. 2009 doi: 10.1021/pr900360j. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang B, Chambers MC, Tabb DL. Proteomic parsimony through bipartite graph analysis improves accuracy and transparency. J Proteome Res. 2007;6:3549–3557. doi: 10.1021/pr070230d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 50.Faddy MJ, Bosch RJ. Likelihood-based modeling and analysis of data underdispersed relative to Poisson distribution. Biometrics. 2001;57:620–624. doi: 10.1111/j.0006-341x.2001.00620.x. [DOI] [PubMed] [Google Scholar]
- 51.Berry GAP. Mid-P confidence intervals a brief review. The Statistician. 1995;44:417–423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Proteins identified in involved gastric mucosa of Ménétrier’s disease patients by shotgun proteomic analysis.
Fig. S1. Decreased proliferation in gastric mucosa of patient 8 one day after first dose of cetuximab. Although this patient opted to leave the trial after one treatment, there was a marked decrease in Ki-67 staining in the involved gastric mucosa. Scale bar is 250 microns.
Fig. S2. Long term changes in the stomach and colon of patient 3. (A) Patient 3 demonstrated partial improvement in foveolar hyperplasia after one month of treatment (top panel). Fovelar hyperplasia continued to regress further with long term cetuximab therapy, along with restoration of normal gastric glandular architecture and improved gastric acidity. Scale bar is 250 microns. (B) There was no evidence of increased severity of ulcerative colitis with long term cetuximab as determined by gross and microscopic (data not shown) examination of the colon at sigmoidoscopy.
Fig. S3. Patient 4 had histologically normal stomach at gastrectomy. Scale bar is 250 microns.