Abstract
Natural isoflavones have demonstrated numerous pharmacological activities in breast cancer cells, including antiproliferative activities and binding affinities for estrogen receptors (ERs). Chemical modifications on the isoflavone ring system have been prepared and explored for the development of new therapeutics for hormone-dependent breast cancer. The antiproliferative actions of the synthesized isoflavones on MCF-7 and MDA-MB-231 breast cancer cells were examined, as well as cytotoxicity, interaction with estrogen receptors, and proapoptotic activity. The compounds were screened in the absence and in the presence of estradiol to evaluate whether or not estradiol could rescue cell proliferation on MCF-7 cells. Several compounds were able to inhibit cell proliferation in a dose-dependent manner, and compounds containing the bulky 7-phenylmethoxy substituent resulted in cell toxicity not only in MCF-7 cells but also in MDA-MB-231 cells. Selected synthetic isoflavones were able to bind to estrogen receptor with low affinity. Apoptotic pathways were also activated by these compounds in breast cancer cells. The majority of the compounds can bind to both ERs. With low affinity, and their effects on hormone-independent breast cancer cells suggest that their ability to inhibit cell growth in breast cancer cells is not exclusively mediated by ERs. Thus, the synthetic trisubstituted isoflavones act on multiple signaling pathways leading to activation of mechanisms of cell death and ultimately affecting breast cancer cell survival.
Keywords: cell proliferation; isoflavones; breast cancer cells (MCF-7, MDA-MB-231); SERMs; apoptosis
Introduction
A marked difference in the incidence rates of breast cancer in women from western countries compared to those in nonwestern countries has been observed [1;2]. Investigators have hypothesized that an Asian diet, which is typically high in soy content, may be one factor that explains the lower incidence of breast cancer in those countries compared with other countries on a diet that lacks soy as a common component [3;4].
Soy products are rich in flavonoids. Flavonoids and isoflavonoids exhibit a range of human health-promoting activities that are currently the focus of intense study [5–7]. The isoflavones are strikingly similar in chemical structure to steroidal and nonsteroidal estrogens and thus exhibit hormonal and anticancer activities. The primary isoflavone component of soybeans associated with chemoprevention is genistein [8]. Genistein’s structure is similar to that of estradiol and therefore is able to bind to estrogen receptors [9]. Moreover, genistein has been found to bind preferentially to ERβ [10]. Animals receiving the highest concentration of dietary genistein developed the lowest number of mammary tumors per rat, suggesting that dietary genistein reduces susceptibility to mammary cancer in rats [11].
Selective estrogen receptor modulators (SERMs) are nonsteroidal compounds that interact with the estrogen receptor and can exert their effects in a tissue specific manner [12]. Hormone-dependent breast cancer tumors contain estrogen receptors and depend on estrogens for tumor growth. Tamoxifen has been the drug of choice to treat this type of cancer by blocking the binding of estrogens to the estrogen receptor. However, in many cases, tumors can develop resistance to the drug after long exposure. The development of drug resistance has encouraged the development and testing of new antiestrogens for the treatment of breast cancer. Because of its tissue specific antiestrogenic/estrogenic properties, genistein has been considered a natural selective estrogen receptor modulator [13–15].
Genistein has demonstrated other biological activities not directly associated with the estrogen receptor. Genistein inhibits the growth of MDA-MB-231 breast cancer cells, regulates the expression of apoptosis-related genes, and induces apoptosis through a p53-independent pathway [16]. Genistein was shown to be a potent growth inhibitor in hormone-independent MDA-MB-468 cells, suggesting that isoflavones can act via an ER-independent pathway [17]. Genistein has also been shown to inhibit mammalian DNA topoisomerase II in L-1210 cells [18].
For the past few years, our research group has been interested in the isoflavone basic ring system as a core of potential therapeutic agents for the treatment of hormone-dependent breast cancer [19–22]. Our hypothesis is to develop a new series of SERMs constructed on the isoflavone scaffold using genistein as our model compound.
Pike et al., have previously described the structure of ERα-LBD in the presence of its natural ligand E2, and the mixed agonist/antagonist RAL. Even more relevant to our design strategy, they further reported the first structural description of ERβ-LBD in complex with RAL and the β-selective partial agonist genistein [23]. Comparison of the ligand-binding mode of genistein in hERβ-LBD and also E2 in hERα-LBD within the cavity is extensively reviewed in their 1999 EMBO paper [23]. Briefly, the interactions of genistein within the hERβ-LBD, which are predominantly controlled by van der Waals contact and hydrogen bonding, allows the residues which align the cavity to yield to more tightly packing around the ligand in ERβ when compared to E2. These interactions attribute to the reported 40-fold higher affinity for ERβ binding. Crystallographic data supports structural views that show the phenolic ring of genistein being able to mimic the A-ring of E2 which is clamped in the narrow cleft between H3, H6 and the β-hairpin. The phenolic hydroxyl (O14) interacts with the side chains of Glu305 and Arg346 along with a buried water molecule. Pike et al. further report that the flavone portion of genistein adopts a conformation that mimics the C- and D-rings of E2 and situated so that the O2 hydroxyl hydrogen bonds with His475 at the distal end of the cavity [23].
In 2004, Manas et al., presented the X-ray structure determinations of the ERα LBD complexed with genistein and ERβ LBD complexed with genistein. The ligand binding mode of genistein bound to either isoform is essentially identical, and the binding site residues influence binding affinity to a great extent [24]. Their overall conclusions are that selectivity regarding ERα binding affinity are chiefly contingent on the design of more selective ligands by introducing functional groups on genistein or a genistein-like scaffold which will allow for a more energetically favorable penetration into the pocket of ERα. The oxygen-oxygen distance in estradiol is 10.9 Å, and genistein contains two phenolic groups separated by approximately 11 Å. Researchers have stated that the optimal pattern of hydroxylation that is necessary for a flavonoid to have estrogenic activity is at 4′ and 7 positions and an additional hydroxyl group at position 5 [25].
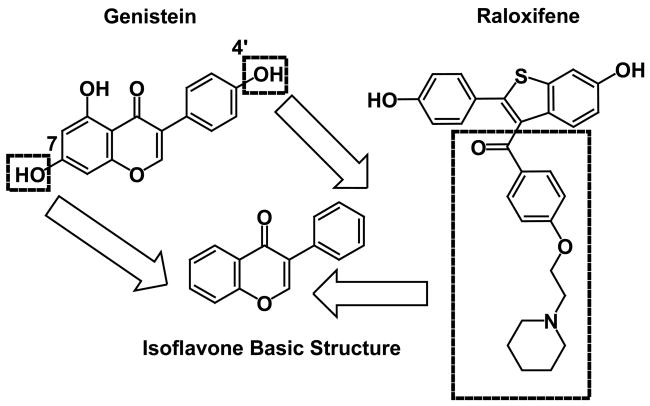
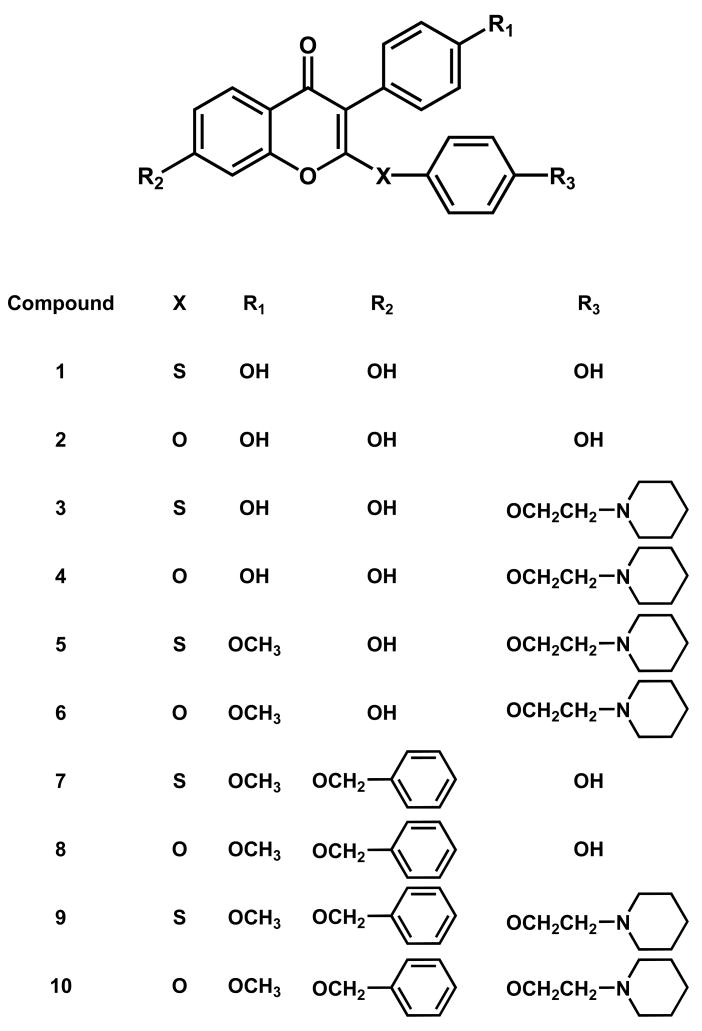
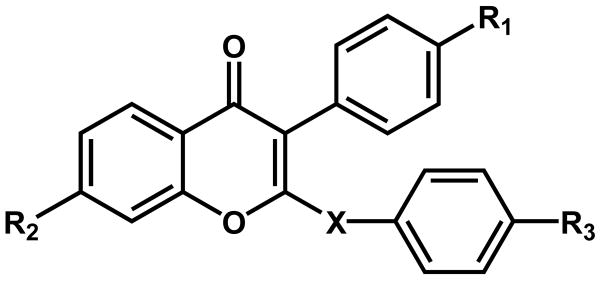
A common feature in many structural SERMs is the ethoxy amine-bearing side chain, and this basic side chain of many typical SERMs (e.g. tamoxifen) plays a key role in their tissue-selective antiestrogenic activity. The basic amine side chain in SERM activity prevents the proper positioning of helix 12 for agonistic activity [26]. In our drug design strategy, this basic chain was connected to the 2-position of the isoflavones scaffold [27]. Our drug design rationale can be summarized in Figure 1. On the basis of this rationale, we synthesized a library of 2,4′,7-trisubstituted isoflavones, as shown in Figure 2. We focused on compounds that contain a sulfur or oxygen as an isostere of the carbonyl group and envisioned these heteroatoms could serve as a hinge to direct the basic side chain to the proper region in the binding pocket of the estrogen receptor for the SERM profile [21].
Figure 1.
Rationale for the synthesis of the isoflavones.
Figure 2.
Chemical structures of the target 2,4′,7-trisubstituted isoflavones.
The present manuscript focuses on the antiproliferative action of a group of synthetic isoflavones on MCF-7 and MDA-MB-231 breast cancer cells. Our results show that these agents are able to inhibit cell proliferation on a dose-dependent manner. Different possible mechanisms were studied to assess their antiproliferative. Our results indicate that some selected isoflavones can bind to the estrogen receptor, as well as induce apoptosis.
Materials and Methods
Chemicals, biochemicals and reagents
Isoflavone analogs were prepared by Y.W. Kim as described [21]. 3,(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt was obtained from Promega (Madison, WI). 17β-Estradiol, 4-hydroxytamoxifen, and phenazine methosulfate were obtained from Sigma (St. Louis, MO). Genistein was obtained from Indofine Chemical Company (Belle Mead, NJ). Radiolabeled [2,4,6,7] 3H estradiol (3H-E2) was purchased from NEN Life Science, (Boston, MA). Human recombinant estrogen receptors, rhERα and rhERβ, were obtained from Invitrogen Corp. (Grand Island, NY). Glucose-6-phosphate dehydrogenase was purchased from Sigma (St. Louis, MO). Scintillation cocktail 3a70B was obtained from Research Products International (Mount Prospect, IL). MEM culture media (B-media), trypsin-EDTA, gentamycin, glutamine, fetal bovine serum (FBS), transferin, bovine insulin, and phosphate-buffered saline (PBS) were obtained from Invitrogen Corp. DMEM-F12 culture media was obtained from Sigma, and the human albumin was obtained from OSU Hospital Pharmacy.
Cell culture
MCF-7 and MDA-MB-231 cell lines were purchased from the American Type Culture Collection (ATCC) (Rockville, MD). Cells were maintained in phenol red-free modified MEM media (MEM, Earle’s salts, 1.5× amino acids, 2× non-essential amino acids, L-glutamine, and 1.5× vitamins), supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and 20 μg/ml gentamycin. Fetal calf serum was heat inactivated for 30 min in a 56°C water bath before use. Cell cultures were grown in monolayers at 37°C, in a humidified atmosphere of 5% CO2 in a Hereaus CO2 incubator. For all experiments, cells were plated in either 24-well or 96-well plates. During drug treatment, cells were grown on defined media containing DMEM/F12 media with 1.0 mg/ml human albumin, 5.0 mg/L human transferin and 5.0 mg/L bovine insulin.
Cell proliferation assay
Cellular proliferation in the presence or absence of experimental compounds was determined using the CellTiter 96® aqueous non-radioactive cell proliferation assay [28]. Rapidly growing cells were harvested, counted, and plated at a concentration of 1 × 104 cells/well for both MCF-7 and MDA-MB-231 cells in 400 μl total volume/well in the modified MEM media with 10% FBS as described earlier in conditions of cell culture. Prior to drug treatment, the modified MEM media were removed and cells were washed with PBS. Defined media was added and cells maintained for 24 hours. Then culture wells (n=6) were treated with the compounds (synthetic isoflavones ± estradiol) in 400 μl defined media every two days for a total of six days. Twenty-four hours after the last treatment, 3,(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt and phenazine methosulfate were prepared in PBS at a final assay concentrations of 333 μg/ml and 25 μM respectively. These solutions were combined and 20 μl of this mixture were added to each well. After 3 hours of incubation at 37°C, absorbance at 490 nm (reference wavelength 700 nm) was measured using a SPECTRAmax plate reader.
Estrogen receptor binding assays
Estrogen receptor binding assays were performed using a modified version of the protocol by Gaido et al. [29]. A 50% w/v hydroxyapatite (HAP) slurry suspension was prepared in 50 mM Tris-HCl (pH 7.5). Assay buffer (10 mM Tris pH 7.5, 10% of glycerol, 2 mM DTT, 1 mg/ml BSA) was prepared fresh before each assay. Immediately before the assay, hrERα or hrERβ stocks were prepared in ER binding buffer at a final concentration of 20 nM. Ligand stock solutions were prepared in 100% ethanol and stored at −20°C. Each reaction (n=3) consisted of 2 nM hrERα or hrERβ, 5 nM 3H-E2 (100,000–120,000 dpm) in binding buffer and different concentration of ligand or 2% of ethanol vehicle. 17β-Estradiol was tested in each assay at concentrations ranging from 10−7 to 10−11 M. Genistein and synthetic isoflavones were tested at concentrations ranging from 10−5 and 10−11 M. Excess of cold β-estradiol (10−6 M) was used to determine the non-specific binding (NSB). The protein-bound radioactivity was separated from free radioactivity by HAP precipitation. After overnight incubation at 4°C, the mixture was incubated with cold 50% HAP slurry for 15 min at 4°C, vortexing three times over this period. Each sample was centrifuged at 10,000 × g for 2 min at 4°C. The supernatant was discarded and the HAP pellet was washed with 1 ml of washing buffer and the suspension was centrifuged at 10,000 × g for 2 min at 4°C. The washing was repeated again twice. For ER-α binding assay the washing buffer was prepared with 40 mM Tris (pH 7.5), 1 mM EDTA, 100 mM KCl. ERβ washing buffer contained 40 mM Tris (pH 7.5). The bound radioactivity was then extracted from HAP by incubating the HAP pellet with 200-proof ethanol at room temperature for one hour (vortexing 4–5 times during incubation). The ethanol suspension was combined with 4 ml 3a70B scintillation cocktail and the amount of radioactivity counted in Beckman LS6500 (Beckman Coulter Inc., Fullerton, CA).
Estrogen receptor binding assay data analysis
The specific binding of 3H-E2 at each concentration of the compound of interest (B) was obtained after subtracting the non specific binding of 3H-E2 (NSB mean value) and expressed as percentage of the specific binding in the absence of the compound of interest (B0 = ethanol vehicle only). The concentration of the compound that reduced the specific binding of 3H-E2 (B0) by 50% (IC50) was determined by computer fitting of the data using nonlinear regression analysis using GraphPad Prism (GraphPad Software Incorporated, San Diego, CA). Data for each competitor and the 17β-estradiol (E2) standard curve were plotted as percent 3H-E2 bound versus Log of molar concentration. The relative binding affinity (RBA) for each ligand was calculated by dividing the IC50 of E2 by the IC50 of the compound and expressed as percent.
Cell cytotoxicity assay
Cellular cytotoxicity in the presence or absence of experimental compounds was determined using the CellTiter 96® aqueous non-radioactive cell proliferation assay. Rapidly growing cells were harvested, counted, and plated at a concentration of 1 × 104 cells/well for both MCF-7 and MDA-MB-231 cells in 100 μl total volume/well into 96-well microtiter plates. After 24 hours, modified MEM media was removed and cells were washed one time with PBS. Culture wells (n=6) were treated with the compounds (100 μl volume), dissolved in defined media and incubated for 48 hours at 37°C. After incubation, the same protocol as described above was followed and absorbance was measured.
Analysis of apoptosis
Apoptosis was determined by selective denaturation of DNA in apoptotic cells by formamide and detection of denatured DNA with a monoclonal antibody to single-stranded DNA using an ELISA kit (CHEMICON, Temecula, CA) [30]. Cells were plated in a 96-well flat bottom plate from 0.5 × 104 to 1 × 104 cells/well in B-media. Cells were allowed to adhere to wells overnight. Following incubation, compounds were made up in defined media and a 10 μM screen was performed in each cell line with respective compounds in triplicate for 48 hours. After 48 hours, the plate was centrifuged at 200 × g for 5 min, media was removed followed by the addition of 200 μl of fixative. The plate was incubated for 30 min at 37°C, at which point the fixative was removed and the plate dried for 1–2 hours at room temperature. Fifty microliters of formamide was added to each well following a brief incubation at room temperature for 10 min. The DNA in apoptotic cells was denatured by heating the plate for 10 min, then briefly cooling the plate for 5 min at 4°C following removal of formamide. The plate was rinsed three times with 200 μl of PBS following one hour incubation at 37°C with 200 μl of 3% blocking agent. After removal of the blocking agent, 100 μl of antibody mixture were added to each well for 30 min at room temperature. The plate was washed three times with 1× wash buffer using 250 μl of wash buffer/well followed by the addition of 200 μl of ABTS solution added to each well for 15–60 minute incubation. The reaction was stopped by the addition of 100 μl of a stop solution and absorbance was measured at 405 nm on a SpectroMax platereader.
Statistical analysis
Statistical and graphical analysis information was determined using GraphPad software and Microsoft Excel (Microsoft Corporation, Redmond, WA). Determination of IC50 values were performed using nonlinear regression analysis. Statistically significant differences were calculated with the two-tailed unpaired Student’s t-test and P values reported at 99% confidence intervals.
Results
Effect of synthetic isoflavones on breast cancer cells proliferation
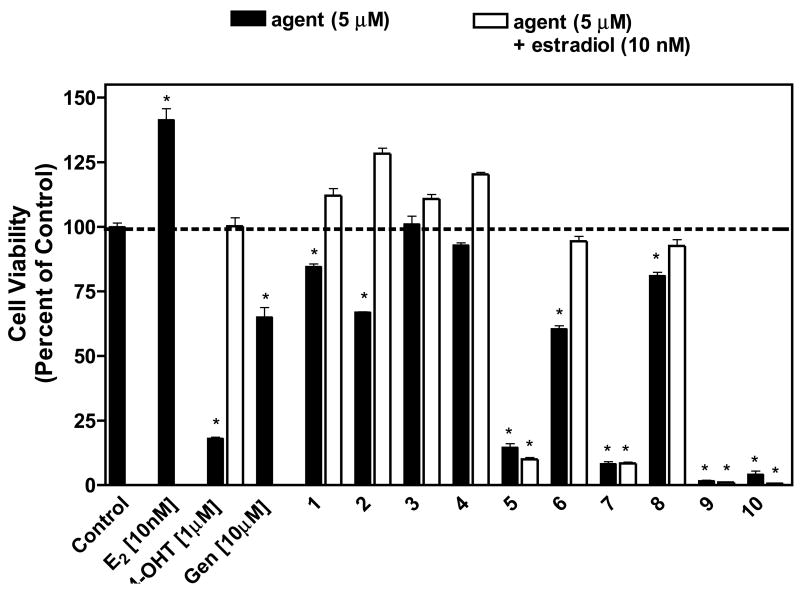
A series of synthetic isoflavones was synthesized and first tested in vitro to assess the ability of the compounds to inhibit cell proliferation of human breast cancer cells exposed to the compounds in the absence and presence of exogenous estradiol for a period of six days. Intrinsic estrogen antagonist activity was determined in vitro by measuring the ability of these compounds to inhibit estrogen-induced proliferation of human MCF-7 breast cancer cells (Figure 3). The addition of exogenous estradiol to hormone-responsive MCF-7 cells resulted in a significant stimulation of cell growth, as expected. When MCF-7 cells were treated with 4-hydroxy-tamoxifen, cell growth was inhibited significantly. When studying the effect of the natural isoflavone genistein, we found that cell growth was significantly inhibited with a greater effect at a higher concentration of 10 μM. Genistein at low concentrations has been demonstrated to act as an estrogen agonist and promote the growth of estrogen-responsive human MCF-7 breast cancer cells in vitro, whereas it produces estrogen antagonist effects at higher concentration [9, 38].
Figure 3. Effect of synthetic isoflavones on cell proliferation in the presence and absence of estradiol.
MCF-7 cells were treated with 5 μM of each of the agents alone and/or in the presence of 10 nM estradiol, and cell viability was measured as described in the experimental section. Estradiol (E2), 4-hydroxytamoxifen (4OHT), and genistein (Gen) were used as controls. The results were normalized against a control treatment with vehicle (DMSO). *, P < 0.0001 vs. control by unpaired t test, n = 6.
Compounds 1, 2, and 6 displayed antiproliferative activity alone but not in the presence of estradiol, whereas compounds 3 and 4 displayed no activity at all. Compound 5 reduced the stimulatory effect of estradiol and suppressed cell proliferation by approximately 90%, and the addition of 10 nM estradiol did not alter its antiproliferative activity. The potency of compound 5 suggests that this compound might be acting through estrogen receptor independent pathways. Compounds sharing the 7-phenylmethoxy substitution, 7, 9 and 10, resulted in a marked decrease in cell proliferation. Again, estradiol did not attenuate the antiproliferative effects. Compound 8, on the other hand, did not show pronounced effects even though it contains the 7-phenylmethoxy substitution.
Dose-dependent study of synthetic isoflavones in breast cancer cells proliferation
The compounds were evaluated for cell proliferation inhibition in a dose-dependent manner. The concentrations used for this study ranged from 0.001 to 20 μM. Compounds 3, 4, and 8 showed no significant activity even at the highest concentration tested. Cell proliferation was inhibited in a dose-dependent manner by 4-hydroxytamoxifen and compounds 1, 2, 5, 6, 7, 9, and 10. For the synthetic isoflavones containing the sulfur-linked side chain, the trend in potency was found to follow this order; 5 > 9 > 7 > 1. For the synthetic isoflavones containing the ether-linked side chain, the trend in potency was found to follow this order; 10 > 6 > 2. The IC50 values for each compound were calculated using a non-linear regression curve (Table 2).
Table 2.
IC50 values for cell proliferation inhibition for the synthetic isoflavones.
| Compound | IC50 ± S.D. (μM) |
|---|---|
| 1 | 11.1 ± 5.0 |
| 2 | 8.2 ± 2.0 |
| 5 | 0.04 ± 0.01 |
| 6 | 6.3 ± 1.0 |
| 7 | 2.1 ± 0.4 |
| 9 | 1.8 ± 0.6 |
| 10 | 2.9 ± 0.2 |
| 4-OHT | 4.2 ± 2.0 |
Estrogen receptor binding affinities of synthetic isoflavones
Several synthetic isoflavones were able to suppress MCF-7 cell proliferation when administered as single agents, whereas co-administration of estradiol with these isoflavones attenuated their antiproliferative effects. These observations suggested that these isoflavones may be altering proliferation via antagonism of an estrogen receptor mediated pathway. The compounds were then evaluated for their ability to bind to human ERα and ERβ in an isotopic ER competitive binding assay measuring the displacement of 3H-17β-estradiol from ER-complex by the tested substance. Human recombinant ERα and ERβ proteins were used to assess the binding selectivity toward the two isoforms of ER. The concentration at which the unlabeled ligand displaces half the specific binding of 3H-E2 to the ER (IC50) was determined by computer fitting of the data using nonlinear regression analysis. The relative binding affinity (RBA) of the tested substance was calculated as IC50(17β-estradiol)/IC50(tested substance) × 100.
The binding data reported reflects only the compounds that exhibited binding in one or either subtype as opposed to no binding at all, which was true of the remaining subset of compounds. Dose-response curves of compounds 1–2 and 4–6 for ERα affinity and for ERβ affinity along with calculated RBA values ERα and ERβ are reported in Table 1. With the exception of 5, which exhibited affinity only for ERα, all the tested compounds showed binding affinities for both ER subtypes. All the compounds with the bulky basic chain at the 2-position of the ring system have higher affinity for ERα than for the other subtype. On the other hand, compounds 1 and 2, which lack the basic side chain, showed stronger binding affinity to ERβ than to ERα. As previously reported by Kuiper et al. [10], genistein, the original lead compound, presented strong binding affinity for ERβ and moderate affinity for ERα, with an RBA for ERβ 13-fold higher than that for ERα.
Table 1. Relative binding affinities (RBA) of the synthetic isoflavones and genistein (GEN) for ERα and ERβ.
For each binding assay, RBA of a specific ligand was calculated by dividing the IC50 of E2 by the IC50 of the tested compound and expressed as percent.
| ||||||
|---|---|---|---|---|---|---|
| Compound | X | R1 | R2 | R3 | RBA (ERα) | RBA (ERβ) |
| 1 | S | OH | OH | OH | 0.01 | 0.19 |
| 2 | O | OH | OH | OH | 0.34 | 0.66 |
| 4 | O | OH | OH | 2-(1-piperidin-1-yl)ethoxy | 14.7 | 1.66 |
| 5 | S | OMe | OH | 2-(1-piperidin-1-yl)ethoxy | 0.02 | no binding |
| 6 | O | OMe | OH | 2-(1-piperidin-1-yl)ethoxy | 3.1 | 0.87 |
| GEN | 1.26 | 16.8 | ||||
| E2 | 100 | 100 | ||||
Effect of synthetic isoflavones on breast cancer cells cytotoxicity
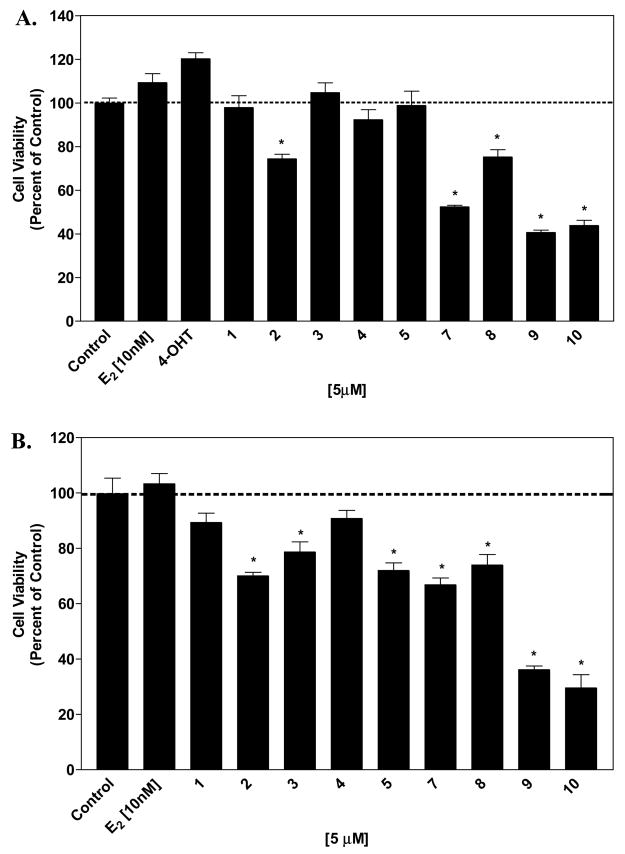
Our previous results imply that the mechanism of action of these agents may involve other pathways that are not associated with estrogen receptor mediated mechanisms. We exposed both MCF-7 and MDA-MB-231 cells for 48 hours to the synthetic isoflavones to study the effect of the agents on cell cytotoxicity. Compounds containing the 7-phenylmethoxy substitution, 7, 8, 9, and 10, resulted in cell cytotoxicity in MCF-7 cells. Compound 2 also resulted in cell cytotoxicity after 48 hours (Figure 4A). At these short exposure times, estradiol and 4-hydroxytamoxifen did not exhibit stimulatory and inhibitory properties. The hormone-independent cell line MDA-MB-231 was more sensitive to the synthetic isoflavones. Compounds containing the 7-phenylmethoxy substitution, 7, 8, 9, and 10, resulted in cell cytotoxicity. In addition, compounds 2, 3 and 5 also showed cytotoxic activities (Figure 4B).
Figure 4. Effect of synthetic isoflavones on cell cytotoxicity.
MCF-7 cells (A) and MDA-MB-231 cells (B) were treated with each of the agents at the indicated concentrations and cell viability was measured as described in the experimental section. The results were normalized against a control treatment with vehicle (DMSO). *, P < 0.0001 vs. control by unpaired t test, n = 6.
Measurement of Apoptosis
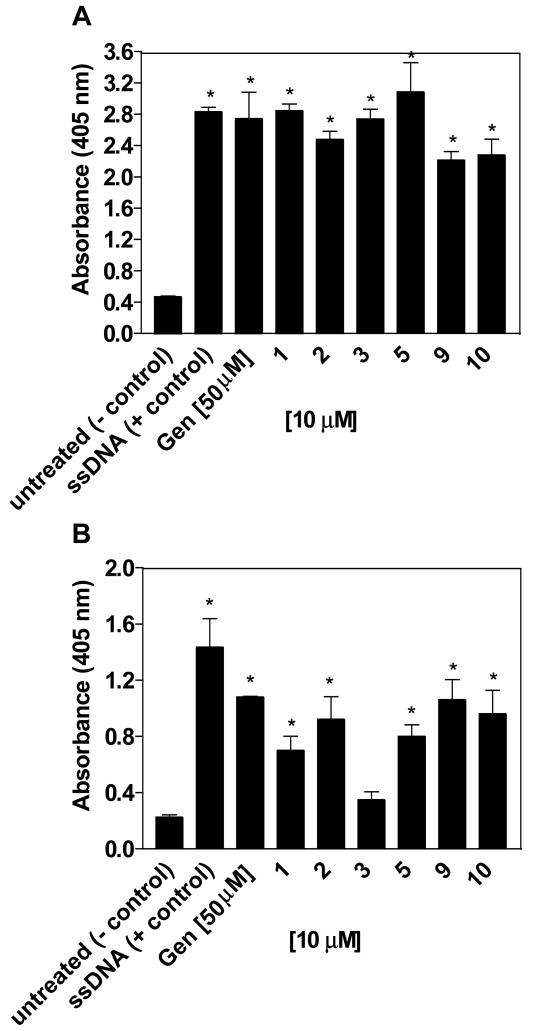
Based on the report of genistein’s ability to induce apoptosis, the isoflavone derivatives were tested to determine whether they played a role in apoptosis induction in both MCF-7 and MDA-MB-231 breast cancer cell lines [16]. Evaluation of synthetic isoflavones at 10 μM over a 48 hour treatment was performed to test the apoptotic inducing ability in both MCF-7 and MDA-MB-231 breast cancer cell lines using the ssDNA ELISA kit. The ELISA kit is able to differentiate between apoptotic and necrotic cells due to the absence of immunoreactivity in necrotic cells. The apoptosis data reported is reflective of only those compounds that demonstrated apoptosis induction in one or both of the breast cancer cell lines and is representative of the entire group of compounds screened in this paper. Several compounds showed a 1 to 2-fold increase or higher in absorbance than negative control and have ability to induce apoptosis. Compounds 1, 2, 3, 5, 9, and 10 showed the ability to induce apoptosis in MCF-7 cells and also to a lesser degree in MDA-MB-231 cells at 10 μM (Figure 5). In each case, the estrogen-dependent MCF-7 cells were more sensitive to the effects of these agents.
Figure 5. Apoptosis induced by 2, 4′-7 trisubstituted isoflavones.
MCF-7 cells (A) and MDA-MB-231 cells (B) were treated with 10 μM of each agent for 48 hours and analyzed by ELISA for detection of ssDNA as described in the experimental section. Genistein at 50 μM and ssDNA were used as controls. Results that showed significant difference in absorbance compared to the negative control were characterized as inducing apoptosis. *, P < 0.01 vs. control by unpaired t test, n = 3.
Discussion
The effect of the synthetic isoflavones cell on breast cancer cell growth were examined in both the hormone-dependent cell line MCF-7 and the hormone-independent cell line MDA-MB-231. The compounds were evaluated in the absence and in the presence of estradiol to determine if the agents could inhibit estradiol-induced cell proliferation on MCF-7 cells (Figure 3). At a concentration of 5 μM, compounds 2 and 6 were able to inhibit cell proliferation in the absence of estradiol, but the addition of exogenous estradiol was able to restore cell proliferation. Compounds 5, 7, 9, and 10 demonstrated in high antiproliferative activity. The fact that their inhibitory potencies were not affected by the addition of estradiol suggests that the antiproliferative activities of these agents may not be mediated by estrogen-receptor dependent pathways.
The synthetic isoflavones were designed based on the isoflavone core of genistein, a molecule that has shown SERM like properties [31]. Our hypothesis was that isoflavones possessing the amine bearing side chain of raloxifene, a clinically used SERM, might exhibit greater antiestrogenic activity in hormone dependent breast cancer cells than genistein. Since SERMs display their activities through binding to the estrogen receptors, the synthetic isoflavones which inhibited cell proliferation alone but not in the presence of estradiol were evaluated for their ability to bind to human ERα and ERβ. The tested compounds, with the exception of 5, exhibited low affinities to both ER subtypes. Compounds with the bulky basic chain at the 2-position of the ring system, compounds 4, 5 and 6, showed higher affinity for ERα than for ERβ (Table 1). In contrast, both compounds 1 and 2, which lack the basic side chain, showed a stronger binding affinity to ERβ than to ERα (Table 1). In addition, the 4′,7-dihydroxy analog 4 binds to ERα and ERβ with higher affinity than the 7-hydroxy-4′-methoxy analogs, 5 and 6 (Table 1). This was expected due to the importance of the 4′-hydroxyl group in the binding of genistein to the ER [23]. In addition, the oxygen linkage appears to be important to enhance the binding affinity to both ERs compared to the sulfur linkage. This result agrees with the previously reported increased affinity for ER for raloxifene analogs containing oxygen linkage [32]. Overall, our ER-binding affinity data of this subset of isoflavones suggest that the basic side chain may provide additional interaction with the ER (in particular with the ERα) and the sulfur linkage may significantly reduce this interaction by altering the orientation of the basic side chain.
Nonetheless, these synthetic isoflavones demonstrated low affinity for the estrogen receptors. Furthermore, these ER binding results do not correlate with the different antiproliferative activities of the flavonoids in the hormone-responsive MCF-7 cells. The weakest antiproliferative compound, compound 4, exhibited the highest binding affinity for the estrogen receptors, whereas the most potent antiproliferative agent, compound 5, showed very low affinity for ERα (Table 1)
The research then focused on the examination of possible mechanisms involved in the antiproliferative activities of synthetic isoflavones in breast cancer cell lines. Cell cytotoxicity assays were conducted to test for compounds that may result in direct toxicity. Compounds containing the bulky 7-phenylmethoxy substituent, resulted in cell toxicity not only in MCF-7 cells but also in MDA-MB-231 cells, suggesting that inhibition of cell proliferation can occur through hormone-independent pathways. Dose-dependent studies revealed that compound 5 is the most potent agent of this group of synthetic isoflavones with an IC50 of 0.04 μM, followed by 6 > 2 > 1. This order of potency suggests that the basic side chain and the 4′-methoxy group are important for the anti-proliferative activity of this set of compounds. The trend in potency for the series of compounds containing the bulky 7-phenylmethoxy substituent were 9 > 7 > 10.
Genistein at physiologically relevant concentrations has been well documented to act upon multiple cell-signaling molecules, and ultimately affecting cell survival by turning on the expression of cell death regulatory genes [18]. Compounds 1, 2, 3, 5, 9 and 10 showed apoptotic inducing effect at 10 μM concentrations in both MCF-7 and MDA-MB-231 cell lines. At higher concentrations, 25 μM and 50 μM genistein treatments over a 48 to 72-hour time period results in the expression of apoptosis relevant genes such as increased expression of p21WAF1, an important cell cycle arrest regulatory protein [33]. As such, our results for these novel synthetic isoflavones are consistent with previously established reports of isoflavones and their ability to induce apoptosis in MCF-7 and MDA-MB-231 breast cancer cell lines [16;34]. Compound 5 showed an apoptotic potential comparable to ssDNA and also demonstrated supportive antiproliferative and cytotoxic activities. Compounds 1, 2, 3, 5, 9 and 10 demonstrated the ability to induce apoptosis at 10 μM in both cell lines tested, with greater apoptosis observed in MCF-7 breast cancer cells. These results from the ELISA apoptosis screening bioassay suggest that the induction of apoptosis may contribute to the antiproliferative and cytotoxic effects of certain synthetic isoflavones. Further studies to validate apoptosis, i.e., TUNEL assay, DNA ladder, immunoblotting of relevant cell death markers, etc., must be performed before a definitive claim of apoptosis is reached.
Conclusions
The significance of this study is that our compounds, derivatives of genistein which itself is well documented as a potential chemopreventive and therapeutic agent against breast cancer, display very similar antiproliferative and cell-death mediated profiles as genistein. We postulate that our isoflavone analogs may in fact complement genistein in regulating gene expression and inhibiting growth of pre-cancer and cancer cells. Our results demonstrated that concentrations as low as 5μM of the isoflavone derivatives were able to inhibit the growth of both breast cancer cell lines, MCF-7 and MDA-MB-231, whereas concentrations of 50μM of genistein have been reported as showing antiproliferative and cytotoxic activity in different cancer cell types. Therefore, these isoflavone derivatives may be an ideal chemopreventive or therapeutic agent for breast cancer. However, much remains to be studied about the efficacy, concentration and activity of these compounds in vivo.
Our research efforts are focused on the elucidation of the mechanism involved in the antiproliferative activity of selected synthetic isoflavones. Our study demonstrated that the majority of the compounds bind to both ERs with low affinity suggesting that the ability of this group of compounds to inhibit cell growth in the hormone dependent cancer cells might not be exclusively mediated by ERs. From the current study, we conclude that the tri-substituted isoflavones tested appear to be acting upon multiple signaling pathways leading to activation of mechanisms of cell death and ultimately affecting breast cancer cell survival. Our results provide sufficient support for further exploration of apoptotic mediated mechanisms at both the protein and gene expression level.
Acknowledgments
The authors would like to acknowledge Dr. Bin Su for the re-synthesis of the 2,4′,7-trisubstituted isoflavones. This work was supported by the National Institutes of Health (NIH) Grant R01 CA73698 (R.W.B.), the USAMRMC Breast Cancer Program DMAD17-00-1-0388 (R.W.B.), the NIH Chemistry and Biology Interface Training Program Grant T32 GM08512 (E.S.D.-C.), and The Ohio State University Comprehensive Cancer Center Breast Cancer Research Fund.
List of Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
- FBS
fetal bovine serum
- Gen
genistein
- HAP
hydroxyapatite
- IC50
inhibitory concentration 50%
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NSB
non specific binding
- 4-OHT
4-hydroxy tamoxifen
- PBS
phosphate buffer saline
- RBA
relative binding affinity
- SERMs
selective estrogen receptor modulators
- ssDNA
single stranded DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 2.Magee PJ, Rowland IR. Phyto-oestrogens. their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91:513–31. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP. Dietary fiber, phytoestrogens, and breast cancer. Nutrition. 1992;8:47–51. [PubMed] [Google Scholar]
- 4.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 5.Dixon RA, Steele CL. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends Plant Sci. 1999;4:394–400. doi: 10.1016/s1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- 6.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–38. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 8.Barnes S, Grubbs C, Setchell KD, Carlson J. Soybeans inhibit mammary tumors in models of breast cancer. Prog Clin Biol Res. 1990;347:239–53. [PubMed] [Google Scholar]
- 9.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–7. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 11.Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19:2151–8. doi: 10.1093/carcin/19.12.2151. [DOI] [PubMed] [Google Scholar]
- 12.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: Estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–44. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ. 1996;7:1345–51. [PubMed] [Google Scholar]
- 14.Hewitt AL, Singletary KW. Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model. Cancer Lett. 2003;192:133–43. doi: 10.1016/s0304-3835(02)00712-7. [DOI] [PubMed] [Google Scholar]
- 15.Constantinou AI, Lantvit D, Hawthorne M, Xu X, van Breemen RB, Pezzuto JM. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001;41:75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–72. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 17.Peterson G, Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun. 1991;179:661–7. doi: 10.1016/0006-291x(91)91423-a. [DOI] [PubMed] [Google Scholar]
- 18.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–7. [PubMed] [Google Scholar]
- 19.Bhat AS, Whetstone JL, Brueggemeier RW. Novel synthetic routes suitable for constructing benzopyranone combinatorial libraries. Tetrahedron Lett. 1999;40:2469–72. [Google Scholar]
- 20.Kim YW, Brueggemeier RW. A convenient one-pot synthesis of 2-(alkylthio)- isoflavones from deoxybenzoins using a phase transfer catalyst. Tetrahedron Lett. 2002;43:6113–15. [Google Scholar]
- 21.Kim YW, Mobley JA, Brueggemeier RW. Synthesis and estrogen receptor binding affinities of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-ones containing a basic side chain. Bioorg Med Chem Lett. 2003;13:1475–8. doi: 10.1016/s0960-894x(03)00132-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim YW, Hackett JC, Brueggemeier RW. Synthesis and aromatase inhibitory activity of novel pyridine-containing isoflavones. J Med Chem. 2004;47:4032–40. doi: 10.1021/jm0306024. [DOI] [PubMed] [Google Scholar]
- 23.Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JA, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J. 1999;18:4608–18. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manas ES, Xu ZB, Unwalla RJ, Somers WS. Understanding the selectivity of genistein for human estrogen receptor-β using x-ray crystallography and computational methods. Structure. 2004;12:2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Miksicek RJ. Estrogenic flavonoids: structural requirements for biological activity. Proc Soc Exp Biol Med. 1995;208:44–50. doi: 10.3181/00379727-208-43830. [DOI] [PubMed] [Google Scholar]
- 26.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 27.Grese TA, Pennington LD, Sluka JP, Adrian MD, Cole HW, Fuson TR, Magee DE, Phillips DL, Rowley ER, Shetler PK, Short LL, Venugopalan M, Yang NN, Sato M, Glasebrook AL, Bryant HU. Synthesis and pharmacology of conformationally restricted raloxifene analogues: highly potent selective estrogen receptor modulators. J Med Chem. 1998;41:1272–83. doi: 10.1021/jm970688z. [DOI] [PubMed] [Google Scholar]
- 28.Barltrop JA, Owen TC. 5-(3-carboxymethoxyphenyl)-2(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg & Med Chem Lett. 1991;1:611–14. [Google Scholar]
- 29.Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140:5746–53. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- 30.Frankfurt OS, Krishan A. Enzyme-linked immunosorbent assay (ELISA) for the specific detection of apoptotic cells and its application to rapid drug screening. J Immunol Methods. 2001;253:133–44. doi: 10.1016/s0022-1759(01)00387-8. [DOI] [PubMed] [Google Scholar]
- 31.Diel P, Olff S, Schmidt S, Michna H. Molecular identification of potential selective estrogen receptor modulator (SERM) like properties of phytoestrogens in the human breast cancer cell line MCF-7. Planta Med. 2001;67:510–4. doi: 10.1055/s-2001-16474. [DOI] [PubMed] [Google Scholar]
- 32.Palkowitz AD, Glasebrook AL, Thrasher KJ, Hauser KL, Short LL, Phillips DL, Muehl BS, Sato M, Shetler PK, Cullinan GJ, Pell TR, Bryant HU. Discovery and synthesis of [6-hydroxy-3-[4-[2-(1-piperidinyl)ethoxy]phenoxy]-2-(4-hydroxyphenyl)]b enzo[b]thiophene: a novel, highly potent, selective estrogen receptor modulator. J Med Chem. 1997;40:1407–16. doi: 10.1021/jm970167b. [DOI] [PubMed] [Google Scholar]
- 33.Chinni SR, Alhasan SA, Multani AS, Pathak S, Sarkar FH. Pleotropic effects of genistein on MCF-7 breast cancer cells. Int J Mol Med. 2003;12:29–34. [PubMed] [Google Scholar]
- 34.Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–82. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]